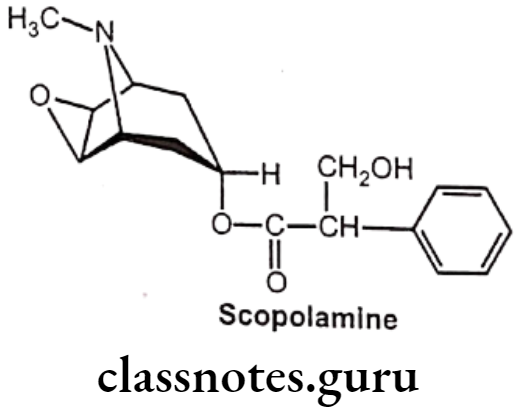
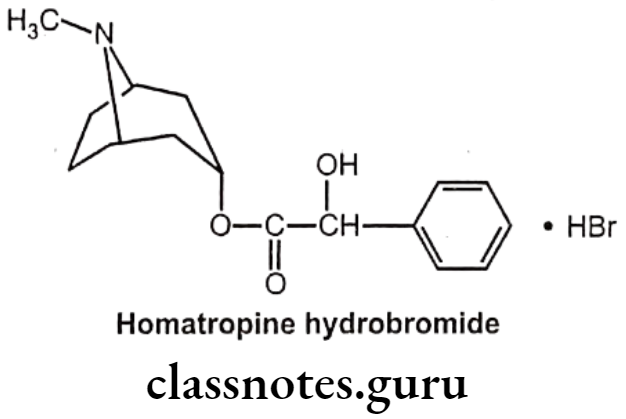
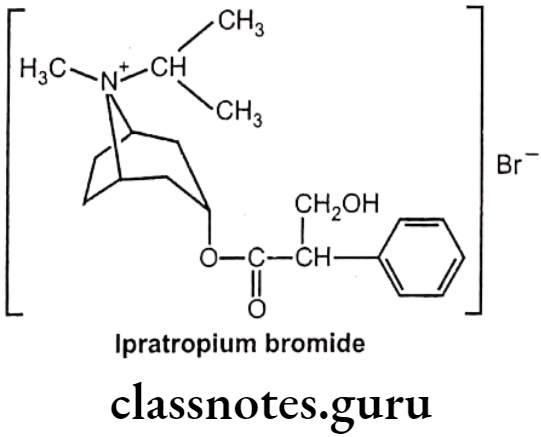
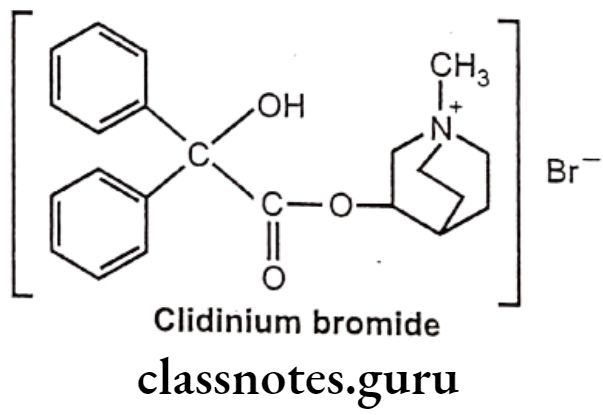
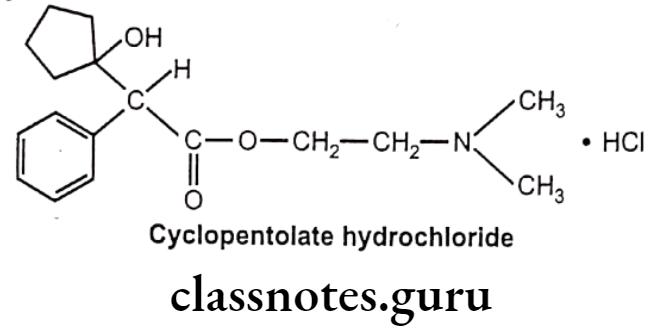
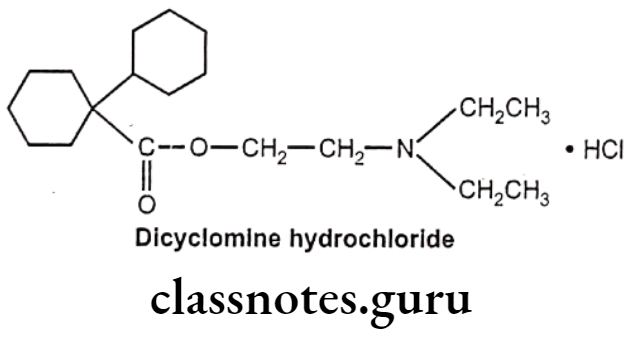
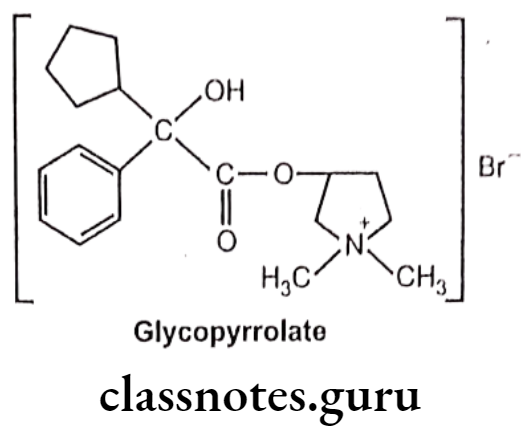
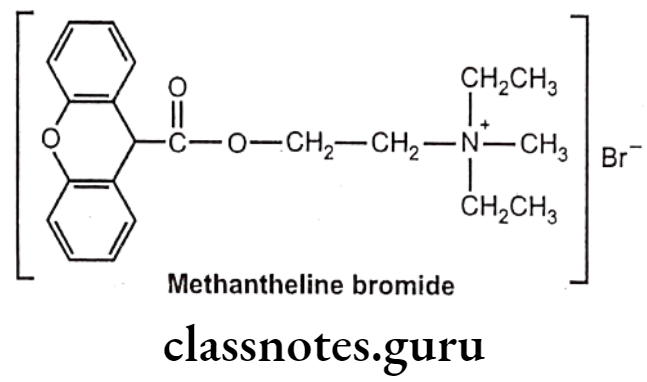
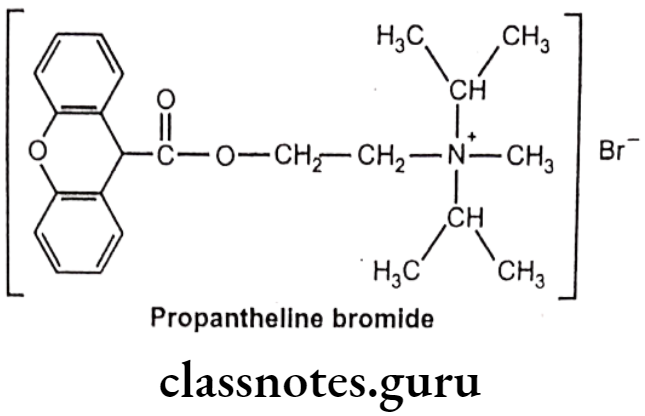
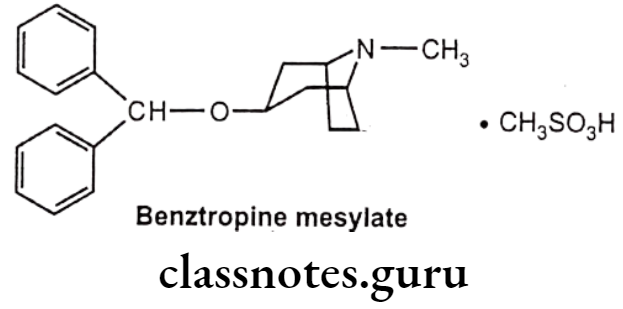
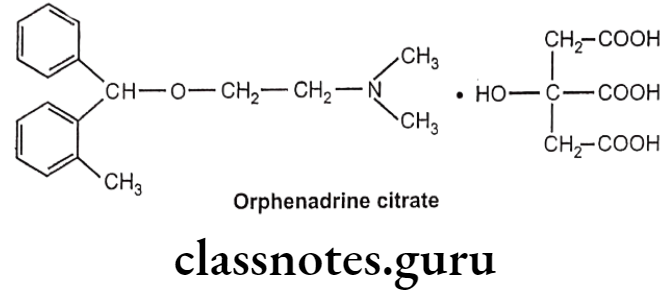
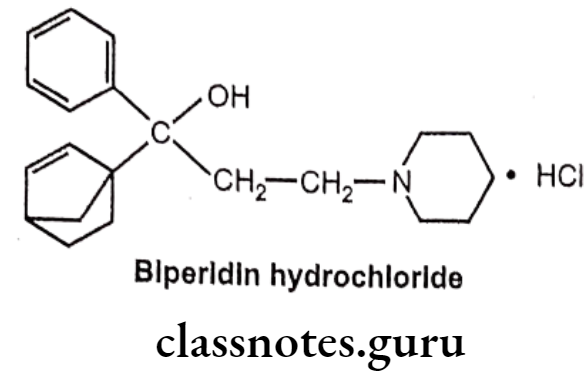
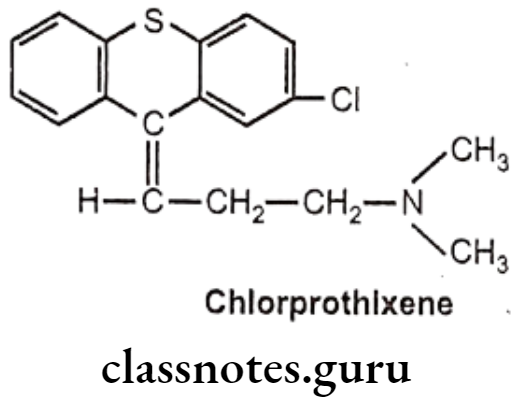
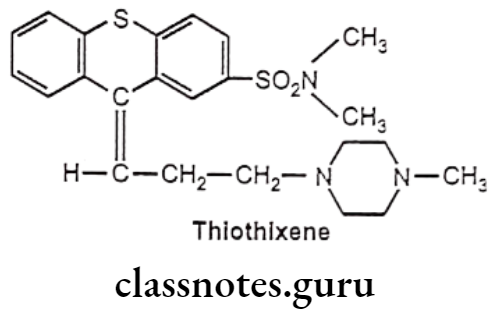
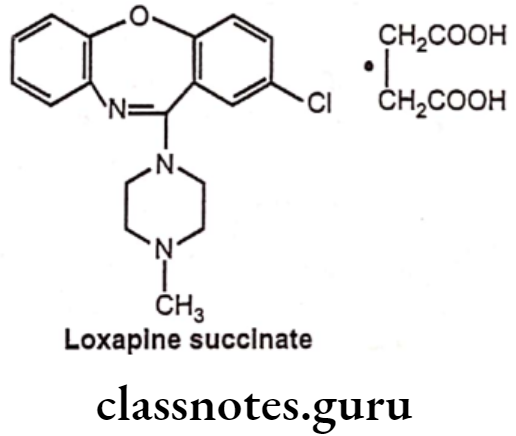
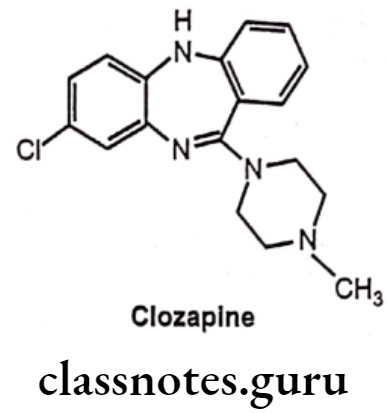
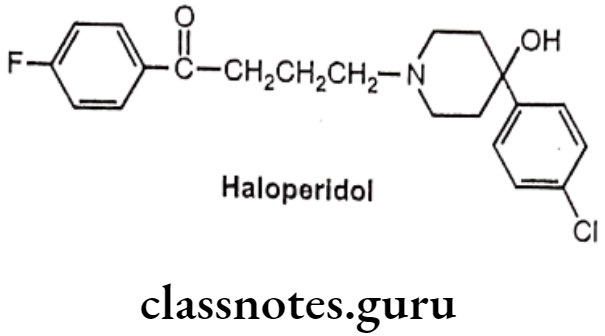
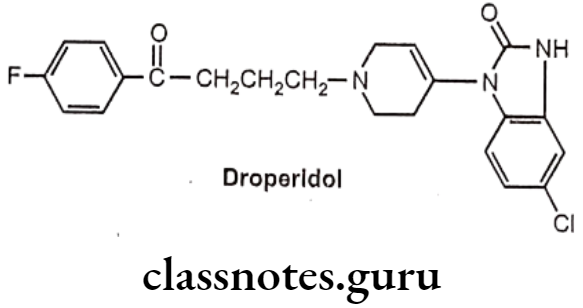
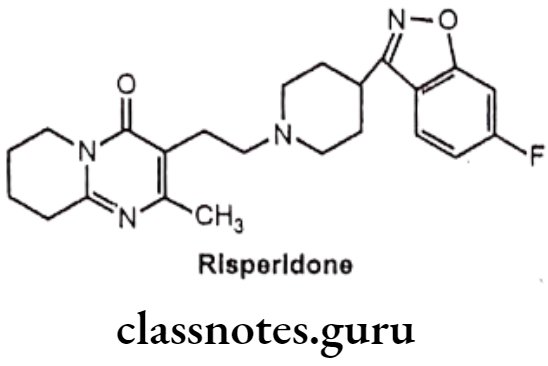
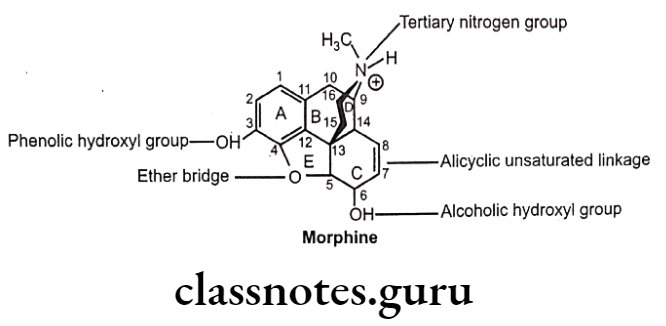
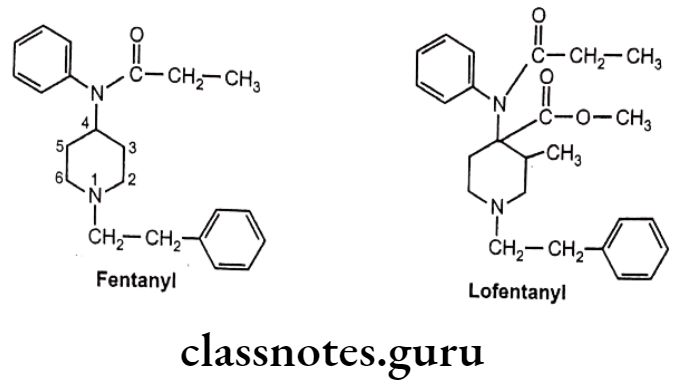
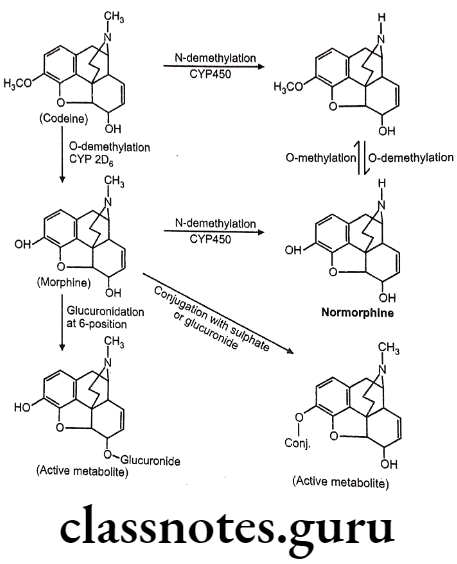
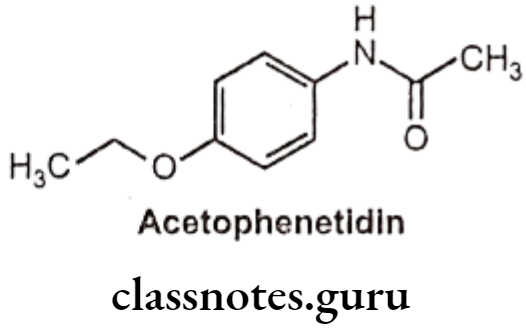
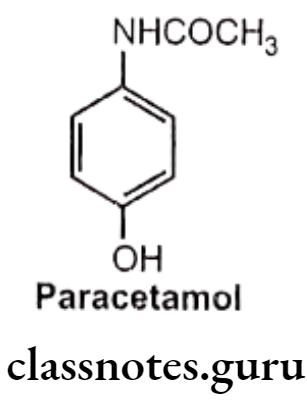
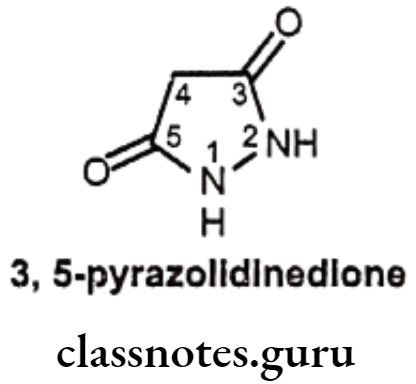
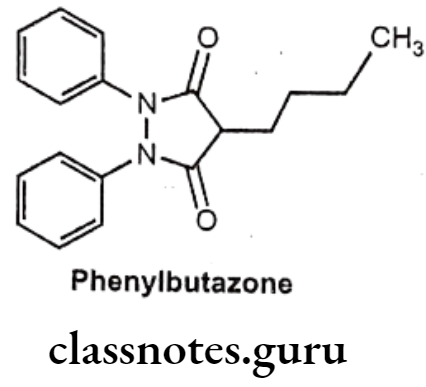
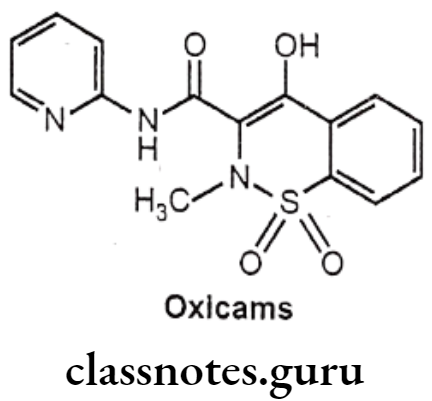
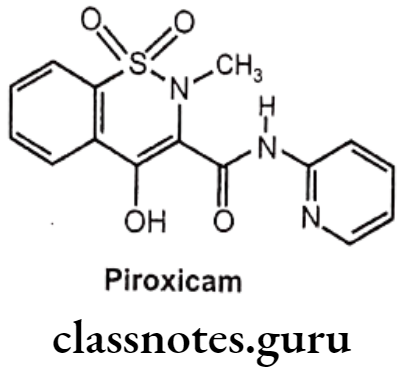
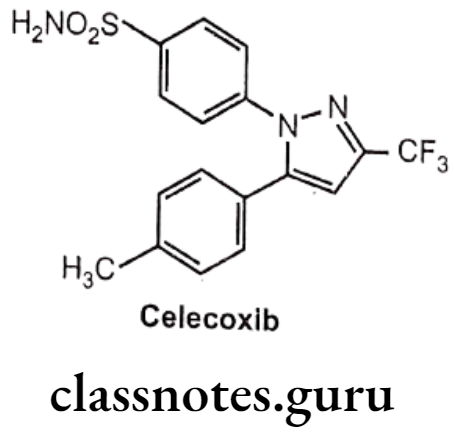
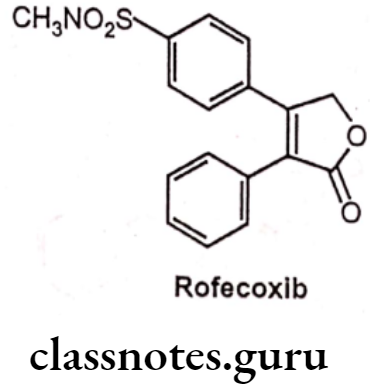
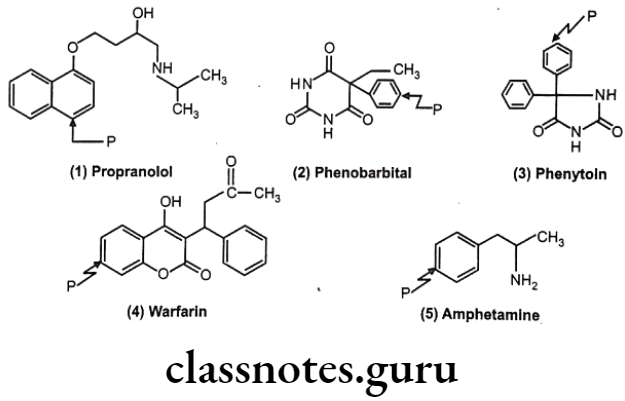
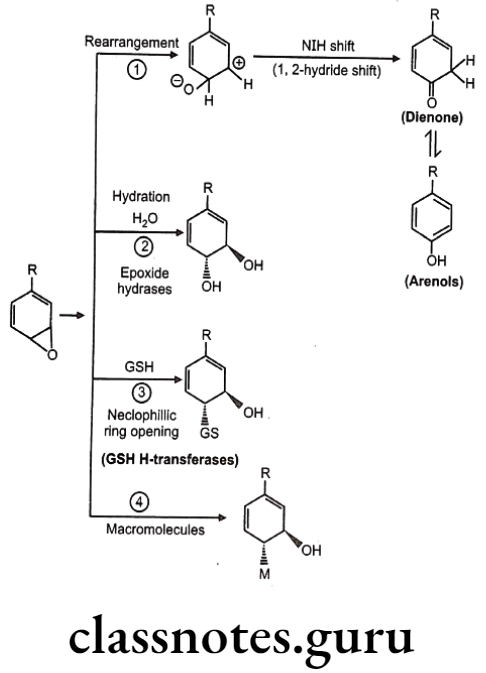
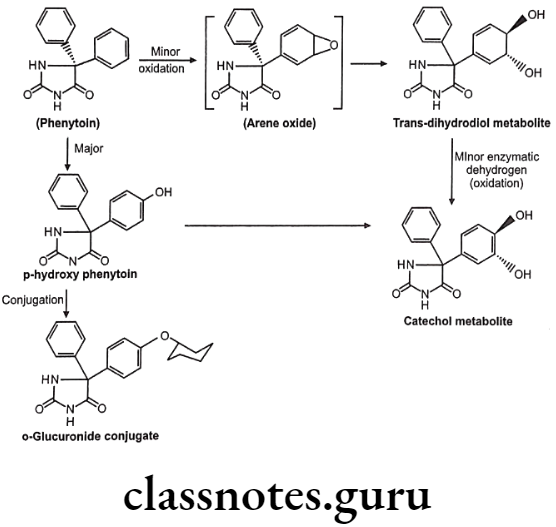
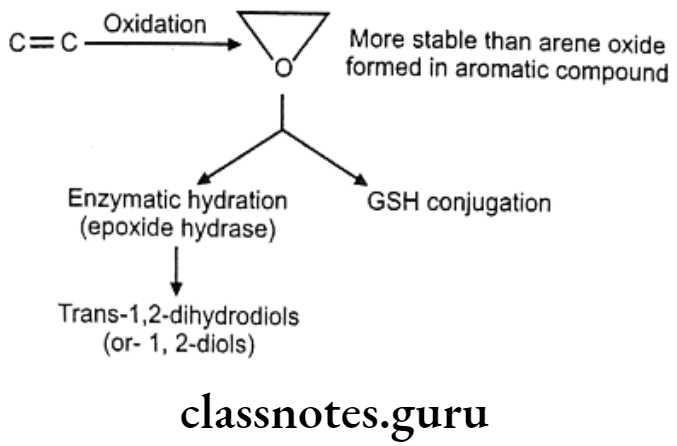
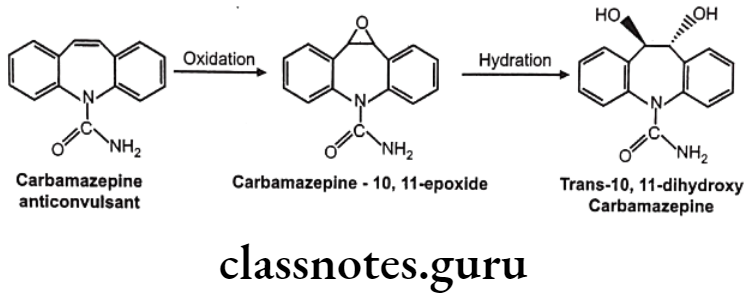
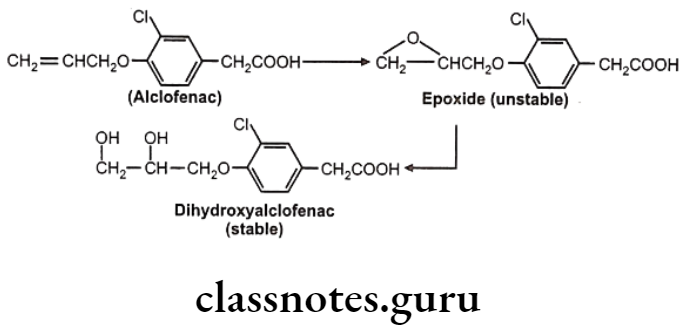
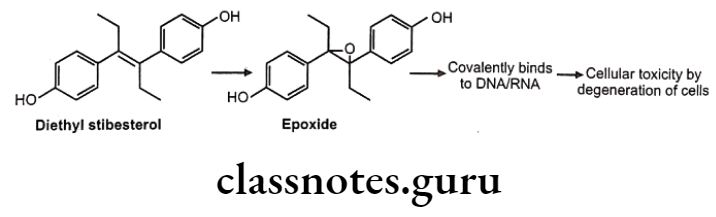
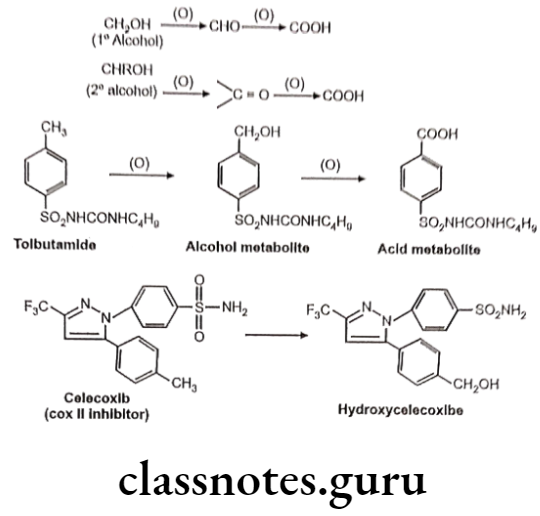
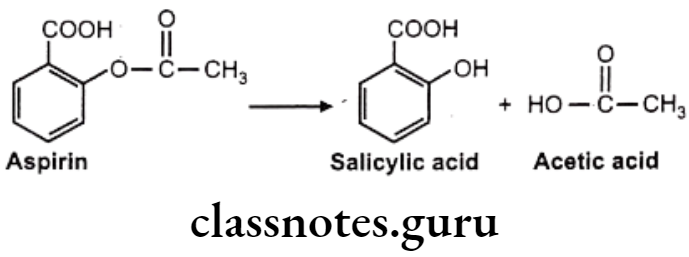
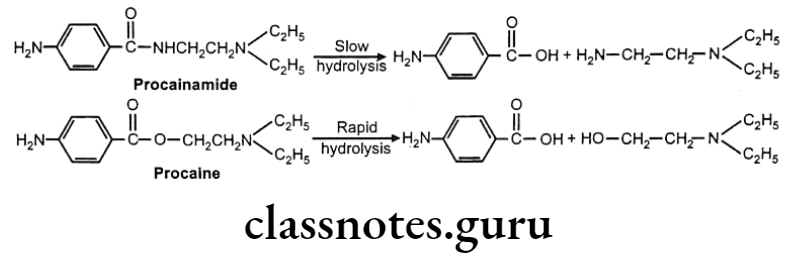
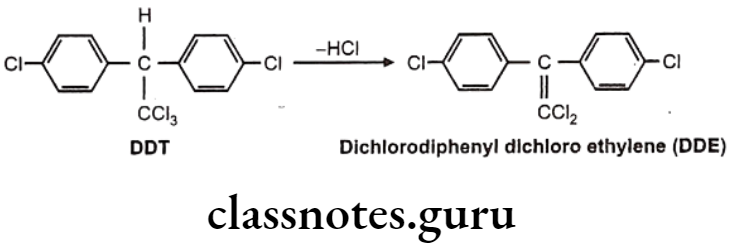
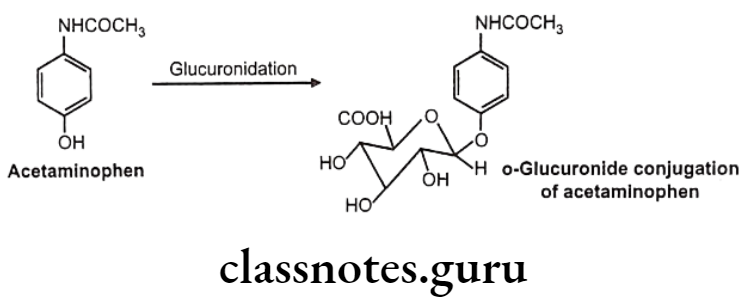
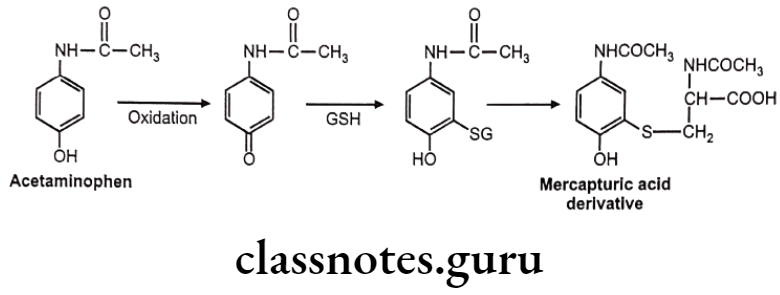
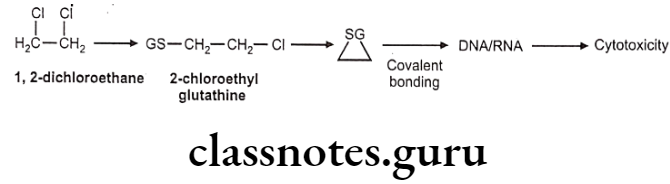
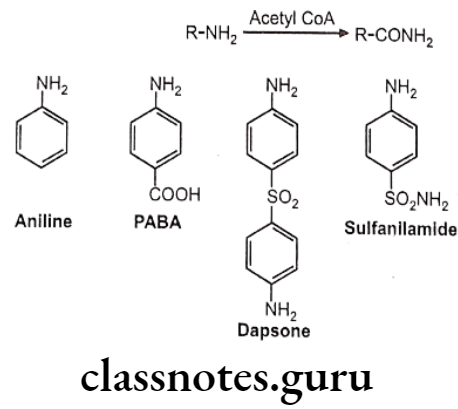
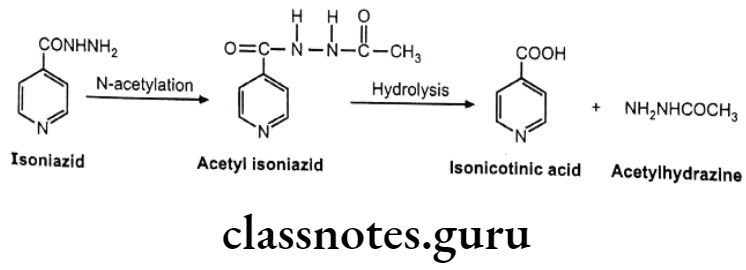
Drugs Acting On Autonomic Nervous System Introduction
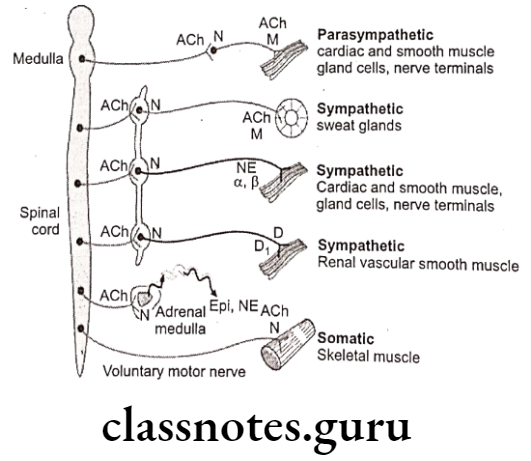
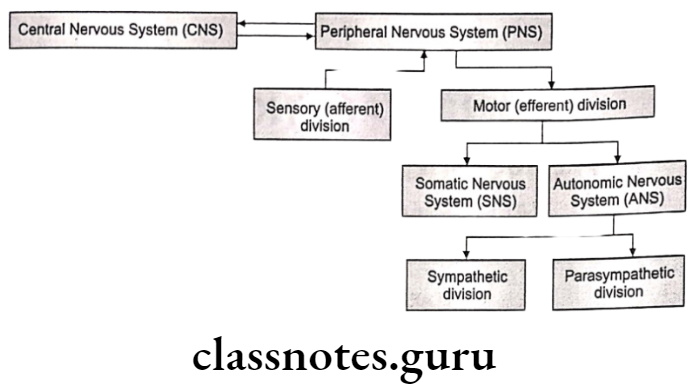
The human nervous system consists of the Central Nervous System (CNS) and the Peripheral Nervous System (PNS). CNS is composed of the brain (located in the cranial cavity) and the spinal cord (located in the vertebral cavity), which serve as the main control centers. for all body activities.
PNS is composed of nerves derived from the brain and spinal cord (12 pairs of cranial nerves and 31 pairs of spinal nerves) which serve as a linkage between the CNS and the body.
PNS can be subdivided into sensory (afferent) nerves and motor (efferent) nerves. Sensory nerves send nerve impulses from the body to the CNS to effector organs.
Motor nerves are divided into the Somatic Nervous System (SNS) which regulates the voluntary contraction of the skeletal muscles and the Autonomic Nervous System (ANS) which regulates the involuntary control of smooth, cardiac muscles and glands.
The Autonomic Nervous System (ANS) can be divided into sympathetic and parasympathetic branches where in general sympathetic nerves stimulate activities of the effect or organs (except digestive organs) and parasympathetic nerves inhibit activities of the effect or organs (except digestive organs).
The Autonomic Nervous System (ANS) controls a variety of involuntary and reflexive regulatory responses, e.g. the beating of the heart, expansion or contraction of blood vessels or pupils, etc.
It is responsible for both the “fight or flight” response that represents the body’s physiological response to crisis or stress and for the less crisis-driven functions of resting, repairing, digesting, and reproductive activities. Many of the drugs used to treat common conditions of the heart, circulation, and blood pressure by altering the functioning of the ANS.
Read and Learn More Medicinal Chemistry III Notes
There are three main components of ANS, viz., Sympathetic Nervous System (SNS), Parasympathetic Nervous System (PNS), and Enteric Nervous System (ENS).
ENS is intrinsic to the digestive system; it carries the key functions in support of systemic neurologic and immunologic well-being and is highly responsible for both physical and emotional stimuli. Comparison chart between Sympathetic Nervous System (SNS) and Parasympathetic Nervous System (PNS):
Adrenergic Drugs
The sympathetic system activates and prepares the body for vigorous muscular activity, stress, and emergencies. Adrenergic drugs are chemical agents that exert their principal pharmacological and therapeutic effects by either enhancing or reducing the activity of the various components of the sympathetic division of the Autonomic Nervous System.
Adrenergic drugs act by stimulation of the peripheral nerve endings of the sympathetic or adrenergic nerves, the action being exerted on the effector cells supplied by postganglionic endings.
As these drugs stimulate the adrenergic nerves directly by mimicking the action of norepinephrine or indirectly by stimulating the release of norepinephrine, they are also called adrenomimetic or sympathomimetic agents.
A sympathomimetic drug plays a vital role in various life-threatening disorders, like acute attacks of bronchial asthma, cardiac arrest, shock and allergic reactions. As most of adrenergic agents contain an intact or partially substituted amino group, they are also called as sympathomimetic amines.
Adrenergic Neurotransmitters
Adrenergic neurons release nor-epinephrine (nor-adrenaline) as the primary neurotransmitter. These neurons are found in the Central Nervous System and also in the Sympathetic Nervous System, where they serve as links between the ganglia and the effector organs.
The adrenergic neurons and receptors located either pre-synaptically on the neuron or post-synaptically on the effector organ, are the sites of action of the adrenergic drugs.
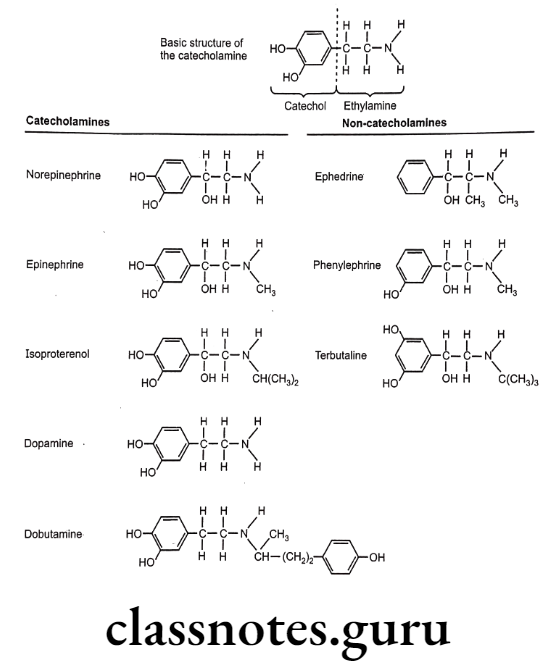
Catecholamines
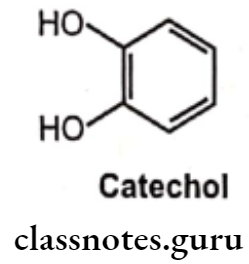
The catecholamines are so named because they contain a catechol group and an amine group. A catechol group is simply a benzene ring with hydroxyl groups on two adjacent carbons. The amine component of the catecholamines is ethylamine.
Because of their chemistry, all catecholamines have three properties in common: (1) They cannot be used orally. (2) They have a long duration of action. (3) They cannot cross the blood-brain barrier.
Catechol is highly susceptible to oxidation in the presence of oxygen to produce an ortho- quinone-like compound, which undergoes further reaction to give a mixture of colored products. Hence solution of catechol amines is stabilized by the addition of antioxidants (reducing agents) such as ascorbic acid or sodium bisulfite.
Synthesis, Storage, and Release of Catecholamine
Catecholamine biosynthesis takes place in adrenergic and dopaminergic neurons in the CNS, in sympathetic neurons in the ANS, and in the adrenal medulla. Epinephrine and norepinephrine each possess chiral carbon atoms, and thus exist an enantiomeric pair of isomers.
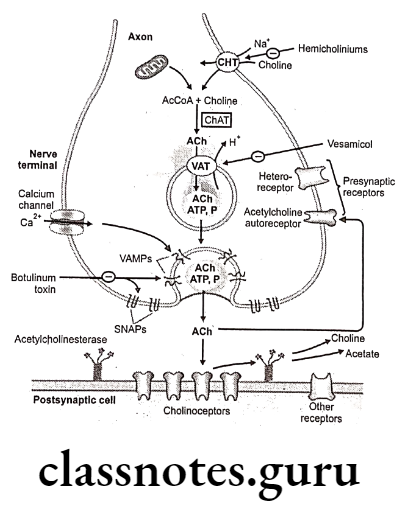
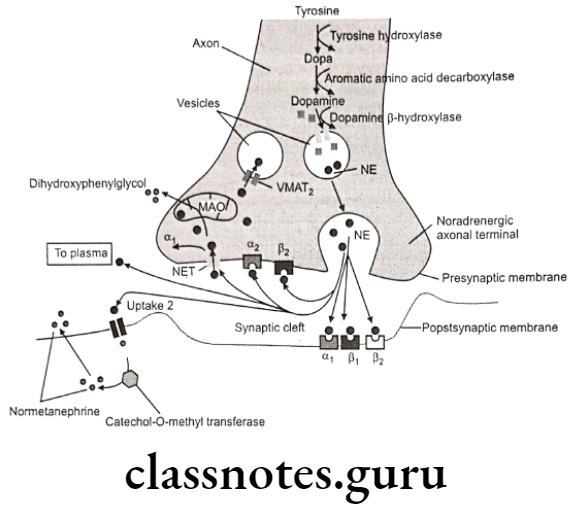
The enantiomer with R-configuration is biosynthesized by the body and possesses biological activity. Neurotransmission in adrenergic neurons is a process that involves five steps: synthesis, storage, release, and receptor binding of norepinephrine, followed by removal of the neurotransmitter from the synaptic gap.
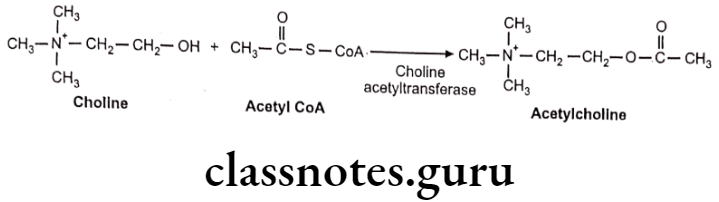
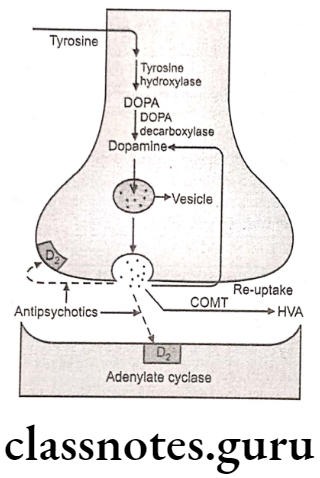
- Synthesis Of Nor-Epinephrine (Nor-Adrenaline): Hydroxylation of tyrosine is rate rate-limiting step.
- Uptake Into Storage Vesicles: Dopamine enters a vesicle and is converted to norepinephrine. Norepinephrine is protected from degradation in the vesicle.
- Release Of Neurotransmitter: Influx of the calcium causes fusion of the vesicle with the cell membrane in a process known as exocytosis.
- Binding To Receptor: The post synaptic receptor is activated by the binding of neurotransmitters.
- Removal Of Norepinephrine: Released norepinephrine is rapidly taken into the neuron.
- Metabolism: Norepinephrine is methylated by COMT and oxidized by MAO.
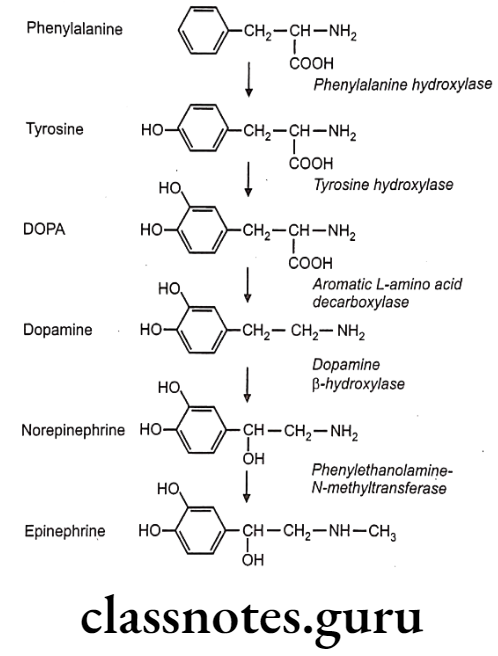
All five enzymes that are involved in the pathway of the biosynthesis of norepinephrine are synthesized within the cell bodies of the adrenergic neurons and transported to their nerve terminals. Phenylalanine-hydroxylase also called phenylalanine-4-monooxygenase coverts phenylalanine to tyrosine.
Conversion of tyrosine to L-B-(3,4-dihydroxy phenyl)-α- alanine (DOPA) is a rate-limiting step catalyzed by the enzyme, tyrosine-hydroxylase. Decarboxylation of DOPA to dopamine takes place by the enzyme aromatic amino-acid decarboxylase. Dopamine formation takes place in the cytoplasm.
In synaptic vesicles, dopamine is hydroxylated stereospecifically by the enzyme dopamine B-monooxygenase (dopamine-ß- hydroxylase) to give noradrenaline (norepinephrine). Norepinephrine undergoes methylation in the presence of the enzyme phenylethanolamine-N-methyl transferase to give adrenaline (epinephrine).
The norepinephrine formed is stored in vesicles as its adenosine triphosphate complex until the depolarization of neuron initiates the process of vesicle fusion with the plasma membrane and extrusion of norepinephrine into the synaptic cleft. Synthesis of norepinephrine and epinephrine also takes place in adrenal medulla.
The release of neurotransmitters occurs when an action potential opens voltage-sensitive calcium channels and increases intracellular calcium. Influx of calcium triggers the fusion of the vesicle with the surface membrane. This causes the release of neurotransmitter (norepinephrine) from the synaptic vesicle through exocytosis.
It then binds to the adrenoreceptor on the effector cells and produces effects by various mechanisms. The excess norepinephrine that are not bound to the postsynaptic receptor, binds to presynaptic receptors to decrease its own release. It then diffuses out, either by metabolism by COMT or is taken back up by the presynaptic neuron.
Uptake And Metabolism Of Catecholamines
The actions of norepinephrine at adrenergic receptors are terminated by a combination of processes, such as
- Uptake into the neuron and into extraneuronal tissues,
- Diffusion away from the synapse and uptake at non-neuronal sites, and
- Metabolism.
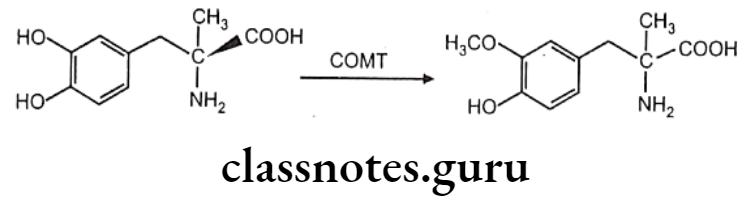
The two principal enzymes involved in catecholamine metabolism are namely monoamine oxidase (MAO) and catechol-o-methyl transferase (COMT).
Both of these enzymes are distributed widely throughout the body, with high concentrations found in the liver and kidney. Catecholamines that are administered orally become inactivated before they can reach to the systemic circulation.
Hence, catecholamines are ineffective if given by mouth. Both enzymes are very active and quickly destroy catecholamines, therefore, three catecholamines viz., norepinephrine, dopamine, and dobutamine are effective only if administered by continuous infusion (by IV route in an emergency), whereas other parenteral routes like subcutaneous and intramuscular will not yield adequate blood levels.
Because of the hydroxyl groups on the catechol portion of the catecholamines, they cannot cross the blood-brain barrier; therefore catecholamines have minimal effects on the CNS.
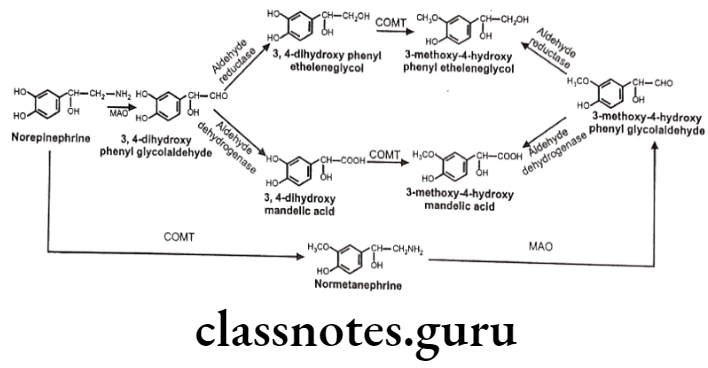
Norepinephrine (NE) is deaminated oxidatively by MAO to give 3,4-dihydroxy- phenylglycoaldehyde which is then reduced by aldehyde reductase to 3,4-dihydroxy- phenylethylene glycol. This glycol metabolite is primarily released into the circulation, where it undergoes methylation by the COMT to give 3-methoxy-4-hydroxyphenylethylene glycol.
3,4-dihydroxyphenylglycolaldehyde undergoes dehydrogenation in the presence of aldehyde dehydrogenase to give 3,4-dihydroxy-mandelic acid, which is then by the action of COMT convert to 3-methoxy-4-hydroxy mandelic acid. This metabolite is commonly referred to as vanillylmandelic acid. It is the end product of several pathways of norepinephrine metabolism.
Non-Catecholamines
The non-catecholamines have ethylamine in their structure but do not contain the catechol moiety that characterizes the catecholamines. The non-catecholamines differ from the catecholamines in three important respects:
- As they do not have a catechol group, non-catecholamines are not substrates for COMT and are metabolized slowly by MAO. As a result, the half-lives of non-catecholamines are much longer than those of catecholamines.
- As they do not undergo rapid degradation by MAO and COMT, non-catecholamines can be administered orally, whereas catecholamines cannot.
- Non-catecholamines are considerably less polar than catecholamines, and hence are more able to cross the blood-brain barrier.
Adrenergic Receptors
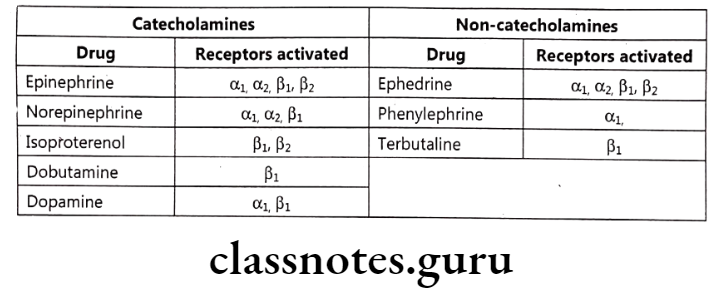
Catecholamines bind to specific receptors known as adrenergic receptors. There are two basic types of receptors: a-adrenergic receptors and B-adrenergic receptors. a-adrenergic receptors have subtypes such as a and α2, whereas ẞ-adrenergic receptors have subtypes such as B1 B2, and B3.
The action of neurotransmitters adrenergic drugs or sympathomimetic drugs upon these receptors is an important means of alternate ANS functions for the therapy of different disease states.
Receptor Distribution:
- α-Adrenergic Receptors: They are found in the smooth muscles of the iris, arteries, arterioles, and veins.
- a-Adrenergic Receptors: They mediate the inhibition of adrenergic neurotransmitter release.
- B1-Adrenergic Receptors: They are found in the myocardium where their stimulation increases the force and rate of myocardial contraction.
- B2-Adrenergic Receptors: They are found in bronchial and vascular smooth muscles where their stimulation causes smooth muscle dilation or relaxation.
- B3-Adrenergic Receptors: These receptors are expressed on fat cells and their stimulation causes lipolysis.
Norepinephrine activates primarily a-receptors and epinephrine activates primarily B-receptors, although it may also activate a-receptors. Stimulation of a-receptors is associated with constriction of small blood vessels in the bronchial mucosa and relaxation of smooth muscles of the intestinal tract. B-receptor activation relaxes bronchial smooth muscles which cause the bronchi of the lungs to dilate.
In addition, B-receptor stimulatory effects cause an increase in the rate and force of heart contractions. As a result, an increased amount of blood leaves the heart and is diverted from non-active organs to areas that actively participate in the body’s reaction to stress such as skeletal muscles, brain, and liver.
Fight Or Flight Response: The changes experienced by the body during emergencies have been referred to as the fight or flight response. These reactions are triggered both by direct sympathetic activation of the effector organs and by stimulation of the adrenal medulla to release epinephrine and lesser amounts of norepinephrine. These hormones enter the bloodstream and promote responses in effector organs that contain adrenergic receptors.
Effects of stimulation of the sympathetic division:
- To increase heart rate and blood pressure.
- To mobilize energy stores of the body.
- To increase blood flow to skeletal muscles and the heart while diverting flow from the skin and internal organs.
- Dilation of the pupils and the bronchioles.
- Affects gastrointestinal motility and the function of the bladder and sexual organs.
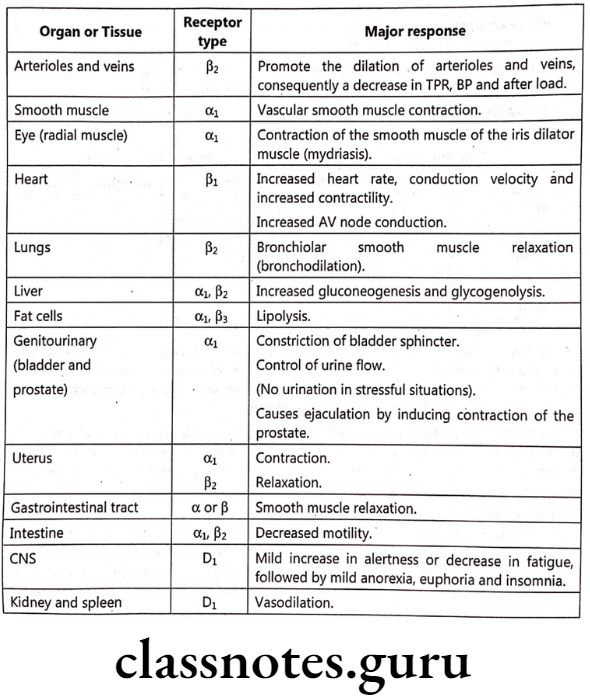
Selected Tissue Responses to Stimulation of Adrenoceptor Subtypes
Classification Of Sympathomimetic Agents
Sympathomimetic agents are subdivided into three classes: direct-acting, indirect-acting and dual (mixed) acting sympathomimetic agents.
Direct-Acting Sympathomimetics
Direct-acting agents elicit a sympathomimetic response by interacting directly with adrenergic receptors. They bind to and activate α1, α2, B1, and B2 receptors.
Naturally occurring molecules that bind to these receptors include,
Norepinephrine: A neurotransmitter which binds to a1, a2, and ẞ1 receptors. It is non-selective sympathomimetic.
Epinephrine: A hormone produced and secreted from the adrenal medulla that binds α1, α2, B1, and B2 receptors. It is non-selective sympathomimetic.
Dopamine: A neurotransmitter that binds to D1 or D2 receptors as well as α1, α2, and ẞ1 receptors.
a-receptor stimulation:
α-agonists act by G-protein activation of the enzyme phospholipase C, resulting eventually in the release of calcium, thereby increasing the intracellular calcium concentration. 2-agonists act by inhibiting adenylyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). This leads to decreased intracellular cAMP levels.
Alpha receptors are further subdivided into α1A, 1B, αic and α1D as well as α2A, 2B and

B-receptor stimulation:
B-agonists are selective to a subtype (B1, B2, and ẞ3) or nonselective- stimulate adenylyl cyclase, causing increased intracellular cAMP levels.

Dopamine-receptor stimulation:
Dopamine receptor agonists can act on D1 or D2 receptors. D1-receptors activate adenylyl cyclase and increase intracellular cAMP mainly in neurons and vascular smooth muscle. D2- receptors reduce intracellular cAMP and are found in the brain and as pre-synaptic receptors. Receptor Drug

Indirect Acting Sympathomimetics
Indirect-acting sympathomimetic agents produce effects, primarily by causing the release of NE (e.g., amphetamine) from adrenergic nerve terminals and preventing its re-uptake (e.g., cocaine and tricyclic antidepressants). The NE that is released by the indirect-acting agent activates the receptors to produce the response.
Mixed-acting Sympathomimetics
Mixed-acting drugs employ both mechanisms i.e., direct and indirect (e.g., ephedrine, metaraminol). They bind directly with adrenergic receptors and cause the release of NE. Ephedrine is used to treat nasal congestion.
Mechanisms For Activation Of Adrenergic Receptor
Drugs can activate adrenergic receptors by four basic mechanisms:
- Direct Receptor Binding: Direct interaction with receptors is the most common mechanism by which drugs activate peripheral adrenergic receptors. The direct-acting receptor stimulants produce their effects by binding to adrenergic receptors and mimicking the actions of natural transmitters (norepinephrine, epinephrine, and dopamine).
- Promotion of Norepinephrine Release: By acting on terminals of sympathetic nerves to cause norepinephrine release, drugs can bring about activation of adrenergic receptors.
Example: Amphetamines and ephedrine. - Inhibition of Norepinephrine Reuptake: Recall that reuptake of norepinephrine into terminals of sympathetic nerves is the major mechanism by which adrenergic transmission is terminated. By blocking norepinephrine reuptake, drugs can cause norepinephrine to accumulate within the synaptic gap, and can thereby increase receptor activation.
Example: Cocaine and tricyclic antidepressants (imipramine). - Inhibition of Norepinephrine Inactivation: Some of the norepinephrine in terminals of adrenergic neurons is subject to inactivation by monoamine oxidase (MAO). Hence, drugs that inhibit MAO can increase the amount of NE available for release, and can thereby enhance receptor activation.
Direct Acting Sympathomimetics
Structure-Activity Relationship:
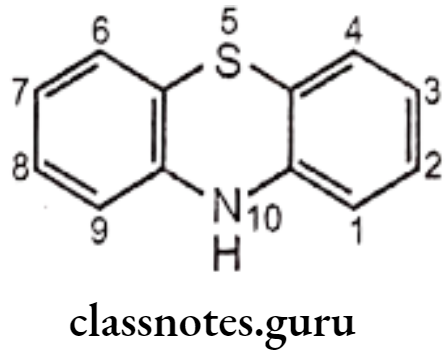
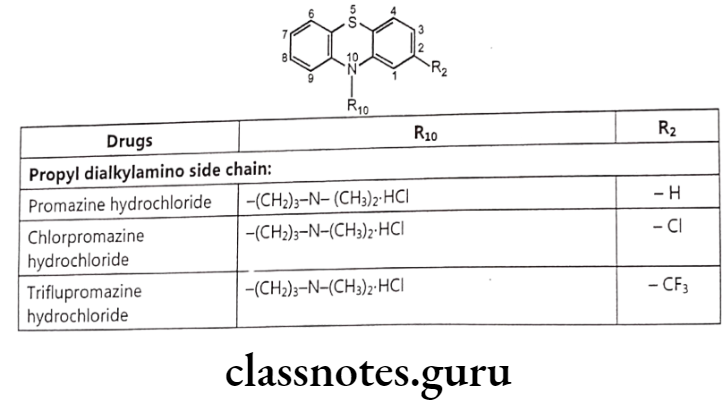
- The sympathomimetic drugs may be divided into catechol and non-catechol amines.
- All catecholamines possess the catechol nucleus (o-dihydroxybenzene). Catechol-amines have only a brief duration of action, and are ineffective orally, due to degradation by COMT.
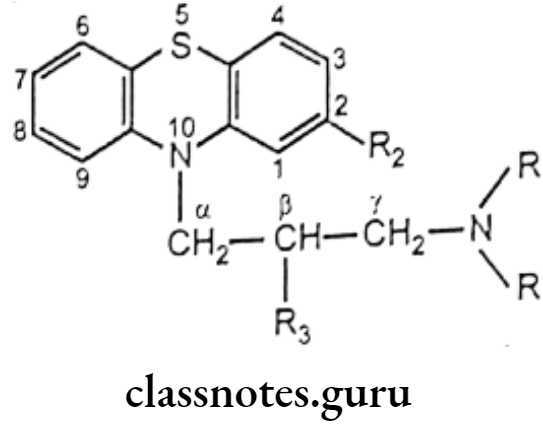
Non-catecholamines consist of a benzene ring and an ethylamine side chain (i.e. B-phenylethylamine). Substitution on the meta and para positions of the aromatic ring and, on a and B-positions of the ethylamine side chain influences not only the mechanism of sympathomimetic action but also the receptor selectivity of the drug.
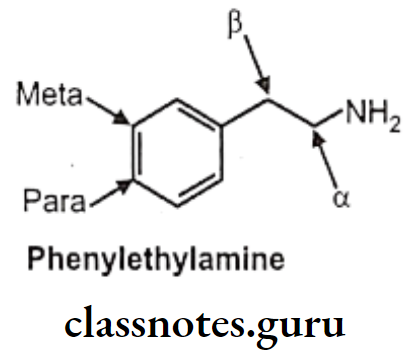
Substitution on the aromatic nucleus, specifically -OH groups at the 3 (meta) and 4 (para) positions of the ring and a B-OH group are required for maximal a and B activity.
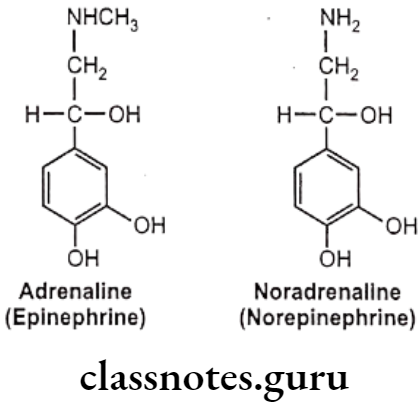
Examples: Adrenaline And Noradrenaline.
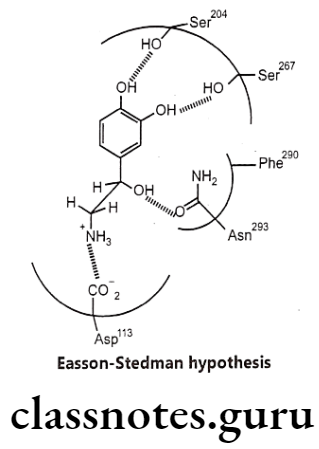
B-hydroxyl (B-OH) Group:
- Due to chirality, they exhibit high stereo selectivity in producing their agonistic effect. The more potent enantiomer i.e. R-configuration is 100 times more potent than S-configuration.
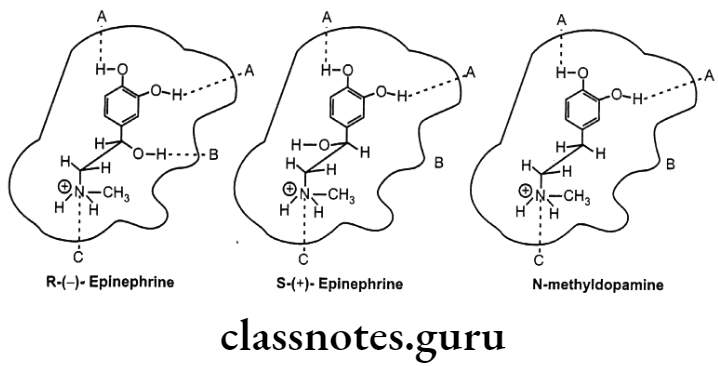
- All direct-acting B-phenylethylamine-derived agonists have the arrangement in space of the catechol group, the amino group, and the B-OH group in the fashion resembling that of R-norepinephrine stereo selectively acts as three critical pharmacophoric groups which bind with three complementary binding areas on the receptor and called as Easson-Stedman Hypothesis (Three point attachment theory).
Amino (-NH2) Group:
- The presence of the amino group in phenylethylamines is important for direct agonist activity.
- The amino group should be separated from the aromatic ring by two carbon atoms for optimal activity.
- Both primary and secondary amines are found among the potent direct-acting agonists, but tertiary or quaternary amines tend to be poor direct agonists.
- Substitution of the bulkier group at the nitrogen decreases a-receptor agonist activity and increases B-receptor activity. Thus, norepinephrine is an effective ẞ-receptor agonist as well as a potent a-receptor agonist, while epinephrine is a potent a, ẞ1 and B2-receptor agonist. Isoproterenol is a potent and ẞ2-receptor agonist, but has little affinity for a-receptors.
Example: Isoproterenol
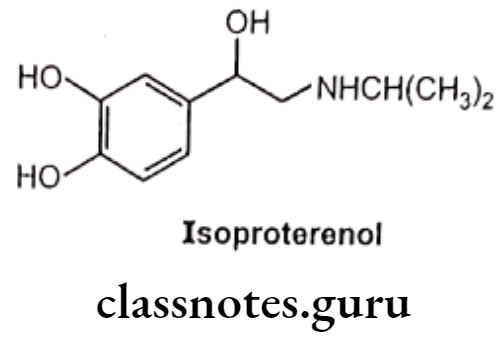
Large substituents on the amino group also protect the amino group front undergoing oxidative deamination by MAO as well as increase ẞ2 selectivity.
Examples: N-tert-butyl norepinephrine (Colterol)
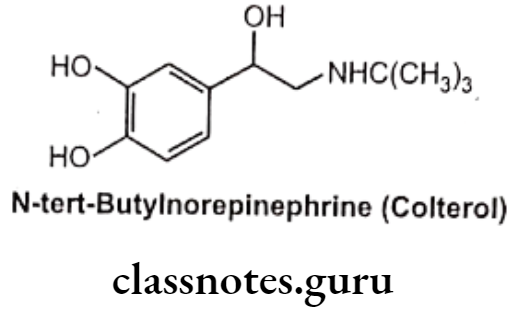
α-Carbon Atom:
- Substitution of CH3 or C2Hs at a-carbon atom reduces direct receptor agonist activity at both a and ẞ receptors.
- a-alkyl group increases the duration of action by making the compound resistant to metabolic deamination by MAO. It also increases oral effectiveness and greater CNS activity.
- Another effect of a-substitution is the introduction of a chiral center i.e. a-methyl norepinephrine, is the erythro isomer that possesses significant activity at a-receptor.
Catechol Moiety:
- Catechol moiety is important for maximal agonist activity. It can be replaced with other substituted phenyl moieties to provide selective adrenergic agonist activity. This approach can be used in the design of selective ẞ2-receptor agonists.
- Replacement of the meta-OH of the catechol structure with a hydroxymethyl group shows B2 selectivity.
Examples: Albuterol (Salbutamol)
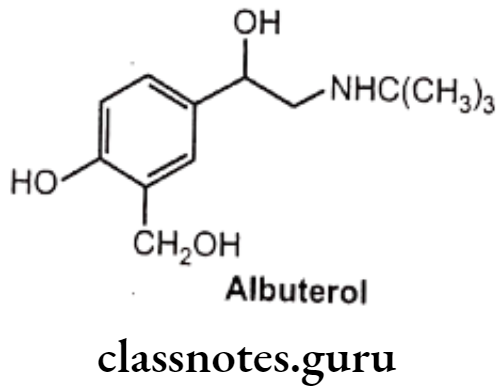
Hydroxyl (-OH) groups at the 3 and 5 positions of the aromatic ring (Resorcinol ring), in compounds with large amino substituents, show ẞ2 selective activity. As the resorcinol ring is not a substrate for COMT, it tends to have better absorption and longer duration of action.
Examples: Metaproterenol, Terbutaline
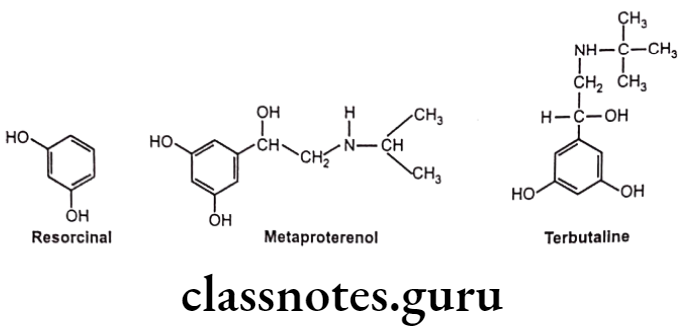
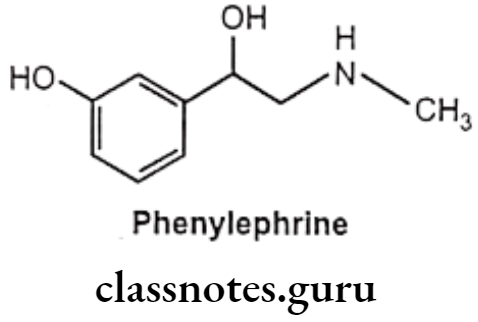
Modification at the catechol ring can also bring about a-selectivity at receptors. Removal of the p-OH group from epinephrine gives phenylephrine, which, in contrast to epinephrine, is selective for the a-adrenergic receptor with B-activity almost entirely absent.
Examples: Phenylephrine
Optical isomerism is conferred by substitution on either of the ethyl carbon atoms.
Laevorotatory: Substitution at the B-carbon atom produces naturally occurring epinephrine and norepinephrine, both of which are over 10 times as potent as their isomers.
Dextrorotatory: Substitution at the a-carbon atom generally confers greater potency in CNS stimulation, e.g. d-amphetamine.
- Agents lacking -OH substitution, especially the compounds with 3-OH, are resistant to COMT and have a longer duration of action and oral effectiveness.
- Lacking of both, aromatic -OH groups and the B-OH on the ethyl chain produce almost all of their effects by NE release, thus their effects are mainly on a and ẞ1.
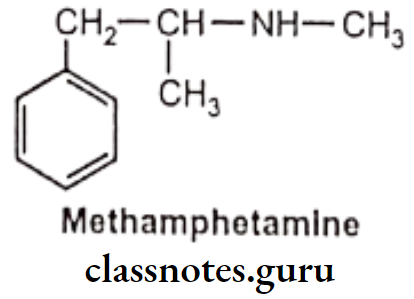
Examples: Ephedrine
Substitution on the B-C atom generally decreases central stimulant action, due to the lower lipid solubility of these agents. However, this also greatly enhances both a and ẞ potency. Thus, ephedrine is less potent than methamphetamine as a CNS stimulant but is more potent vasoconstrictor and bronchodilator.
Absence of the benzene ring reduces the CNS stimulant action, without reducing peripheral effects. e.g. Cyclopentamine, and many of these agents are used as nasal decongestants.
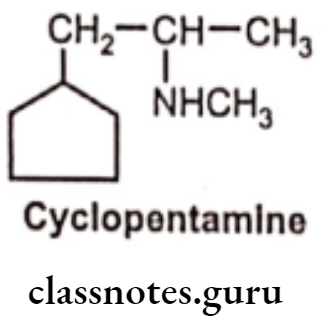
Endogenous Catecholamines
Adrenaline (Epinephrine)
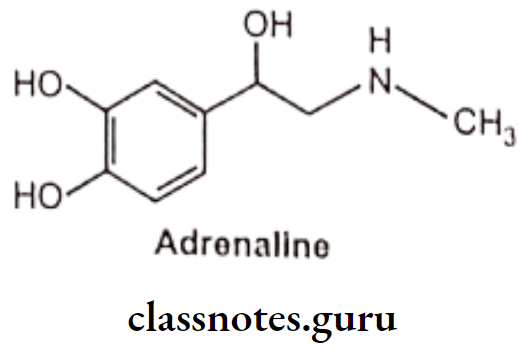
- Adrenaline is chemically 1-(3,4-dihydroxyphenyl)-2-methylaminoethanol and available as adrenaline acid tartarate.
- Adrenaline is a potent stimulator of both a and ẞ receptors.
- It is light sensitive and easily oxidized on exposure to air because of the catechol ring system and turns to brown colour.
- It is ineffective by the oral route because of poor absorption and rapid metabolism by MAO and COMT.
- Adrenaline is rapidly inactivated in the body, despite its stability in blood.
- It shows prominent actions on the heart and vascular smooth muscle.
- Adrenaline is one of the most potent vasopressors known, given by IV route, it evokes a characteristic rise in blood pressure.
- Increase in blood pressure is due to direct myocardial stimulation (positive inotropic effect), an increased heart rate (positive chronotropic effect) and peripheral vasoconstriction.
Adrenaline Uses:
- As it activates ẞ1 receptors, epinephrine is used to overcome AV heart block and restore cardiac function in patients experiencing cardiac arrest.
- Activation of ẞ2 receptors in the lung promotes bronchodilation, which can be useful in patients with asthma.
- Used to prevent bleeding during surgery or in the case of inner organ bleeding.
- It can cause. α-mediated vasoconstriction; it is administered in combination with local anaesthetics which leads to longer lasting effect of local anaesthetics in smaller doses.
- Used in the treatment of open-angle glaucoma, where it apparently reduces intraocular pressure increasing the rate of outflow of aqueous humour from its anterior chamber of the eye.
- Used to produce mydriasis during ophthalmologic procedures.
- It activates a combination of a and ẞ receptors, epinephrine used as treatment of choice for anaphylactic shock.
Noradrenaline (Norepinephrine):
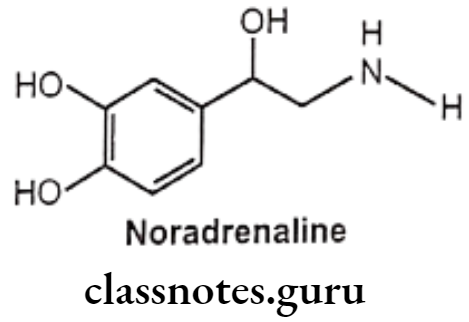
- Noradrenaline is chemically 1-(3,4-dihydroxyphenyl) amino ethanol, available as acid tartarate salt.
- Both adrenaline and noradrenaline are approximately equipotent at cardiac ẞ1 receptors. Noradrenaline is a potent agonist for a-receptors but has little action on B2 receptors.
- Noradrenaline should be protected from air and light as it darkens on exposure to air and light.
- Like the other. endogenous catecholamines, it is a substrate for both MAO and COMT and thus is not effective by the oral route of administration. It is given by intravenous injection.
- Levo-isomer is pharmacologically active.
Noradrenaline Uses:
- Noradrenaline is used to maintain blood pressure in acute hypotensive states resulting from surgical or non-surgical trauma, central vasomotor depression and haemorrhage.
Dopamine:
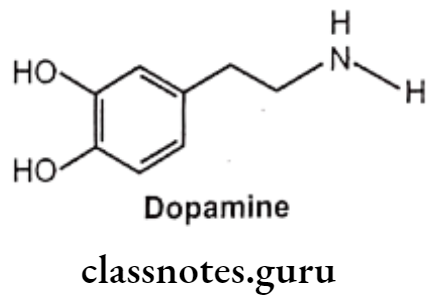
- Dopamine is chemically 3,4-dihydroxyphenylethylamine, is a central neurotransmitter. It differs from the other naturally occurring catecholamines, lacking the B-OH group on the ethylamine side chain.
- It is the metabolic precursor of noradrenaline and adrenaline..
- It is a substrate for both MAO and COMT and is thus ineffective orally.
- As it cannot cross blood brain barrier, it has minimal effects on the CNS.
- It stimulates ẞ1-receptors of heart and exerts a positive inotropic effect on the heart.
- It increases blood flow to the kidney in doses that have no chronotropic effect on the heart or that cause no increase in blood pressure.
Dopamine Uses:
- Dopamine is used in acute congestive cardiac failure with imminent renal failure, septic shock, cardiogenic shock, surgical shock and acute pancreatitis.
α-adrenergic Receptor Agonists
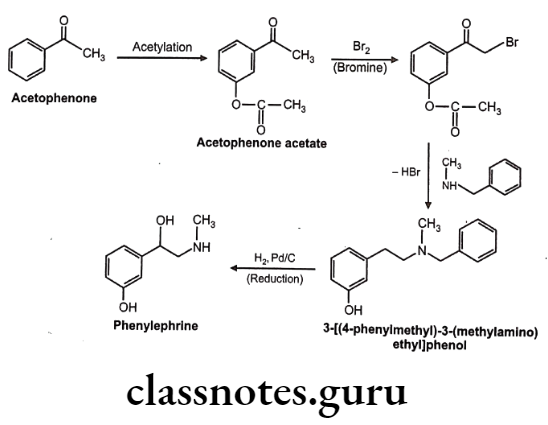
Phenylephrine:
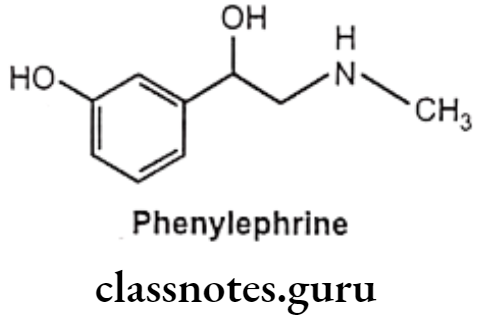
- Phenylephrine is chemically 1-(3-hydroxyphenyl)-2-methylaminoethanol and available as hydrochloride salt.
- It should be stored in airtight container to protect from light because it is decomposed by light.
- Phenylephrine differs from adrenaline only by lacking the 4-OH group on the benzene ring and subsequently is resistant to COMT and has predominantly α-agonist effects.
- It gets metabolized by MAO.
- Phenylephrine is a selective α1-receptor agonist.
- Oral absorption is not reliable, and so it is given parenterally or topically as eye or nasal drops.
Phenylephrine Uses:
- Phenylephrine is potent vasoconstrictor, but is less potent than epinephrine and norepinephrine.
- It is used as a nasal decongestant, mydriatric and as a vasopressor agent.
- It is used to dilate the pupil and to treat open-angle glaucoma.
- It is used in spinal anesthesia, to prolong the anesthesia and to prevent a drop in blood pressure.
- It is used in the treatment of severe hypotension resulting from either shock or drug administration.
Methoxamine:
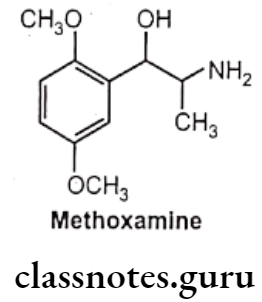
- Methoxamine is chemically, 2-amino-1-(2,5-dimethoxyphenyl)propan-1-ol.
- It is a selective direct acting a1-receptor agonist.
- It is less potent than phenylephrine as a vasoconstrictor that has no stimulant action on the heart.
- It is not substrate for COMT, its duration of action is significantly longer than that of norepinephrine.
Methoxamine Uses:
- It is used primarily during surgery or shock to maintain adequate arterial blood- pressure.
Imidazolines:
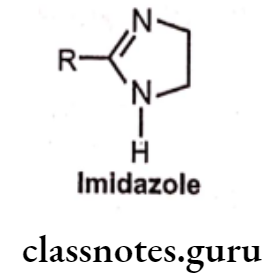
Structure-Activity Relationship:
- These compounds interact differently with receptors to that of B-phenylethylamine. The heterocyclic imidazoline ring is linked to a substituted aromatic moiety.
- Modification in imidazoline ring decreases the activity.
- Open ring imidazoline are highly active i.e. Guanabenz and Guanfacine.
- Aromatic substitution is flexible. Agonist activity increases on substitution of aromatic ring with halogen (CI) or small alkyl group (CH3), particularly when placed at two ortho positions.
- Naphazoline: (2-(1-naphthylmethyl)-2-imidazoline}
- Tetrahydrozoline: 2-(1,2,3,4-tetrahydronaphthalen-1-yl)-4,5-dihydro-1H-imidazole}
- Xylometazoline: (2-(4-tert-butyl)2, 6-dimethylbenzyl)-2-imid ‘ine}
- Oxymetazoline: (6-tert-butyl-3-(2-imidazoline-2-ylmethyl)-2,4-dimethylphenol}
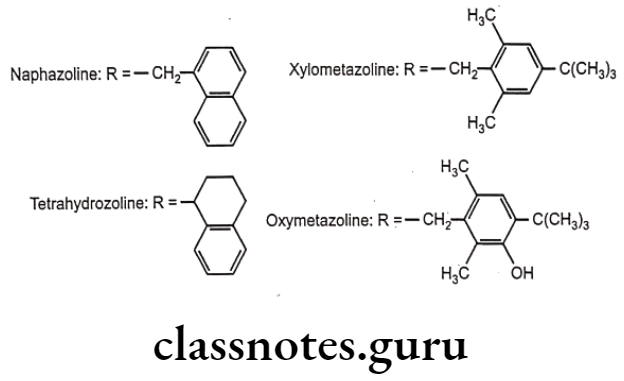
- These drugs are agonists at both α, and a2-adrenergic receptors.
- Because of the basic nature of the imidazoline ring, these drugs essentially exist in an ionized form at physiological pH.
Imidazolines Uses:
- These agents are used for their vasoconstrictive effects as nasal decongestants and ophthalmic decongestants.
- (e) Guanabenz: (2-[(2,6-dichlorophenyl) methylideneamino] guanidine}
- (f) Guanfacine: (2-[(2,6-dichlorophenyl) acetamido] guanidine}
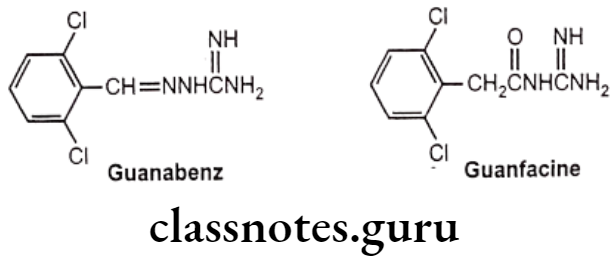
- Structurally, these two compounds can be considered “open-ring imidazolidines.
- 2,6-dichlorophenyl moiety is connected to a guanidino group by a two-atom bridge.
- In guanabenz the bridge is -CH-N- group and in guanfacine the bridge is -CH2CO-group.
- Conjugation of the guanidine moiety with the bridging moiety helps to decrease the pka, therefore because of basic group these drugs exist in their non-ionized form at physiological pH.
Imidazolines Uses:
- Guanabenz and guanfacine are used as antihypertensive drugs.
Clonidine:
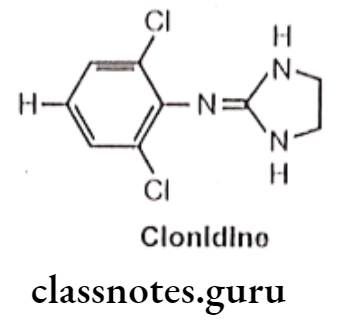
- Clonidine is chemically, 2-(2,6-dichloroanilino)-2-imidazoline.
- It has selective action on α2 receptor.
- It exhibits vasoconstrictive activity by stimulation of peripheral a-adrenergic receptors.
- It enters into the CNS and stimulates 2 receptors located in regions of the brain leads to decrease in sympathetic outflow from the CNS, which in turn, decreases in peripheral vascular resistance and blood pressure.
- It produces bradycardia due to stimulation of cardiac a2-adrenergic receptor.
Clonidine Uses:
- Clonidine is useful in the treatment of hypertension.
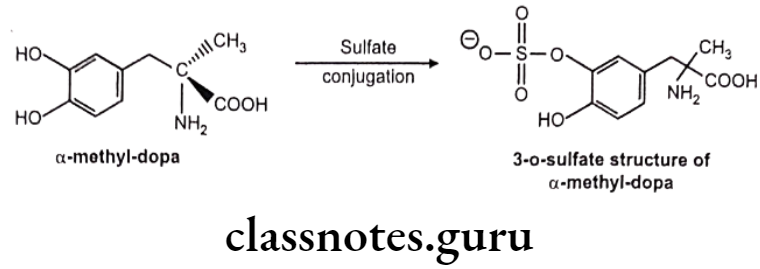
Methyldopa:
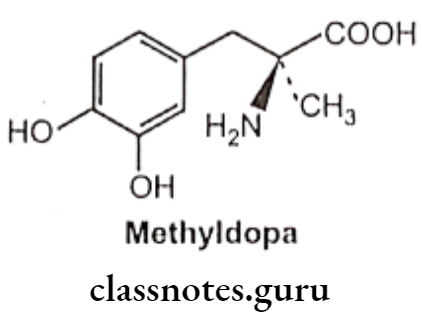
- Methyldopa is 3-(3,4-dihydroxyphenyl)-2-methyl-L-alanine.
- Methyldopa is used only by oral administration since its zwitter ionic character limits its solubility.
- a-methyl norepinephrine is the metabolic product of the drug methyldopa. It shows selectivity towards the α1 receptor.
- a-methyl norepinephrine acts on a2 receptors in the CNS in the same manner as clonidine to decrease sympathetic outflow and to lower blood pressure.
Methyldopa Uses:
- Methyldopa is useful in the treatment of hypertension.
α and ẞ-adrenergic Receptor Agonists
Dobutamine:
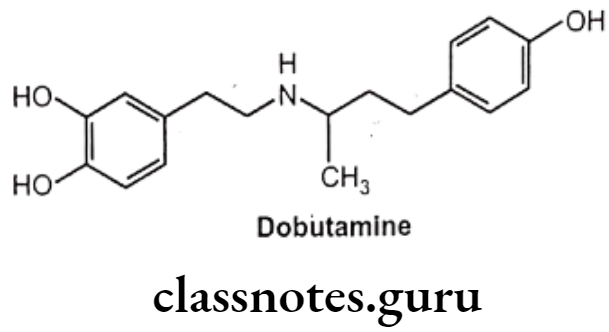
- Dobutamine is chemically 3,4-dihydroxy-N-[3-(4-hydroxyphenyl)-1-methylpropyl]-b- phenylethylamine.
- Dobutamine is a synthetic catecholamine derivative.
- Dobutamine is given by intravenous infusion since it is not effective orally.
- Dobutamine gets metabolized by COMT and conjugation, but not by MAO.
- Dobutamine resembles dopamine chemically but possesses a bulky aromatic residue on the amino group i.e. 1-(methyl)-3-(4-hydroxyphenyl) propyl and the absence of a B-OH group.
- Dobutamine is a selective ẞ1 receptor agonist and has only slight indirect actions.
- Dobutamine increases cardiac output without any effect on heart rate and blood pressure.
- Dobutamine is a racemic mixture of two enantiomeric forms. The (+) isomer has potent B-agonistic actions. The (-) isomer has potent agonistic and poor ẞ-agonistic actions.
- Racemic dobutamine increases the inotropic activity of the heart to a much greater extent than chronotropic activity.
- Dobutamine does not act as an agonist at the dopaminergic receptors that mediate renal vasodilation.
Dobutamine Uses:
- Dobutamine is used in patients with heart failure associated with myocardial infarction, open heart surgery and cardiomyopathy.
B-adrenergic Receptor Agonists
Isoproterenol:
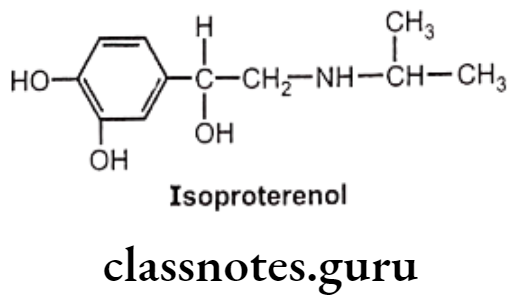
- Isoproterenol is chemically, 4-[1-hydroxy-2-[isopropylaminoethyl]-1,2-benzenediol. It acts on both ẞ1 and B2 receptors.
- As it contains an isopropyl substitution on the nitrogen atom, it has virtually no effect on a-receptors.
- Isoproterenol increases cardiac output by stimulating cardiac ẞ1 receptors and can bring about bronchodilation through stimulation of B2 receptors in the respiratory tract.
- Isoproterenol is available for use by inhalation and injection.
- Isoproterenol is metabolized by COMT.
Isoproterenol Uses:
- Isoproterenol is clinically used for the relief of bronchospasms associated with bronchial asthma.
- It produces adverse effect as cardiac stimulation which leads to use it sometimes in the treatment of heart block.
Selective ẞ2-adrenergic Receptor Agonists:
- Modification of the catechol portion of a ẞ-agonist (isoproterenol) has resulted in the development of selective ẞ2 receptor agonists.
- Further the shift of the OH group produces resistance to MAO and prolongs the duration of action.
- Increased ẞ2 agonist activity is also conferred by the substitution of increasing bulky lipophilic groups on the amino group of isoproterenol.
- Because of their relative selectivity, these agents relax the smooth muscle of the bronchi, uterus and blood vessels.
- They have far less action on the heart than Isoproterenol and other agents.
Metaproterenol:
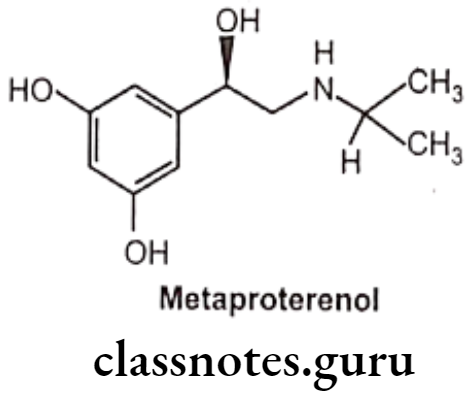
- Metaproterenol is chemically,1-(3,5-dihydroxy phenyl)-2-isopropylaminoethanol.
- Metaproterenol is a photosensitive compound and, hence should be protected from light and air.
- Metaproterenol is less ẞ2 selective than terbutaline.
- Metaproterenol is not metabolized by either COMT or MAO, therefore more effective when given orally, and have a longer duration of action.
Terbutaline:
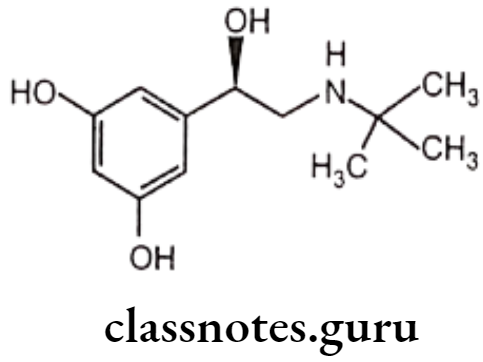
- Terbutaline is chemically, N-tert-butyl-N-[2-(3,5-dihydroxyphenyl)-2-hydroxy-methyl]amine or 1-(3,5-dihydroxyphenyl)-2-(tert-butyl amino) ethanol.
- It is a non-catecholamine, therefore is resistant to COMT.
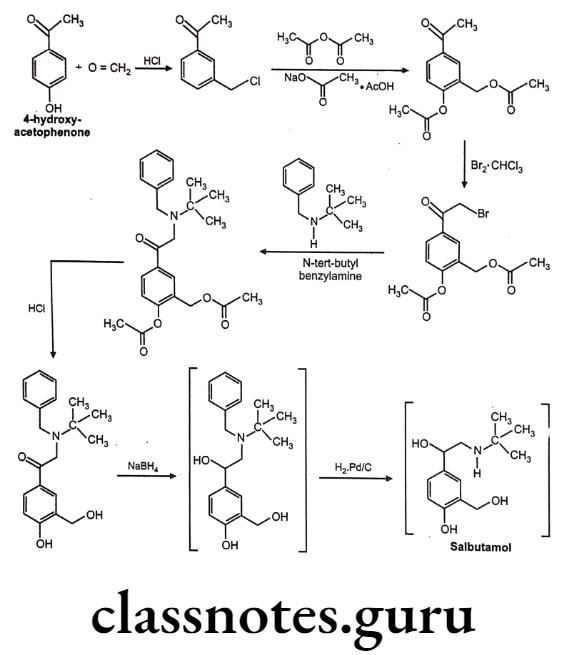
Salbutamol (Albuterol):
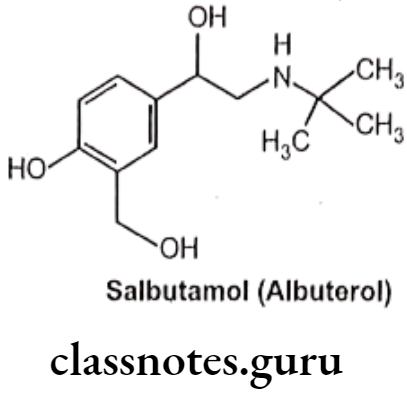
- Salbutamol is chemically, 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(tert-butylamino) ethanol.
- It is a selective ẞ2 receptor agonist whose selectivity results from the replacement of the meta-hydroxyl group of the catechol ring with a hydroxyl methyl moiety.
- It is not metabolized by either COMT or MAO.
- It is active orally and they exhibit a longer duration of action.
Salbutamol Uses:
- Metaproterenol, Terbutaline, and Salbutamol (albuterol) possess strong ẞ2 agonistic properties.
- They are used in the treatment of bronchial asthma.
Bitolterol:
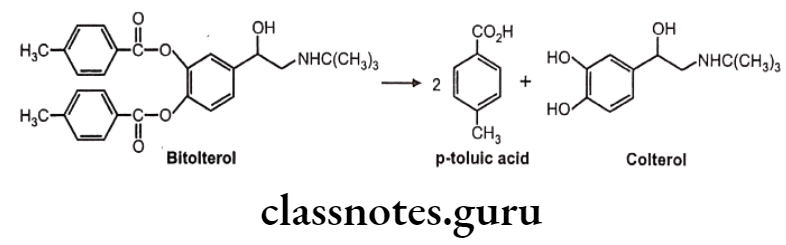
- Bitolterol is a prodrug of the ẞ2 selective adrenergic agonist colterol.
- It is chemically, [4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(4-methylbenzoyl)-oxyphenyl] 4-methylbenzoate.
- The presence of the two p-toluic acid esters in bitolterol makes it considerably more lipophilic than colterol.
- It is. hydrolyzed by esterases in the lung and other tissues to produce the active agent, colterol.
- It has a longer duration of action and is metabolized by COMT and conjugation after hydrolysis of the esters.
Bitolterol Uses:
- Bitolterol is administered by inhalation for bronchial asthma and reversible bronchospasm.
Selective ẞ3 adrenergic receptor agonists
Selective ẞ3 receptor agonists have been developed, but not approved for therapeutic use. B3 receptor stimulation promotes lipolysis. These agents act as anti-obesity drugs and are used for the treatment of non-insulin-dependent diabetes.
Indirect Acting Sympathomimetics
Indirect-acting sympathomimetics act by releasing endogenous NE. They enter the nerve endings by way of the active uptake process and displace NE from its storage granules. This class is comprised of non-catecholamines.
These compounds are resistant to COMT and MAO enzymes due to the lack of phenolic hydroxyl groups and the presence of a-methyl groups. These compounds pass more readily through blood blood-brain barrier because of increased lipophilicity.
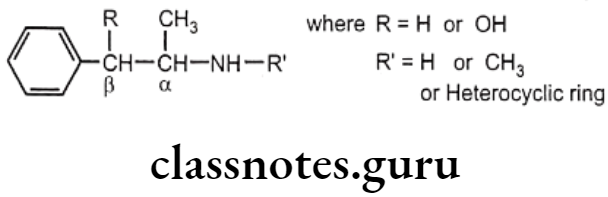
Structure-Activity Relationship:
- Most of these drugs retain phenyl ethylamine skeleton.
- The presence of a B-OH group decreases, and an α-CH3 group increases the effectiveness of indirect-acting agents.
- The presence of nitrogen substituents decreases indirect activity,
- Substituent larger than the methyl group at nitrogen renders the compound virtually inactive.
- Tertiary amino group substitution is ineffective as an NE-releasing agent.
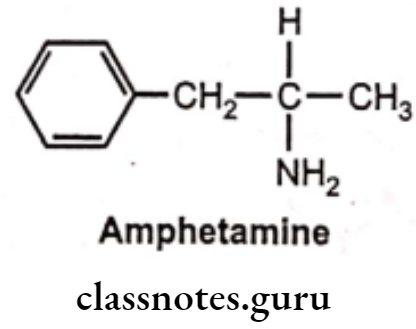
Amphetamine:
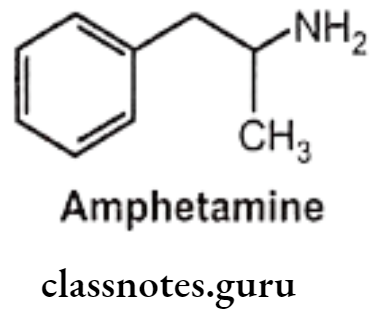
- Amphetamine is chemically, 1-phenylpropan-2-amine.
- It is one of the most potent sympathomimetic.
- It is an indirect-acting sympathomimetic amine and its action depends on the release of norepinephrine from adrenergic nerves.
- CNS stimulant effects are thought to be due to stimulation of the cortex.
- The d-isomer of amphetamine is 3-4 times more potent than the I-isomer.
Amphetamine Uses:
- Amphetamine causes increased wakefulness, elevated mood, increased initiative, self- confidence and ability to concentrate.
- It has an anorexic action and can be used in the treatment of obesity.
Hydroxyamphetamine:
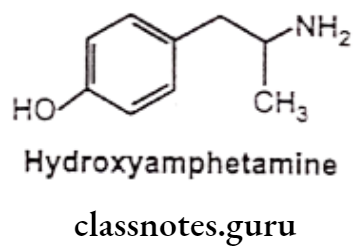
- Hydroxyamphetamine is chemically, 4-(2-aminopropyl)phenol or 1-(4-hydroxy- phenyl)-propan-2-amine.
- It possesses a-receptor stimulant activity, but lacks CNS activity.
- It is a powerful vasoconstrictor.
Hydroxyamphetamine Uses:
- Hydroxyamphetamine is used as an anorexiant in the treatment of obesity.
- It is used to dilate the pupil for diagnostic eye examinations and for surgical procedures on the eye.
- It is used sometimes with cholinergic blocking drugs like atropine to produce a mydriatic effect.
- It is used in hyperkinetic syndrome in children.
- It is used in narcolepsy (sudden attack of sleep in completely inappropriate situations).
Pseudoephedrine:
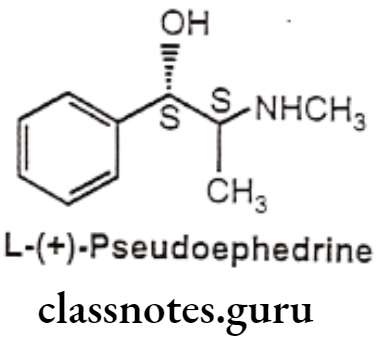
- L-(+)-Pseudoephedrine is chemically, (15,25)-2-(methylamino)-1-phenylpropan-1-ol. It is the (S,S) diastereoisomer of ephedrine in which B-OH group is having S-configuration.
- It is a naturally occurring alkaloid from the Ephedra species.
- Ephedrine has a mixed mechanism of action whereas pseudoephedrine acts predominantly by an indirect mechanism.
Pseudoephedrine Uses:
- Pseudoephedrine is used as a nasal decongestant and in the treatment of cold.
- Pseudoephedrine can also be used in the treatment of hypertension.
Propylhexedrine:
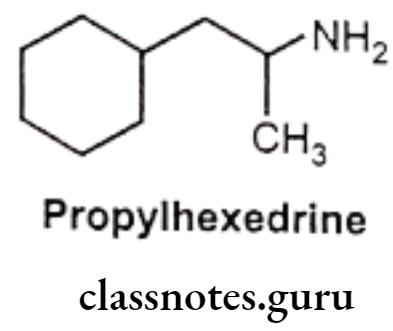
- Propylhexedrine is an analogue of amphetamine in which the aromatic ring has been replaced with a cyclohexane ring.
- It is chemically, 1-cyclohexyl-2-methylaminopropane.
Propylhexedrine Uses:
- Propylhexedrine is used as nasal decongestant.
- It has local vasoconstrictor effect on nasal mucosa in the symptomatic relief of nasal congestion caused by the common cold, allergic rhinitis or sinusitis.
Sympathomimetics With Mixed Action
These drugs act both, directly with the receptor sites and partly by the release of endogeneous norepinephrine. These agents have no -OH on the aromatic ring, but do have 6-OH group.
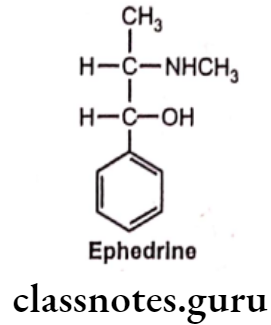
Ephedrine:
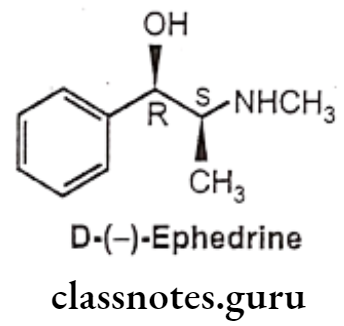
- Ephedrine is chemically, (1R, 25)-2-(methylamino)-1-phenylpropan-1-ol.
- It occurs naturally in many plants, being the principal alkaloid obtained from various species of Ephedra.
- Ma Huang, the plant containing ephedrine, has been used in China for over 2000 years.
- It contains two asymmetric carbon atoms, thus there are four optically active forms. The erythro racemate is called as ephedrine, whereas threo racemate is known as pseudoephedrine.
- Among four compounds available, D(-) isomer is clinically most active.
- It has agonist activity at both a. and B-receptors.
- Ephedrine decomposes gradually and darkens when exposed to light.
- It is not metabolized by either MAO or COMT.
Ephedrine Uses:
- The pharmacological activity of ephedrine resembles with epinephrine. Ephedrine differs from adrenaline mainly by its
- effectiveness after oral administration,
- longer duration of action,
- more pronounced central actions,
- much lower potency.
- It produces a sharp rise in systolic, diastolic and pulse pressures, with a reflex bradycardia, similar to adrenaline, but lasting for 10 times as long.
- Ephedrine is mainly used as a bronchodilator in asthma.
- It is used to treat narcolepsy and depressive state.
- It is also used as nasal decongestant, mydriatic and in certain allergic disorders. • It is also used to treat hay fever and urticaria.
Phenylpropanolamine:
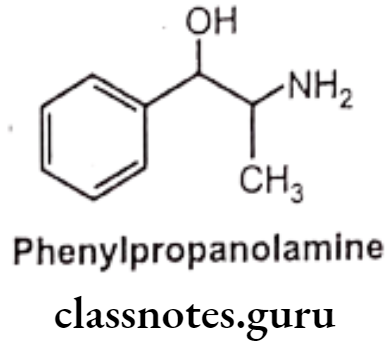
- Phenylpropanolamine is similar in structure to ephedrine except that, it possesses primary amine instead of a secondary amine.
- It is chemically, (1S 2R)-2-amino-1-phenyl propan-1-ol.
- This structural difference makes Phenylpropanolamine to slightly higher vassopressive and lower central stimulatory action than ephedrine.
Phenylpropanolamine Uses:
- Phenylpropanolamine is used as nasal decongestant with prolonged action than that of ephedrine.
- Earlier it was used as appetite suppressant and also used in the treatment of cough and cold. But because of increased risk of hemorrhagic stroke in young women, FDA recommended its removal from such medication.
Metaraminol:
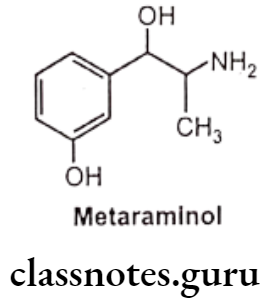
- Metaraminol is chemically, 3-[(1R,25)-2-amino-1-hydroxypropyl]phenol.
- It is an isomer of phenylephrine.
- It possesses a mixed mechanism of action.
- It’s direct-acting effects mainly on a-adrenergic receptor.
Metaraminol Uses:
- Metaraminol is used for its vasopressor action for maintaining blood pressure during spinal anesthesia and hemorrhage.
- It has also been used to treat severe hypotension brought on by other traumas that induce shock.
Adrenergic Receptor Antagonists
Adrenergic receptor antagonists are also called as adrenergic blocking agents or adrenergic receptor blockers or sympatholytic agents.
α-Adrenergic Receptor Antagonists
Drugs that attach to a-adrenergic receptors and block the effect of norepinephrine and epinephrine are categorized as a- adrenergic antagonists.
These drugs consist of a number of compounds of diverse chemical structure that bear little obvious resemblance to the a-adrenergic receptor agonist. a-adrenergic receptor antagonists may be non-selective or selective for α1 receptor.
Non-selective a-adrenergic receptor antagonists block both a1 and α2 adrenergic receptors resulting in reducing blood pressure as a major cardiovascular effect.
Generally, a-adrenergic receptor antagonists are useful in the management of hypertension, prostatic hypertrophy (prostate enlargement) and heart failure.
Non-selective a-Receptor Antagonists:
Reversible a-Receptor Antagonists:
Example: Tolazoline and Phentolamine
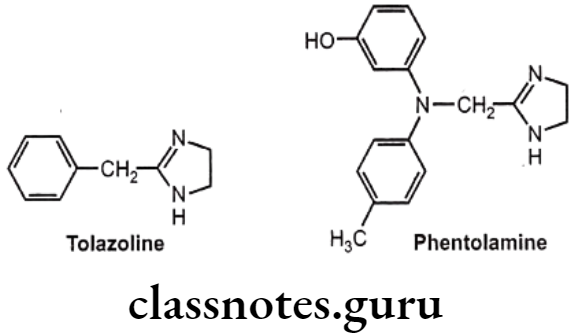
- Tolazoline and phentolamine are imidazoline a-antagonists that also have antihypertensive activity.
- They have structural similarities to the imidazoline α-agonists, such as naphazoline and xylometazoline, but does not have the lipophilic substituents required for agonist activity.
- They are potent non-selective competitive (reversible) a-adrenergic receptor antagonists.
- They have both α1- and a2- antagonistic activity and produce tachycardia.
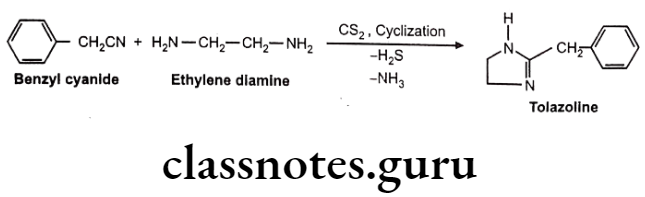
- Tolazoline is chemically, 2-benzyl-4,5-dihydro-1H-imidazole.
- Tolazoline is relatively weak a-antagonist, its histamine like and acetylcholine like action contributes to its vasodilatory activity.
- Tolazoline stimulates stimulation of gastric acid secretion, render its use in patients who have gastric or peptic ulcer.
- Phentolamine is chemically, 3-[N-(4,5-dihydro-1H-imidazol-2-ylmethyl)-4- methylanilino] phenol.
- Phentolamine is the more effective a-antagonist. The vasodilatory effect of phentolamine is used in the management of hypertension.
Reversible a-Receptor Antagonists Uses:
- Tolazoline is a vasodilator that is used to treat spasms of peripheral blood vessels.
- Tolazoline is used in treatment of persistent pulmonary hypertension of the newborn. Tolazoline is used to treat Raynaud’s syndrome and other conditions involving peripheral vasospasm.
- Tolazoline is also used to treat thrombophlebitis.
- Phentolamine is used to prevent or control hypertensive episodes that occur in patients with pheochromocytoma.
- Phentolamine can be used as an aid in the diagnosis of pheochromocytoma (Tumor of the adrenal medulla).
- Phentolamine is used in combination with papaverine to treat impotence.
Irreversible a-Receptor Antagonists:
Example: Phenoxybenzamine
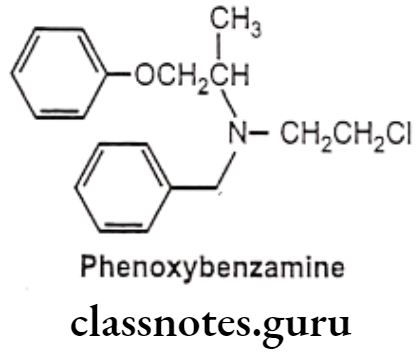
- Phenoxybenzamine is chemically, N-benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-amine.
- It is non-competitive, non-selective and irreversible a-adrenergic receptor antagonist. It is a B-haloalkylamine that alkylates a-receptors.
- It is non-selectively and irreversibly blocks the postsynaptic a-adrenergic receptor in smooth muscle, leading to a muscle relaxation and a widening of the blood vessels.
- It has small onset of action, but long duration of action which may last for 3-4 days.
- As phenoxybenzamine forms covalent bonds with the receptors is irreversible, new receptors must be synthesized before the effects can be overcome, therefore, the a-receptor blocking action is long-lasting.
Irreversible a-Receptor Antagonists Uses:
- Phenoxybenzamine is used to treat hypertension and as a peripheral vasodilator.
- It is used in alleviating the sympathetic effects of pheochromocytoma.
Selective α-receptor Antagonists:
Example: Prazosin, Terazosin, Doxazosin
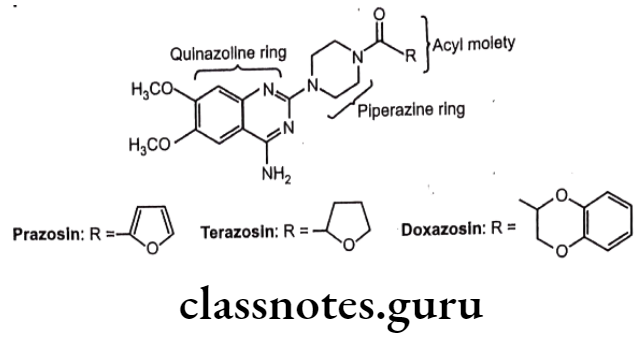
- These agents contain quinazoline ring, the piperazine ring, and the acyl moiety.
- The 4-amino group on the quinazoline ring is very important for α-rcceptor affinity. Replacement of piperazine ring with other heterocyclic ring like piperidine does not lose the activity.
- The pharmacokinetic activity of these agents is significantly affected by nature of acyl group.
- These drugs produce peripheral vasodilation without an increase in heart rate or cardiac output.
- Prazosin is chemically, [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]- (furan-2-yl)methanone.
- Terazosin is chemically, [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]- (oxolan-2-yl)methanone.
- Doxazosin is chemically, [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]- (2,3-dihydro-1,4-benzodioxin-3-yl)methanone.
- They are selective adrenergic α- antagonist.
Selective α-receptor Antagonists Uses:
- Prazosin is used in the treatment of heart failure; hypertension; pheochromocytoma; Raynaud’s syndrome; prostatic hypertrophy and urinary retention.
- Terazosin is used for treatment of symptoms of an enlarged prostate. It also acts to lower the blood pressure, and is therefore a drug of choice for men with hypertension and prostate enlargement.
- Doxazosin is used to treat high blood pressure and urinary retention associated with prostate enlargement.
Selective α2-receptor Antagonists:
Example: Yohimbine
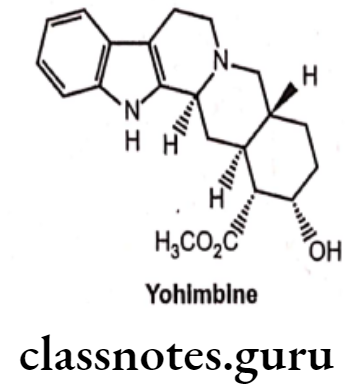
- Yohimbine, an indole alkaloid is isolated from Pausinystalia yohimbe bark and Rauwolfia roots.
- It is an α2-antagonist with greater selectivity for a2- than for α1-adrenoceptors.
- It is also used as a serotonin antagonist.
- It has actions both in the CNS and in the periphery, inducing hypertension and increase in heart rate.
Selective α2-receptor Antagonists Uses:
- Yohimbine is used to treat male erectile impotence and postural hypotension.
- It increases heart rate and blood pressure as a result of its blockade of a2-receptors in the CNS.
- It is also used in research to induce anxiety.
Other α-receptor antagonists:
Dihydroergotamine:
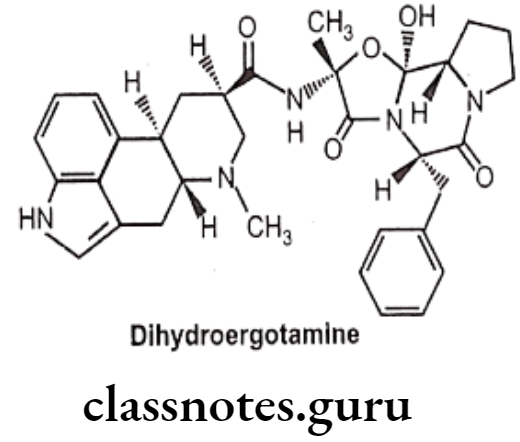
- Dihydroergotamine is an ergot derivative with agonistic activity for alpha- adrenergic, serotonergic, and dopaminergic receptors.
- It acts by stimulating the 5-HT1D receptor subtype and causes constriction of cerebral blood vessels.
- It is administered as a nasal spray or injection. Nausea is a common side effect.
Dihydroergotamine Uses:
- Dihydroergotamine is used to treat migraine headaches and cluster headaches.
Methysergide:
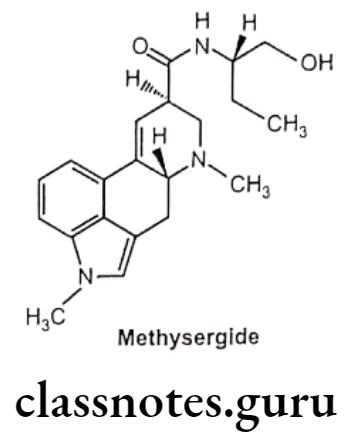
- Methysergide is an ergot derivative.
- It is chemically, (6aR,9R)-N-[(2S)-1-hydroxybutan-2-yl]-4,7-dimethyl-6,6a,8,9- tetrahydro-indolo[4,3-fg]quinoline-9-carboxamide.
- It antagonizes the effects of serotonin in blood vessels and gastrointestinal smooth muscle.
Methysergide Uses:
- Methysergide is used prophylactically in migraine and other vascular headaches.
B-Adrenergic Receptor Antagonists
B-adrenergic receptor antagonists are drugs that attach to B-adrenergic receptors and block the effect of agonists (e.g. Norepinephrine and epinephrine) at those B-receptor sites. B-antagonists are classified as non-selective and cardioselective.
Non-selective ẞ- antagonist blocks both B1 and B2-receptors; whereas cardio-selective ẞ-antagonist preferentially blocks B1-receptors and decreases heart rate and myocardial contraction. ẞ2-receptor antagonistic effects include inhibition of vascular dilation and inhibition of bronchodilation.
B-adrenergic receptor antagonists are useful in the management of hypertension, arrhythmia, ischemic heart disease, chronic open-angle glaucoma, migraine, and thyrotoxicosis. It is also useful in the management of chronic but not acute heart failure.
The common side effects of B-adrenergic receptor antagonists are bradycardia, bronchospasm, fatigue, sleep disturbance, impotence in men, and attenuated responses to hypoglycemia. Non-selective ẞ-antagonists are generally avoided in patients with asthma, as it may lead to progressive heart block, bronchoconstriction, and heart failure.
Sudden discontinuation of ẞ-antagonist may result in “rebound” increases in heart rate and blood pressure, which may lead to myocardial ischemia, infarction, or sudden death in susceptible individuals, therefore for termination of ẞ-antagonist therapy gradual reduction of the dose is recommended.
Structure-Activity Relationships:
- Structural modification by replacing the aromatic hydroxyl groups of Isoproterenol by chlorines leads to the formation of dichloro isoproterenol, which is a ẞ-antagonist that blocks the effects of sympathomimetic amines on bronchodilation, uterine relaxation, and heart stimulation.
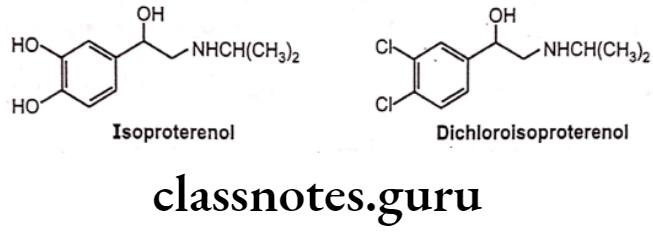
- Dichloroisoproterenol is a partial agonist, it has no clinical utility, therefore it is not used as a clinically useful drug.
- Pronethalol, 3,4-dichloro substitutes with a carbon bridge to form a naphthyl-ethanolamine derivative which is a clinically useful drug. It has less intrinsic sympathomimetic activity than dichloro isoproterenol, but it was withdrawn from clinical testing because of tumor induction in animal tests.
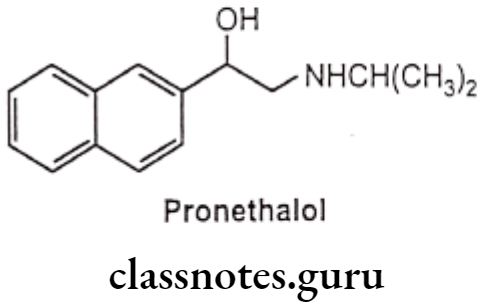
- Introduction of an oxymethylene bridge, (-OCH2-) between the aromatic ring and the ethylamino side chain and changing the side chain from C2 to C position of the naphthyl group of pronethalol (arylethanolamines) increases the B-blocker activity, i.e. propranolol (an aryloxypropanolamine).
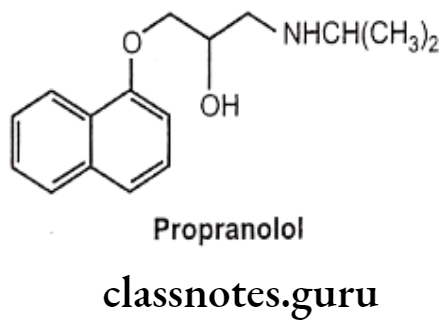
- Oxymethylene bridge (-OCH2-) is responsible for the antagonistic properties of the molecules which depend on the nature of the aromatic ring and its substituents.
- Lengthening the side chain would prevent the appropriate binding of the required functional groups to the same receptor site.
- The nature of the aromatic ring is also a determinant in the ẞ1-selectivity of the antagonists.
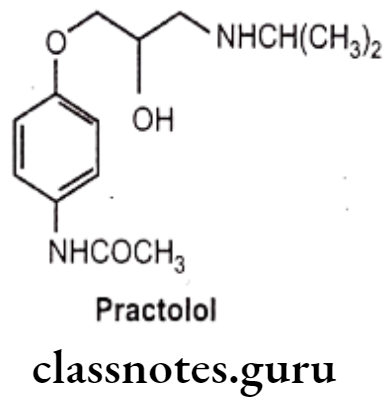
- Substitution of sufficient size on the para and absence at the meta position of an aromatic ring is a common structural feature of cardio-selective antagonist (B) i.e., practolol.
- Bulky aliphatic groups, such as the tert-butyl and isopropyl groups are essential for B-receptor antagonist activity.
- Secondary amine is essential for optimal activity. N, N-disubstituted compounds are inactive.
- B-blocking agent exhibits its effect based on stereoselectivity.
- The -OH-bearing C-atom possesses S-configuration for optimal activity to ẞ-receptor. The enantiomer with the R-configuration is typically 100 times less potent.
- Although most of the B-antagonistic activity resides in one enantiomer, propranolol, and most other B-blockers are used clinically as racemic mixtures. The only exceptions are levobunolol, timolol, and penbutolol with which the S-enantiomer is used.
Non-Selective ẞ-Blockers:
Example: Propranolol
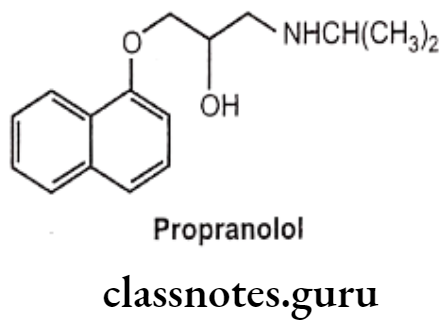
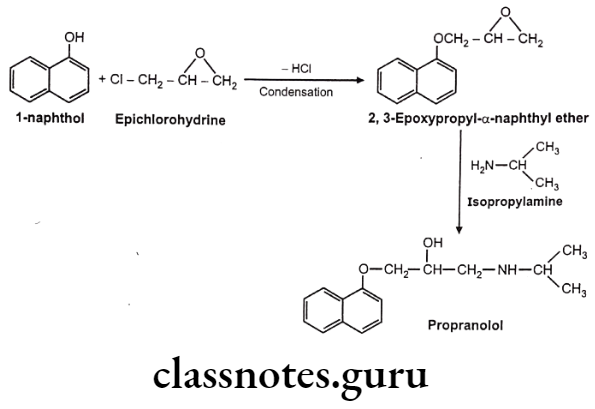
- Propranolol is chemically, 1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol.
- Non-Selective ẞ-Blockers is a non-selective ẞ-adrenergic receptor antagonist, which blocks both B1 and B2 receptors.
- Non-Selective ẞ-Blockers act by blocking the B-receptor in the heart. It slows down the heart rate, reduces the force of contraction, and reduces cardiac output.
- Its antihypertensive action is due to its ability to reduce cardiac output and suppress renin release from the kidney.
- Non-selective ẞ-Blockers is contraindicated in the presence of conditions such as asthma and bronchitis.
- Its receptor blocking action can be reversed with a sufficient quantity of B-agonist.
- Non-selective ẞ-Blockers is well absorbed after oral administration, but undergo extensive first-pass metabolism before it reach the systemic circulation.
Non-Selective ẞ-Blockers Uses:
- Propranolol is used in the treatment of hypertension, cardiac arrhythmias, angina pectoris, myocardial infarction, hypertrophic cardiomyopathy, pheochromocytoma, and migraine prophylaxis.
- Non-Selective ẞ-Blockers have high lipophilicity and the ability to penetrate the CNS and found to be useful in treating diseases of the CNS, such as anxiety.
- Non-Selective ẞ-Blockers is under investigation for the treatment of schizophrenia, alcohol withdrawal syndrome, and aggressive behavior.
Other Non-selective ẞ-blockers:
Several other nonselective ẞ-blockers, such as pindolol, nadolol, penbutolol, carteolol, timolol, levobunolol, sotalol, and metipranolol are found to be clinically useful agents.
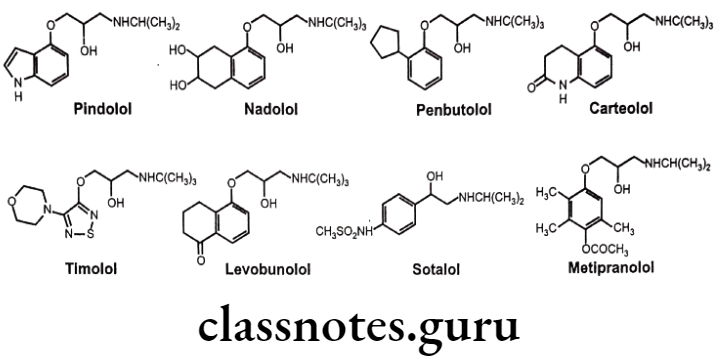
These drugs are chemically,
- Pindolol: 1-(1H-indol-4-yloxy)-3-(propan-2-ylamino)propan-2-ol.
- Nadolol: (2R,3S)-5-(3-(tert-butylamino)-2-hydroxypropoxy]-1,2,3,4-tetrahydro naphthalene-2,3-diol.
- Penbutolol: (25)-1-(tert-butylamino)-3-(2-cyclopentylphenoxy)propan-2-ol.
- Carteolol: 5-[3-(tert-butylamino)-2-hydroxypropoxy]-3,4-dihydro-1H-quinolin-2-one.
- Timolol: (25)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy] propan-2-ol.
- Levobunolol: 5-[(25)-3-(tert-butylamino)-2-hydroxypropoxy]-3,4-dihydro-2H-naphthalen-1-one.
- Sotalol: N-[4-[1-hydroxy-2-(propan-2-ylamino)ethyl]phenyl]methanesulfonamide.
- Metipranolol: [4-[2-hydroxy-3-(propan-2-ylamino)propoxy]-2,3,6-trimethylphenyl] acetate.
Other Non-selective ẞ-blockers Uses:
- Pindolol, nadolol, penbutolol, carteolol and timolol are used to treat hypertension.
- Nadolol is found to be useful in the long-term management of angina pectoris.
- Timolol is used in the prophylaxis of migraine headaches and in myocardial infarction.
- In addition to its ẞ-adrenergic blocking activity, sotalol also blocks the inward K* current which delays cardiac repolarization and leads to be a useful agent in the treatment of ventricular arrhythmias and atrial fibrillation.
- Carteolol, timolol, levobunolol and metipranolol are used topically to treat open-angle glaucoma. This agent lowers intraocular pressure by decreasing the amount of aqueous humour fluid produced in the eye by the ciliary body.
B1-Selective Blockers:
Cardioselective ẞ-antagonist drugs have a greater affinity for the ẞ1-receptors of the heart than for B2-receptors in other tissues. These agents are useful in the treatment of cardiovascular diseases, such as hypertension. These drugs do not possess ẞ2-receptor antagonistic properties and, therefore can be used safely in patients who have bronchitis or bronchial asthma.
Because of the absence of vascular ẞ2-receptor antagonistic activity, these agents reduce the increase in peripheral resistance that sometimes occurs after the administration of non-selective ẞ-antagonists. These drugs show its ẞi-receptors blocking cardio selectivity at relatively low doses only. At normal therapeutic doses, most of the selective activity is lost.
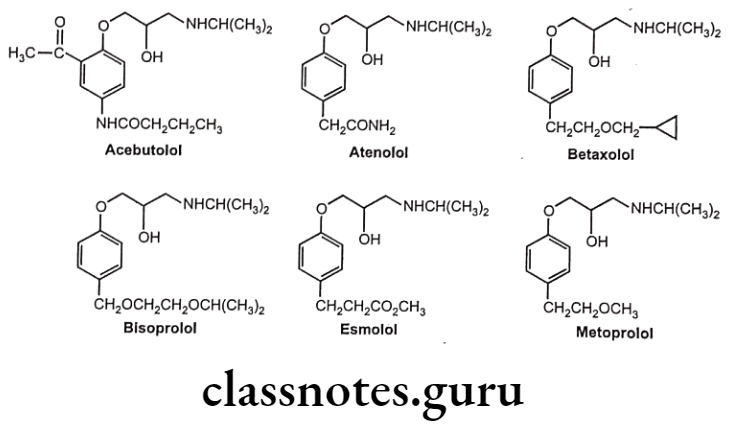
These drugs are chemically,
- Acebutolol: N-[3-acetyl-4-[2-hydroxy-3-(propan-2-ylamino) propoxy]phenyl]butanamide. Atenolol: 2-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide.
- Betaxolol: 1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-(propan-2-ylamino) propan-2-ol.
- Bisoprolol: 1-(propan-2-ylamino)-3-[4-(2-propan-2-yloxyethoxymethyl)phenoxy] propan-2-ol. Esmolol: Methyl 3-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]propanoate.
- Metoprolol: 1-[4-(2-methoxyethyl)phenoxy]-3-(propan-2-ylamino)propan-2-ol.
B1-Selective Blockers Uses:
- All drugs except esmolol are used in the treatment of hypertension. Acebutolol and esmolol are used for treating certain cardiac arrhythmias.
- Atenolol and metoprolol are useful in treating angina pectoris and in myocardial infarction. Atenolol has low lipid solubility and does not readily cross the blood brain barrier.
- Betaxolol is used in the treatment of glaucoma.
- Esmolol possesses a rapid onset of action and a very short duration of action. Its effects disappear within 20 to 30 minutes after the infusion is discontinued. Therefore it is used in controlling heart rate during surgery after operation.
Mixed α / ẞ-Adrenergic Receptor Antagonists
These drugs possess both ẞ- and a-receptor blocking within the same molecule. Labetalol and carvedilol are anti-hypertensives with α1, B1 and B2-blocking activity.
Labetalol:
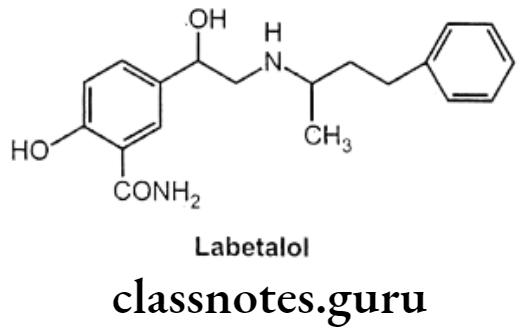
- Labetalol is chemically, 2-hydroxy-5-(1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl] benzamide.
- It is a phenyl ethanolamine derivative that is a competitive inhibitor of both ẞ1 and B2-adrenergic receptors as well as adrenergic receptors.
- It is a more potent ẞ-antagonist than a-antagonist.
- It has two asymmetric carbon atoms, therefore it exists as a mixture of four isomers. The different isomers possess different a and ẞ antagonistic activities. The ẞ-blocking activity is seen only in the (1R, 1’R) isomer, whereas α-antagonistic activity is seen in the (15, 1’R) isomer.
- It is very well absorbed and it undergoes extensive first-pass metabolism.
Labetalol Uses:
- Labetalol is a clinically useful as an antihypertensive drug.
- Due to its a-receptor blocking effect, it produces vasodilation and because of the B-receptor blocking effect, it prevents the reflex tachycardia usually associated with vasodilation.
Carvedilol:
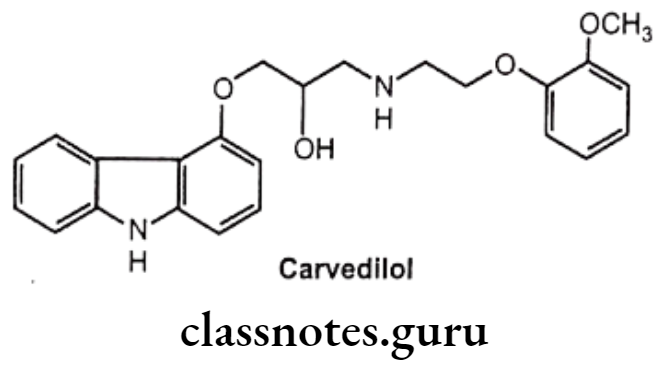
- Carvedilol is chemically, 1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)-ethyl- amino] propan-2-ol.
- It is a B-blocker that also possesses α- adrenergic receptor blocking activity.
- Its only S-enantiomer possesses the ẞ-blocking activity, whereas both enantiomers possess α1-receptor antagonistic activity.
- It also possesses antioxidant activity and an anti-proliferative effect on vascular smooth muscle cells.
Carvedilol Uses:
- It is used in the treatment of hypertension and congestive cardiac failure.
- It has neuro-protective effect and the ability to provide major cardiovascular protection.
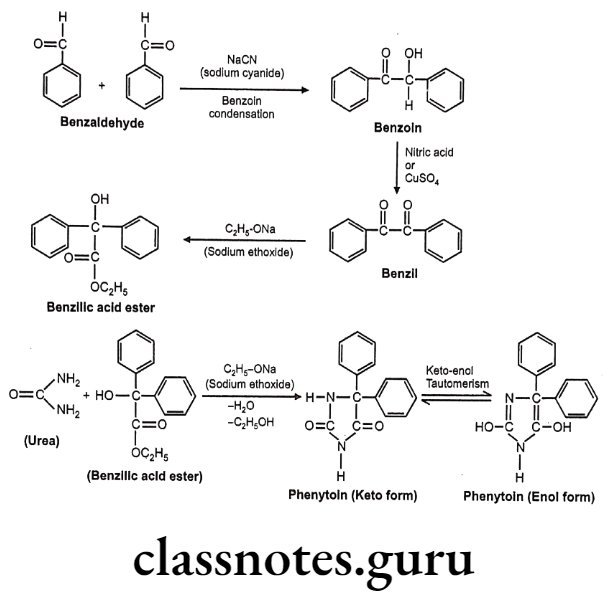
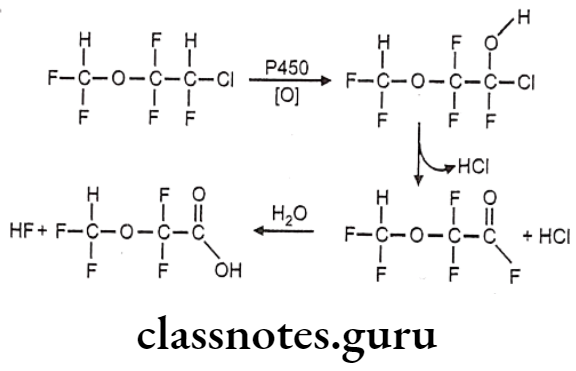
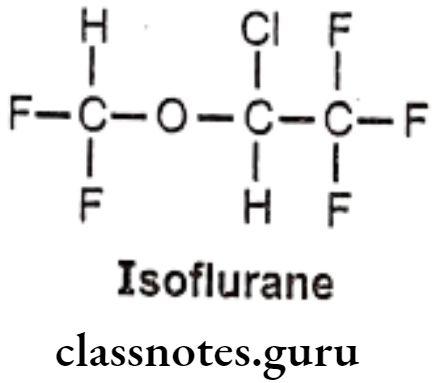
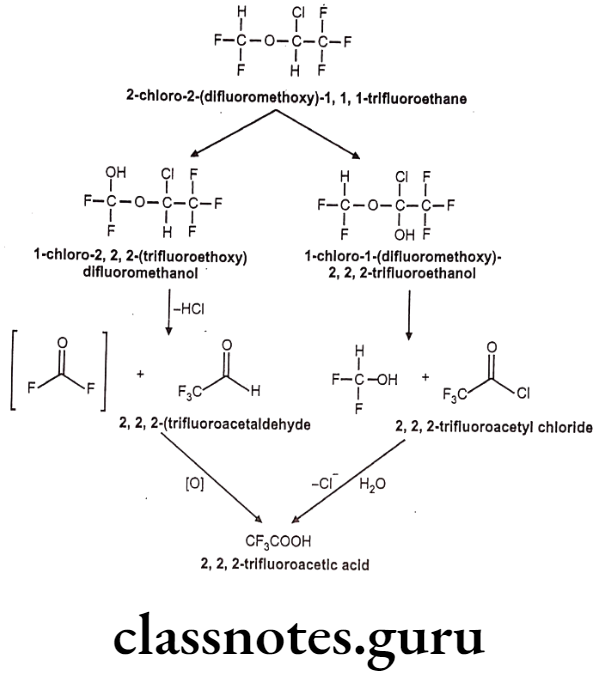
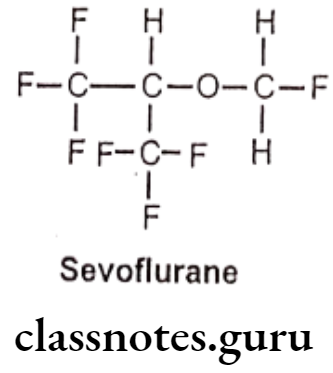
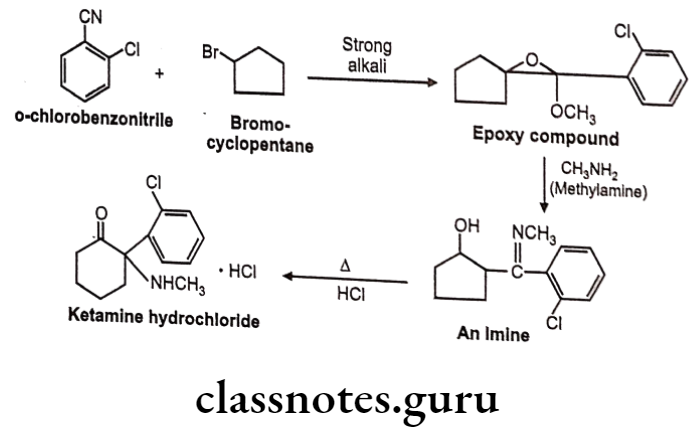
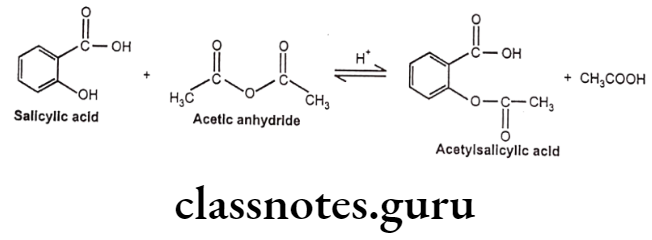
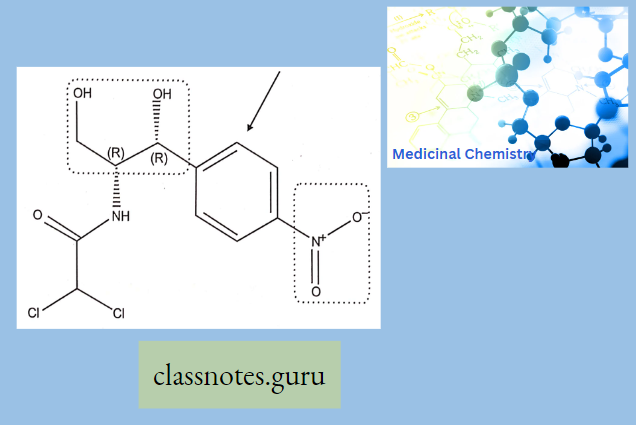
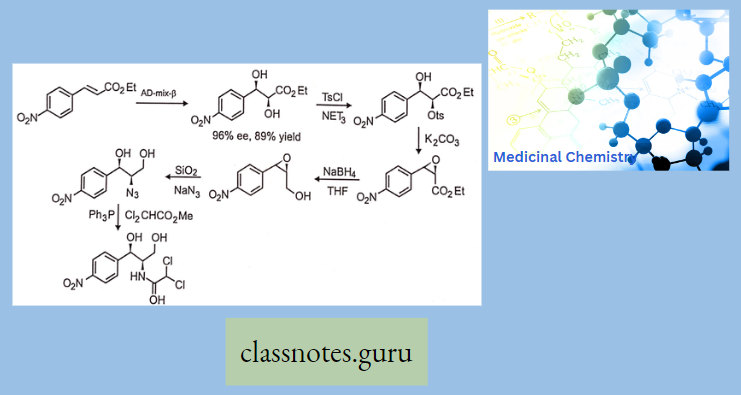
Synthesis
Phenylephrine
Salbutamol
Tolazoline
Propranolol
Multiple Choice Questions
Question 1. Sympathetic stimulation is mediated by:
- release of norepinephrine from nerve terminals
- activation of adrenoreceptors on postsynaptic sites
- release of epinephrine from the adrenal medulla
- all of the above
Answer. 4. all of the above
Question 2. Clonidine hydrochloride IP is:
- monoamine oxidase inhibitor which contains an imidazoline ring system
- arterial and venous vasodilator which contains an imidazoline ring system
- monoamine oxidase inhibitor which contains a pyrimidine ring system
- monoamine oxidase inhibitor which contains a phthalazine ring system
Answer. 2. arterial and venous vasodilator which contains an imidazoline ring system
Question 3. DOPA is an important natural amino acid and a precursor in the biosynthesis of
- Dopamine
- Adrenaline
- Nor-adrenaline
- Methyldopa
Answer. 3. Nor-adrenaline
Question 4. Characteristics of epinephrine include all of the following EXCEPT:
- It is synthesized into the adrenal medulla.
- It is mainly synthesized into the nerve ending.
- It is transported in the blood to target tissues.
- It directly interacts with and activates adrenoreceptors.
Answer. 2. It is mainly synthesized into the nerve ending.
Question 5. Chemically a-methyldopa is:
- 2-methyl-3-hydroxy-2-tyrosine
- 3-methyl-2-hydroxy-2-tyrosine
- 6-methyl-4-hydroxy-2-tyrosine
- 3-hydroxy-a-methyl-2-tyrosine
Answer. 4. 3-hydroxy-a-methyl-2-tyrosine
Question 6. Which of the following sympathomimetics acts indirectly?
- Epinephrine
- Norepinephrine
- Ephedrine
- Methoxamine
Answer. 3. Ephedrine
Question 7. The raw material for the synthesis of Propranolol is
- a-Naphthylamine
- B-Naphthol
- α-Naphthol
- a-Naphtholdehyde
Answer. 3. α-Naphthol
Question 8. Indirect action includes all of the following properties EXCEPT:
- Displacement of stored catecholamines from the adrenergic nerve ending.
- Inhibition of reuptake of catecholamines already released.
- Interaction with adrenoreceptors.
- Inhibition of the metabolism of endogenous catecholamines from peripheral adrenergic neurons.
Answer. 3. Interaction with adrenoreceptors.
Question 9. Tyrosine is a p-hydroxy derivative of phenylalanine. It loses carbon dioxide on heating with formation of:
- Dopamine
- 2-p-Hydroxypropionic acid
- 2-p-Hydroxy benzoic acid
- 2-p-Hydroxyphenylethylamine
Answer. 4. 2-p-Hydroxyphenylethylamine
Question 10. Catecholamine includes the following EXCEPT:
- Ephedrine
- Epinephrine
- Isoproterenol
- Norepinephrine
Answer. 1. Ephedrine
Question 11. Identify the biologically active isomer of adrenaline:
- R (+) enatiomer
- R (-) enatiomer
- S (+) enatiomer
- S (-) enatiomer
Answer. 2. R (-) enatiomer
Question 12. Epinephrine decreases intracellular cAMP levels by acting on:
- a receptor
- a2 receptor
- B1 receptor
- B2 receptor
Answer. 2. a2 receptor
Question 13. IUPAC name of terbutaline is:
- 5-[2-[(1,1-dimethyl ethyl)amino]-1-hydroxy ethyl]-1,3-benzenediol
- 5-[2-[(2,2-dimethyl ethyl)amino]-1-hydroxy ethyl]-1,3-benzenediol
- 5-[3-[(1,1-dimethyl ethyl)amino]-1-hydroxy ethyl]-1,3-benzenediol
- None of above
Answer. 1. 5-[2-[(1,1-dimethyl ethyl)amino]-1-hydroxy ethyl]-1,3-benzenediol
Question 14. Direct effects on the heart are determined largely by:
- α1 receptor
- a2 receptor
- B1 receptor
- B2 receptor
Answer. 3. B1 receptor
Question 15. Which isomer of the Propranolol is more active?
- Dextro isomer
- Levo isomer
- Meso isomer
- Racemic isomer
Answer. 1. Dextro isomer
Question 16. Which of the following effects is related to direct ẞ1-adrenoreceptor stimulation?
- Bronchodilation
- Vasodilation
- Tachycardia
- Bradycardia
Answer. 3. Tachycardia
Question 17. The aromatic nucleus present in Propranolol is
- Benzene
- Naphthalene
- Anthracene
- Pyridine
Answer. 2. Naphthalene
Question 18. The most appropriate starting material for propranolol is
- a-naphthol, epichlorohydrin, and isopropyl amine
- B-naphthol, epichlorohydrin, and isopropyl amine
- a-naphthol, epichlorhydrine and butyl amine
- a-naphthol, epichlorohydrin and dimethyl amine
Answer. 1. a-naphthol, epichlorohydrin and isopropyl amine
Question 19. Distribution of a-adrenoreceptor subtypes is associated with all of the following tissues except those of:
- Heart
- Blood vessels
- Prostate
- Pupillary dilator muscle
Answer. 1. Heart
Question 20. B-adrenoreceptor subtypes are contained in all of the following tissues EXCEPT:
- Bronchial muscles
- Heart
- Pupillary dilator muscle
- Fat cells
Answer. 3. Pupillary dilator muscle
Question 21. In which of the following tissues both a and ẞ1 adrenergic stimulation produce the same effect?
- Blood vessels
- Intestine
- Uterus
- Bronchial muscles
Answer. 1. Blood vessels
Question 22. The effects of sympathomimetics on blood pressure are associated with their effects on:
- The heart rate
- The peripheral resistance
- The cardiac output
- All of the above
Answer. 4. All of the above
Question 23. A relatively pure a-agonist causes all of the following effects EXCEPT:
- Increase in peripheral arterial resistance
- Increase in venous return
- Has no effect on blood vessels
- Reflex bradycardia
Answer. 3. Has no effect on blood vessels
Question 24. A non-selective ẞ-receptor agonist causes all of the following effects EXCEPT:
- Increase in cardiac output
- Increase in peripheral arterial resistance
- Decrease in peripheral arterial resistance
- Decrease in mean pressure
Answer. 2. Increase in peripheral arterial resistance
Question 25. Which of the following statements is not correct?
- a-agonists cause miosis.
- a-agonists cause mydriasis.
- B-antagonists decrease the production of aqueous humor.
- a-agonists increase the outflow of aqueous humor from the eye.
Answer. 1. a-agonists cause miosis.
Question 26. A bronchial smooth muscle contains:
- a1-receptor
- α2-receptor
- B1-receptor
- B2-receptor
Answer. 4. B2-receptor
Question 27. All of the following agents are ẞ-receptor agonists EXCEPT:
- Epinephrine
- Isoproterenol
- oxymetazoline
- Dobutamine
Answer. 3. oxymetazoline
Question 28. Which of the following drugs causes bronchodilation without significant cardiac stimulation?
- Isoproterenol
- Terbutaline
- Xylometazoline
- Clonidine
Answer. 2. Terbutaline
Question 29. B1-receptor stimulation includes all of the following effects EXCEPT:
- Increase in heart contractility
- Bronchodilation
- Tachycardia
- Increase in conduction velocity in the atrioventricular node
Answer. 2. Bronchodilation
Question 30. B2-receptor stimulation includes all of the following effects EXCEPT:
- Stimulation of renin secretion
- Fall of potassium concentration in plasma
- Relaxation of bladder, uterus
- Tachycardia
Answer. 1. Stimulation of renin secretion
Question 31. Hyperglycemia induced by epinephrine can be due to:
- Gluconeogenesis (B2)
- Inhibition of insulin secretion (α2)
- Stimulation of glycogenolysis (B2)
- All of the above
Answer. 4. All of the above
Question 32. Which of the following effects is associated with ẞ3-receptor stimulation?
- Lipolysis
- Decrease in platelet aggregation
- Bronchodilation
- Tachycardia
Answer. 1. Lipolysis
Question 33. Which of the following statements is not correct?
- Epinephrine acts on both a- and ẞ-receptors.
- Norepinephrine has a predominantly ẞ-action.
- Phenylephrine has a predominantly a-action.
- Isoproterenol has a predominantly ẞ-action.
Answer. 2. Norepinephrine has a predominantly ẞ-action.
Question 34. Indicate the drug, which is a direct-acting both a- and B-receptor agonist:
- Norepinephrine
- Methoxamine
- Isoproterenol
- Ephedrine
Answer. 1. Norepinephrine
Question 35. Which of the following agents is an a2-selective agonist?
- Norepinephrine
- Clonidine
- Ritodrine
- Ephedrine
Answer. 2. Clonidine
Question 36. Which of the following agents is a non-selective ẞ-receptor agonist?
- Norepinephrine
- Terbutaline
- Isoproterenol
- Dobutamine
Answer. 3. Isoproterenol
Question 37. Indicate the ẞ1-selective agonist:
- Isoproterenol
- Dobutamine
- Metaproterenol
- Epinephrine
Answer. 2. Dobutamine
Question 38. Which of the following sympathomimetics is a ẞ2-selective agonist?
- Terbutaline
- Xylometazoline
- Isoproterenol
- Dobutamine
Answer. 1. Terbutaline
Question 39. Indicate the indirect-acting sympathomimetic agent:
- Epinephrine
- Phenylephrine
- Ephedrine
- Isoproterenol
Answer. 3. Ephedrine
Question 40. Epinephrine produces all of the following effects EXCEPT:
- Decrease in oxygen consumption
- Bronchodilation
- Hyperglycemia
- Mydriasis
Answer. 1. Decrease in oxygen consumption
Question 41. Epinephrine is used in the treatment of all of the following disorders EXCEPT:
- Bronchospasm
- Anaphylactic shock
- Cardiac arrhythmias
- Open-angle glaucoma
Answer. 4. Open-angle glaucoma
Question 42. Which of the following direct-acting drugs is a relatively pure a-agonist, an effective mydriatic and decongestant and can be used to raise blood pressure?
- Epinephrine
- Norepinephrine
- Phenylephrine
- Ephedrine
Answer. 3. Phenylephrine
Question 43. Isoproterenol is:
- Both an a- and B-receptor agonist
- B1-selective agonist
- B2-selective agonist
- Non-selective ẞ-receptor agonist
Answer. 4. Non-selective ẞ-receptor agonist
Question 44. Ephedrine causes:
- Miosis
- Bronchodilation
- Hypotension
- Bradycardia
Answer. 2. Bronchodilation
Question 45. Which of the following sympathomimetics is preferable for the emergency therapy of cardiogenic shock?
- Ephedrine
- Dobutamine
- Isoproterenol
- Methoxamine
Answer. 2. Dobutamine
Question 46. Which of the following sympathomimetics is related to short-acting topical decongestant agents?
- Albuterol
- Phenylephrine
- Terbutaline
- Norepinephrine
Answer. 3. Terbutaline
Question 47. Indicate the long-acting topical decongestant agent:
- Epinephrine
- Norepinephrine
- Isoproterenol
- Xylometazoline
Answer. 4. Xylometazoline
Question 48. Which of the following topical decongestant agents is an a1-selective agonist?
- Phenylephrine
- Clonidine
- Ephedrine
- Epinephrine
Answer. 1. Phenylephrine
Question 49. Indicate the agent of choice in the emergency therapy of anaphylactic shock:
- Methoxamine
- Terbutaline
- Phenylephrine
- Epinephrine
Answer. 4. Epinephrine
Question 50. Which of the following sympathomimetics is an effective mydriatic?
- Salmeterol
- Phenylephrine
- Dobutamine
- Norepinephrine
Answer. 2. Phenylephrine
Question 51. Sympathetic stimulation is mediated by.
- Release of norepinephrine from nerve terminals
- Activation of adrenoreceptors on postsynaptic sites
- Release of epinephrine from the adrenal medulla
- All of the above
Answer. 4. All of the above
Question 52. Which of the following sympathomimetics acts indirectly?
- Epinephrine
- Norepinephrine
- Ephedrine
- Methoxamine
Answer. 3. Ephedrine
Question 53. Catecholamine includes following EXCEPT:
- Ephedrine
- Epinephrine
- Isoprenaline
- Norepinephrine
Answer. 1. Ephedrine
Question 54. Which of the following agents is an a1-selective agonist?
- Norepinephrine
- Methoxamine
- Ritodrine
- Ephedrine
Answer. 2. Methoxamine
Question 55. All of the following agents are B-receptor agonists EXCEPT:
- Epinephrine
- Isoproterenol
- Methoxamine
- Dobutamine
Answer. 3. Methoxamine
Question 56. Indicate the direct-acting sympathomimetic, which is an a12 and B1 receptor agonist:
- Isoproterenol
- Ephedrine
- Dobutamine
- Norepinephrine
Answer. 4. Norepinephrine
Question 57. Indicate the indirect-acting sympathomimetic agent:
- Epinephrine
- Phenylephrine
- Ephedrine
- Isoproterenol
Answer. 3. Ephedrine
Question 58. Which of the following agents is an a1, a2, B1, and B2 receptor agonist?
- Methoxamine
- Albuterol
- Epinephrine
- Norepinephrine
Answer. 3. Epinephrine
Question 59. Which of the following sympathomimetics is a B2-selective agonist?
- Terbutaline
- Xylometazoline
- Isoproterenol
- Dobutamine
Answer. 1. Terbutaline
Question 60. Isoproterenol is:
- Both a- and ẞ-receptor agonist
- B1-selective agonist
- B2-selective agonist
- Nonselective ẞ-receptor agonist
Answer. 4. Nonselective ẞ-receptor agonist
Question 61. Ephedrine causes:
- Miosis
- Bronchodilation
- Hypotension
- Bradycardia
Answer. 2. Bronchodilation
Question 62. Indicate the long-acting topical decongestant agents:
- Epinephrine
- Norepinephrine
- Phenylephrine
- Xylometazoline
Answer. 4. Xylometazoline
Question 63. Indicate the agent of choice in the emergency therapy of anaphylactic shock:
- Methoxamine
- Terbutaline
- Norepinephrine
- Epinephrine
Answer. 4. Epinephrine
Question 64. Which of the following sympathomimetics is an effective mydriatic?
- Salmeterol
- Phenylephrine
- Dobutamine
- Norepinephrine
Answer. 2. Phenylephrine
Question 65. Which of the following drugs is a nonselective a-receptor antagonist?
- Prazosin
- Phentolamine
- Metoprolol
- Reserpine
Answer. 2. Phentolamine
Question 66. Indicate the α-selective antagonist:
- Phentolamine
- Dihydroergotamine
- Prazosin
- Labetalol
Answer. 3. Prazosin
Question 67. Which of the following drugs is a non-selective ẞ-receptor antagonist?
- Metoprolol
- Atenolol
- Propranolol
- Acebutolol
Answer. 3. Propranolol
Question 68. Indicate the ẞ1-selective antagonist:
- Propranolol
- Metoprolol
- Carvedilol
- Sotalol
Answer. 2. Metoprolol
Question 69. Which of the following drugs is a reversible non-selective a, ẞ-antagonist?
- Labetalol
- Phentolamine
- Metoprolol
- Propranolol
Answer. 1. Labetalol
Question 70. The principal mechanism of action of adrenoreceptor antagonists is:
- Reversible or irreversible interaction with adrenoreceptors
- Depletion of the storage of catecholamines
- Blockade of the amine reuptake pumps
- Non-selective MAO inhibition
Answer. 1. Reversible or irreversible interaction with adrenoreceptors
Question 71. Non-selective a-receptor antagonists are most useful in the treatment of:
- Asthma
- Cardiac arrhythmias
- Pheochromocytoma
- Chronic hypertension
Answer. 3. Pheochromocytoma
Question 72. Which of the following drugs is useful in the treatment of pheochromocytoma?
- Phenylephrine
- Propranolol
- Phentolamine
- Epinephrine
Answer. 3. Phentolamine
Question 73. Indicate adrenoreceptor antagonist agents, which are used for the management of pheochromocytoma:
- Selective ẞ2-receptor antagonists
- Non-selective ẞ-receptor antagonists
- Indirect-acting adrenoreceptor antagonist drugs
- a-receptor antagonists
Answer. 4. a-receptor antagonists
Question 74. Propranolol is used in the treatment all of the following diseases EXCEPT:
- Cardiovascular diseases
- Hyperthyroidism
- Migraine headache
- Bronchial asthma
Answer. 4. Bronchial asthma