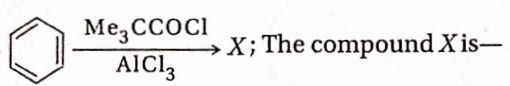CBSE Class 11 Chemistry Notes For Chapter 5 States Of Matter Gases And Liquids States Of Matter Gases And Liquids Introduction
Anything that possesses mass and occupies space is called matter. Based on physical state, they can be of three types i.e., solid, liquid, and gas. Any substance can exist in any one ofthe three states, depending on the temperature and pressure.
Along with these three states, matter can exist in two other states.
They are plasma and Bose-Einstein condensate. However, the physical state of a substance at normal temperature (usually 25°C) and pressure {i.e. 1 atm) depends on its normal melting point and normal boiling point.
- A substance is said to be in a gaseous state if its normal boiling point is below room temperature.
- According to kinetic molecular theory, matter is composed of minute particles (like atoms, molecules, or ions), which are held together by intermolecular forces of attraction.
- However, particles of matter also possess thermal energy due to temperature.
- The forces arising from thermal energy have a disruptive effect and tend to cause the particles to get separated from each other.
Read and Learn More CBSE Class 11 Chemistry Notes
Thus, the effect of intermolecular forces of attraction and that of thermal energy are just opposite, and they counteract each other.
- The relative magnitudes of those counteracting effects determine whether a substance would exist in a solid, liquid, or gaseous state at a given temperature and pressure.
- If the effect of intermolecular forces of attraction is much less than that of the thermal energy of the molecules in a substance, then the substance exists in the gaseous state.
- Because intermolecular forces of attraction in a gas are very weak or negligible, gas molecules do not occupy fixed positions.
- Instead, they are always in ceaseless, rapid, and random motion, and move out independently throughout the entire volume ofthe container holding the gas.
- This explains why gases do not have any definite shape or volume.
When the effect of intermolecular forces of attraction is comparatively greater than that of the thermal energy of the molecules of a substance, then the substance exists in the liquid state.
- Because intermolecular forces attract liquids that are not so strong for their molecules to be held at well-defined positions, liquids do not have a definite shape.
- They assume the shape of the container in which they are kept.
- However, intermolecular forces of attraction in a liquid are strong enough to hold the molecules together and prevent them from moving apart. This is why liquids have a definite volume.
- When the effect of intermolecular forces of attraction is much stronger compared to that of thermal energy in a substance, then the substance exists in a solid state.
- In a solid due to strong intermolecular forces of attraction, particles in I Solid state making up the solid are held at fixed locations and remain very close to each other.
Particles, being held at fixed positions, do not possess translational motion although they can vibrate about their mean positions. As the particles in a solid have fixed positions and lack translational motion, solids have a definite shape and volume.

NCERT Class 11 Chemistry Notes Liquids, States of Matter, Gases
Various Kinds Of Intermolecular Attractive Forces And Their Nature
Attractive Forces Acting Between The molecules (atoms in the case of monoatomic gas) in a substance are called intermolecular forces of attraction.
These forces are different from the electrostatic force existing between the two oppositely charged ions and the forces that hold the atoms together with molecular covalent bonds intermolecular forces of attraction are relatively weaker than the forces of attraction by which atoms are held in covalent bonds or the electrostatic forces of hydrogen by which oppositely charged ions are held.
Different Types Of Intermolecular Forces of Attraction
Intermolecular forces of attraction are classified as follows:
- Instantaneous induced dipole-instantaneous induced dipole attractions.
- Dipole-dipole attractions.
- Dipole-induced dipole attractions
- Ion-dipole attractions
- Hydrogen bond. Among the above forces, the first three are collectively known as the van der Waals forces because van der Waals explained the deviation of real gases from ideal behaviour in terms of these forces. In this chapter, we will focus only on the van der Waals forces.
Instantaneous induced dipole-instantaneous induced dipole attraction (London forces or dispersion forces)
These forces are found to occur in all substances because they exist between all atoms, molecules, and ions. In the case of non-polar substances, however, these are the only intermolecular forces that operate.
Due to the existence of these forces, it is possible to transform non-polar gases (such as H2, N2, O2, CH4, etc.) or inert gases (such as He, Ne, Ar, etc.) into liquid, when cooled to very low temperatures.
These forces are commonly known as London forces, after the name of a German physicist. Frit London, explained the origin and nature of these forces.
Origin:
To understand the origin of London forces, let us consider the generation of a dipole in an atom. Electrons are symmetrically distributed around the nucleus of an atom.
This symmetrical distribution is a time average distribution and the centers of gravity of the positive charge and negative charge (electron cloud) remain at the same point in this distribution.
However, at any instant, the density of the electron cloud on one side of the nucleus may be greater than the other.
Consequently, an instantaneous dipole develops in the die atom due to the separation of charge centers and as a result, a partial negative charge (O’-) on one side ofthe atom and a partial positive charge (5+) on the other side are created.
This short-lived dipole continuously changes its direction with the movement of electrons in such a way that the time average dipole moment becomes zero. The instantaneous dipole formed in one atom distorts the symmetrical distribution of the electron cloud of a neighbouring atom and induces an instantaneous dipole in that atom.
Similar temporary dipoles are induced in polar molecules also. This leads to an interatomic or intermolecular attraction. Such forces of attraction are known as instantaneous-induced dipole-instantaneous induced dipole attraction. Illustrates how instantaneous induced dipole-instantaneous induced dipole forces develop between the atoms in He gas.

Characteristics:
- The strength of London forces decreases very rapidly as the distance between particles increases. If the distance between two interacting particles is r then the strength of London forces varies as 1/r6.
- The strength of this force generally lies between 0.05 to 40 kj.mol-1
- The magnitude of London’s force depends on the polarisability ofthe atoms or molecules. The polarisability of an atom or a molecule is a measure of the ease with which its electron clouds can be distorted.
An atom or a molecule with a large size (or large molar mass) contains a large number of electrons and hence possesses a large diffused electron cloud, which can get distorted easily. This is why atoms or molecules with larger size (or molar mass) are more polarisable and found to possess stronger London forces.
For the halogens, molecular sizes follow the order: F2 < Cl2 < Br2 < I2:
Accordingly, the order of their increasing strength of London forces is F, < Cl, < Br2 < I2. Because of this, their boiling points follow the order F2 < Cl2 < Br2< I2 Similarly, the order of boiling points of inert gases is He < Ne < Ar < Kr < Xe. This is because the atomic size of inert gases increases in die same order.
Dipole-dipole attraction
These forces exist between neutral polar molecules (Example: H2O, NH3, HC1, etc.). However, in addition to these forces, London forces also act between the molecules.
Origin:
Polar molecules have permanent dipoles.
- They possess a partial positive charge at one end and a partial negative charge at the other end.
- The dipole-dipole attractive force causes the polar molecules to align themselves in such a manner that the positive end of one polar molecule is directed towards the negative end of another polar molecule.
- As a result, a net attractive force acts between the two polar molecules.
This attractive force is called the dipole-dipole attractive force.

Dipole-dipole attraction Characteristics:
- Dipole-dipole attractive forces are generally stronger than London forces and have a magnitude ranging from 5-25 kj.mol-1
- The strength of dipole-dipole attractive forces increases with the increasing polarity of the molecules
- As the distance between the two dipolar molecules increases, the strength of these forces decreases. If the distance between two dipoles is r, then dipole-dipole attractive forces will be proportional to l/r6.
Dipole-induced dipole attractions
These forces act between polar molecules having permanent dipole moments and non-polar molecules or polar molecules having very low dipole moments. For example— H2O….I2, Kr….(phenol)2, etc.
Origin:
Whenever a polar molecule comes closer to a polarisable non-polar molecule, the polar molecule induces a dipole moment in the non-polar molecule by deforming or polarising its electron cloud.
Thus, the non-polar molecule becomes a dipole. We call it an induced dipole as it is induced by the polar molecule. As a result, attractive forces are generated between the permanent dipole and the induced dipole. This attractive force is called dipole-induced dipole attractive force.

Dipole-induced dipole attractions Characteristics:
- If the distance between the molecule with a permanent dipole and that with an induced dipole is r, then the dipole-induced dipole attractive force between them is found to be proportional to 1/r6.
- The strength of this force increases with the increasing dipole moment of a polar molecule and the polarisability of a non-polar molecule.
- The magnitude of this force is generally 2-10 kj.mol-1.
Physical And Measurable Properties Of Gaseous Substances
The physical properties of different gaseous substances are generally the same although their chemical properties may differ Some important properties
The physical properties of a gas can be explained with the help of four measurable properties such as pressure, temperature, mass, and volume. Different gas laws are based on the relationships among these properties.

Mass And Volume Of Gas
- Mass of gas: The mass of a gas enclosed in a container can be determined by subtracting the mass of the empty container from the mass of the container filled with the gas. In the gas laws, the amount of a gas is generally expressed in terms of moles. The number of moles of a gas enclosed in a container is obtained by dividing the mass of the gas by its molar mass.
- Volume of a gas: At a particular temperature and pressure, the volume of a gas enclosed in a container is equal to the volume ofthe container
- Units of volume: Generally, volume is expressed in the (L), millilitre (mL), cubic centimetre (cm3), cubic meter (m3), or cubic decimetre (dm3).
The relationship among these different units are—
⇒ \(1 \mathrm{~L}=10^3 \mathrm{~mL}=10^3 \mathrm{~cm}^3=10^3 \times\left(10^{-1}\right)^3 \mathrm{dm}^3=1 \mathrm{dm}^3\)
In the SI system, the unit of volume is a cubic meter (m3).
⇒ \(1 \mathrm{~m}^3=\left(10^2 \mathrm{~cm}\right)^3=10^6 \mathrm{~cm}^3=10^6 \mathrm{~mL}=10^3 \mathrm{dm}^3=10^3 \mathrm{~L}\)
The pressure of a gas
The pressure of a gas arises due to the collisions of the gas molecules with the walls of the container in which it is kept. It is defined as the force exerted by the gas molecules per unit area ofthe walls of the container.
Measurement of atmospheric pressure:
Atmospheric pressure is measured with the help of an apparatus known as a barometer To construct a barometer, a glass tube, about 80 cm long, with one end closed is filled with dry mercury.
- And placed vertically with its open end immersed in a dish of mercury. As some mercury lows out of the tube, the height of the mercury level in the tube drops gradually.
- This continues until the downward pressure exerted by the mercury column and the atmospheric pressure over the mercury in the dish become equal.
- When the mercury level inside the tube becomes fixed, it is nd placed vertically with its open end immersed in a dish of mercury. As some mercury lows out of the tube, the height of the mercury level in the tube drops gradually.
- This continues until the downward pressure exerted by the mercury column and the atmospheric pressure over the mercury in the dish become equal.
When the mercury level inside the tube becomes fixed, it is

- Inside The Tube Is a Perfect Vacuum, The Pressure is Due To The. Mercury Column Is Equal To The Pressure Of The Atmosphere.
- Thus, The Height Of The Mercury Level In The Barometer Tube Is
- A Direct Measure Of The Atmospheric Pressure.
- For This Reason, Atmospheric Pressure Is Usually Expressed In
- Terms Of Height Of The Mercury Level In The Barometer Tube. For Example, The Atmospheric Pressure Of 76 Cm (Or 760 Mm) Hg Means That The Atmospheric Pressure Is Equal To The Pressure Exerted By 76 Cm (Or 760 Mm) mercury column.
The pressure exerted by the mercury column:
Let us consider a mercury column of hem height in a glass tube with a uniform cross-sectional area of Acm². The downward force exerted by the mercury column is equal to its weight, and this force per unit area is the pressure exerted by the column.
Therefore, the pressure exerted by the mercury column,
P = \(\frac{\text { force }}{\text { area }}=\frac{\text { mass } \times \text { acceleration due to gravity }}{A}=\frac{m \times g}{A}\)
Where m and g are the mass of the mercury inside the tube and acceleration due to gravity respectively. If the volume and density of mercury inside the tube are V and d, respectively,
Then- V= A × h and m = V × d = A × h × d
And,
P = \(\frac{m \times g}{A}=\frac{A \times h \times \mathrm{d} \times g}{A}\)
- By using equation (1), we can calculate the pressure exerted by a mercury column of height if the density (d) of mercury and the acceleration due to gravity (g) is known of a place depending on the height of that place from the sea level.
- The higher the altitude, the lower the atmospheric pressure. Atmospheric pressure also depends on the temperature and weather conditions.
- The pressure exerted by a mercury column of 76 cm or 760 mm height at sea level is defined as the standard or normal atmospheric pressure.
Determination of the pressure of gas:
The instrument used for measuring the pressure of a gas in a vessel is called a manometer. Using this instrument, the pressure of gaseous reactants or products in a chemical reaction can be measured. Manometers are of two types: open-end manometers and closed-end manometers.
Open-end manometer:
It consists of aU-tube partly filled with mercury. One arm ofthe tube is longer than the other. The shorter arm is connected to a container holding a gas whose pressure is to be determined. The longer arm is open to the atmosphere, Three possibilities may arise during the determination of the pressure of a gas using such a manometer.

- The level of mercury in both the arms of the U-tube is the same. Therefore, the pressure of the gas enclosed in the bulb is equal to the atmospheric pressure, i.e., Pgas = Patm.
- The level of mercury in the longer arm of the U-tube is above that in the shorter arm. This occurs when the pressure of the gas inside the bulb is greater than the atmospheric pressure i.e., Pgas >Patm.
- The pressure of the gas = atmospheric pressure + difference between the heights of mercury levels in the two arms of the U-tube i.e., Pgas = Patm.
- The level of mercury in the longer arm is below that in the shorter arm, indicating the pressure of the gas inside the bulb is less than the die atmospheric pressure i.e., Pgas < Patm.
- Therefore, the pressure of the gas = atmospheric pressure – the difference between the heights of mercury levels in the two arms of the tube, ie., Psas = Paatmh
Closed-end manometer:
If the pressure of the gas inside the die container is less than the die atmospheric pressure, then this type of manometer is generally used to determine the pressure of the gas.
- It also consists of a U-tube with arms of different heights, The space above the mercury level ofthe closed-end arm of the U-tube is made perfectly vacuum by partially filling up the tube with mercury.
- The shorter arm is connected to the container filled with gas whose pressure is to be determined.
Because of the pressure exerted by the gas, the mercury level goes down in the shorter arm and goes up in the longer arm. The difference between the mercury levels in the two arms gives the pressure of the gas.

Therefore, the pressure of the gas= The difference between the mercury levels in the two arms = h i.e., Pgas= h
Units of pressure:
1. The pressure of a gas is generally expressed in the unit of the atmosphere (atm). The pressure exerted by exactly 76 cm (or 760 mm) of a mercury column at the level of 0°C is called 1 atmosphere (1 atm).
2. Sometimes, pressure is also expressed in torr [named after Torricelli], The pressure exerted by exactly 1 mm of mercury column at sea level at 0°C is called 1 torr.
1 atm = 760 torr
The value of 1 atm pressure in torma of dyn/cm² and N/m²:
At 0°C, the density of pure mercury, d = 13.5951 g. cm-3, standard acceleration due to gravity, g =9110.665 cm. s¯³; height of mercury column, h = 76cm.
Applying the relation P = h × d × g gives,
1 atm =76 cm × 13.5951 g cm¯³ × 980.665 cm. s¯²
= 1.013 × 106 dyn cm¯² [∴ dyn = g. cm. s¯²)
= 1.013 × 105 N m‾²
1N = 105 dyn and 1m = 100 cm]
The SI unit of pressure is Pascal.
3. The pressure exerted when a force of 1 newton acts on an area of lm² is called 1 pascal. Therefore,[1 Pa = 1 N m‾²J, and 1 atm
= 1.013 ×105 N.m² = 1.013 ×105 Pa
= 101.3 kPa
1 atm = 76.0 cm Hg = 760 mm Hg = 760 torn = 1.013 × 105 N m-2
= 1.013 × 105 Pa Another unit used for gas pressure is a bar.
1 bar = 105 Pa = 0.9869 atm = 750.062 torr and atm = 1.013 bar
The pressure is also expressed in the unit pound per square inch or psi. 1 atm = 14.7 psi
Numerical Examples
Question 1. At a certain place, the atmospheric pressure is 740 mm Hg. What will be the value of this pressure in the units of— torr atm Pa and bar
Answer: Given:
Atmospheric pressure = 740 mm Hg.
1 torr =1 mm Hg.
Therefore, 740 mm Hg = 740 torr
1 atm = 760 mm Hg
Thus, 740 mm Hg \(=\frac{1 \mathrm{~atm}}{760 \mathrm{~mm} \mathrm{Hg}} \times 740 \mathrm{~mm} \mathrm{Hg}\)
= 0.97 atm
Atm = 760 mm Hg = 1.013 X 105 Pa
Therefore, 740mm Hg \(=\frac{1.013 \times 10^5 \mathrm{~Pa}}{760 \mathrm{~mm} \mathrm{Hg}} \times 740 \mathrm{~mm} \mathrm{Hg}^{-10}\)
= 9.86 ×104 Pa
1 atm = 760 mm Hg
= 1.013 bar
Hence, 740 mm Hg \(=\frac{1.013 \text { bar }}{760 \mathrm{~mm} \mathrm{Hg}} \times 740 \mathrm{~mm} \mathrm{Hg}\)
= 0.986 bar.
Liquids and Gases States of Matter Class 11 Chemistry Notes
Question 2. A bulb filled with a gas is connected to an open-end manometer. The level of mercury in the arm attached to the gas bulb is 20 cm lower than that in the open-ended arm. Calculate the pressure of the gas in the bulb in the units of atm and Pa. Consider the atmospheric pressure to be 76 cm Hg.
Answer:
Since the height of the mercury level in the open-end arm is higher than that in the arm attached to the bulb,
⇒ \(P_{\text {gas }}=P_{\text {atm }}+h=(76+20) \mathrm{cm} \mathrm{Hg}=96 \mathrm{~cm} \mathrm{Hg}\)
h = 20cm, p atm = 76cm hg]
As 1 atm = 76 cm Hg
= 1.013 × 105 Pa
⇒ \(96 \mathrm{~cm} \mathrm{Hg}=\frac{1 \mathrm{~atm}}{76 \mathrm{~cm} \mathrm{Hg}} \times 96 \mathrm{~cm} \mathrm{Hg}=1.26 \mathrm{~atm}\)
Question 3. An open-end manometer was used to determine the pressure of a gas present in a container. It was found that the height of the mercury level in the arm attached to the gas-filled container was 4 cm higher than that in the open-end arm. If the atmospheric pressure was measured to be 76 cm Hg, then what would be the pressure of the gas inside the container in the atm unit
Answer:
As the height of the mercury level in the arm attached to the container is higher than that in the open-end arm
⇒ \(P_{\text {gas }}=P_{\text {atm }}-h\)
⇒ \(P_{\text {gas }}=P_{\text {atm }}-h=(76-4.0) \mathrm{cm} \mathrm{Hg}=72 \mathrm{~cm} \mathrm{Hg}\)
Thus, the pressure of gas Inside the container
⇒ \(=72 \mathrm{~cm} \mathrm{Hg}=\frac{72 \mathrm{~cm} \mathrm{Hg}}{76 \mathrm{~cm} \mathrm{Hg}} \times 1 \mathrm{~atm}=0.94 \mathrm{~atm}\)
Temperature of a gas
Temperature is a measure of the degree of hotness or coldness ofa the body. It is a property that determines the direction of heat flow from one body to another.
The temperature of a body is measured by an instrument known as a thermometer. Three types of temperature scales are generally used. These are the Celsius scale, Fahrenheit scale, and Kelvin scale.
Celsius scale:
On the Celsius scale, the normal freezing and the normal boiling temperatures of pure water are O’C and 100°C, respectively. On this scale, 0°C (lower fixed point) and ItMTC (upper fixed point) are used as reference points, and the space between these two temperatures is divided into 100 equal divisions. Each division corresponds to 1°C.

Fahrenheit scale:
On the Fahrenheit scale, the normal freezing and the normal boiling temperatures of pure water are 32°F and 212°F respectively.
On this scale, 32°F (lower fixed point) and 212°F (upper fixed point) are used as reference points, and the space between these two temperatures is divided into 180 equal divisions. Each division corresponds to 1°.
Kelvin or absolute scale:
On this scale, -273°C temperature is considered as zero point and is termed as absolute zero temperature. Hence, the Kelvin scale is also called the absolute scale of temperature.
Each division on this scale is called IK [Note the temperature unit is K, not °K. The conventional degree symbol (°) is not written] and is equal to each division on the Celsius scale (i.e. equal to 1°C). So, the zero point on the Kelvin scale is 273-degree units below the zero point on the Celsius scale [i.e., 0°C).
Hence, 0°C = 273K and 0 K = -273°C. According to the absolute scale, the value of temperature is called absolute temperature and is generally expressed by the letter, T.
Relation between Celsius and Kelvin scale:
Let, a particular temperature in the Celsius scale be t°C and in the Kelvin scale be 7K.
As 0°C = 273 K, so (0 + f)°C = (273 + 1)K or’l t°C = (273 + t)K
Therefore, if the temperature on the Celsius scale is t°C, then this temperature kelvin scale will be (273 + t)K.
Example: 25 °C = (273 + 25) = 298 K,
-40°C = (273-40) = 233K
- The SI unit oftemperatureiskelvin(K).
- The value of temperature on the Kelvin scale is always positive.
- The numerical values of the change in temperature in both Celsius and Kelvin scales are the same.
Gas laws
The gaseous state is the simplest state of matter. All gases irrespective of their chemical nature, obey some general laws called gas laws. These laws are related to four measurable properties viz., pressure (P), volume (V), temperature (I), and amount or number of moles (n) of a gas.
Boyle’s law:
Relation between volume & pressure Robert Boyle performed a series of experiments to know how the volume of a given mass of gas at a constant temperature is changed with the pressure. The results of his experiments led him to put forward a law which is known as Boyle’s law.
Boyle’s law: At constant temperature, the volume of a given mass of gas is inversely proportional to its pressure.
Mathematical expression of Boyle’s law:
Suppose, at a constant temperature, the pressure and volume ofa definite mass of gas are P and V, respectively. According to Boyle’slaw,
⇒ \(V \propto \frac{1}{p}\) [when mass and temperature are constant]
⇒ \(\text { or, } V=K \times \frac{1}{P} \quad \text { or, } P V=K\)
Pressure Of A Gas
The equation is the mathematical expression of Boyle’s law. In this equation, If is a constant, whose value depends on the mass and temperature ofthe gas.
- Therefore, according to Boyle’s law, at a constant temperature, the product of pressure and volume of a given mass of gas is always constant.
- Let us consider, that at a particular temperature, the pressure and volume of a definite mass of gas are P1 and Vl, respectively.
- Keeping the temperature constant, if the volume of the gas is changed from V1 to V2 by changing the pressure from P1 to P2, then according to Boyle’s law, at the initial state, P1V1 = K, and at the final state, P2V2 = K.
Therefore, P1V1 = P2V2 or, P1/P2 = V2/V1
Explanation of Boyle’s law:
Suppose, the pressure and volume of a definite amount of gas at a particular temperature are P and V, respectively. Thus, according to Boyle’s law, at a constant temperature, if the pressure of the gas is doubled (2P), the volume of the gas will become half of its initial volume ( V/2), and if the pressure is increased by a factor of four, then the volume will become one-fourth of its initial value ( V/4).
On the other hand, at a constant temperature, the pressure of the gas is halved (P/2), and the volume of the gas will become twice its initial volume (2V). Similarly, if the pressure is decreased to one-fourth of its initial pressure (P/4) the volume will become four times its initial volume (4 V).

Graphical representations of Boyle’s law:




Applicability of Boyle’s law:
At normal temperature and pressure, H2, N2, and light inert gases obey this law with some degree of approximation but gases such as NH3, CO2, etc. do not obey this law. Most gases follow Boyle’s law only at very high temperatures or at relatively low pressures.
States of Matter and Gases Class 11 Chemistry Notes
Molecular Interpretation of Boyish Law:
The pressure of a gas is the result of collisions of gas molecules with the walls of the container.
- At constant temperature if the volume of a given mass of gas is reduced, the number of molecules per unit volume of the gas increases.
- This results in an enhanced frequency of collisions of molecules with the walls of the container.
- As a result, the pressure of the gas increases. Alternatively, the frequency of collisions with the walls decreases with the increasing volume of a given mass of gas at a constant temperature, resulting in a decrease in the pressure of the gas.
Corollary of Boyle’s law—Relation between pressure and density:
Suppose, a gas with a mass of m has a pressure, volume, and density of P, V, and d, respectively, at a constant temperature. According to Boyle’s law, PV = K (constant); when the mass and temperature of the gas are constant.
⇒ \(\text { Density }(d)=\frac{\text { mass }}{\text { volume }}=\frac{m}{V} \text { or, } V=\frac{m}{d}\)
Therefore, \(P \times \frac{m}{d}=K \text { or, } \frac{P}{d}=\frac{K}{m}\)
Since m is fixed and K is constant at a given temperature
for a fixed mass of a gas, so \(\left(\frac{K}{m}\right)\) is also a constant. Thus, \(\frac{P}{d}\)= Constant or \(P \propto d\)
Hence, at a constant temperature, the density of a gas is directly proportional to its pressure i.e., the density ofa gas increases with increasing pressure and decreases with decreasing pressure.
If at a constant temperature, the densities of gas at pressures P1 and P2 are d1 and d2, respectively, then according to equation [1], we obtain
p1/p2=d1/d2
Numerical Examples
Question 1. A balloon contains 1.2L of air at a particular temperature and 90 cm Hg pressure. What will be the volume of air if the pressure is reduced to 70 cm Hg while keeping the temperature constant?
Answer:
Given, P1 = 90 cmHg, P2= 70 cmHg, V1= 1.2 L & V2 = ?
From the relation, P1V1 – P2V2, We have
⇒ \(V_2=\frac{P_1 V_1}{P_2}=\frac{90 \mathrm{~cm} \mathrm{Hg} \times 1.2 \mathrm{~L}}{70 \mathrm{~cm} \mathrm{Hg}}=1.54 \mathrm{~L} .\)
Thus, the volume of air at 70 cm Hg is 1.54L
Question 2. The volume of a certain amount of gas containing an incompressible solid is 100 cc at 760 mm Hg and 80 cc at 1000 mm Hg. What is the volume of the solid?
Answer:
Suppose the volume of the solid Vcc
So, the volumes ofthe gas at 760 mm Hg and 1000mm Hg are (100- V)cc and (80- V)cc, respectively.
Using the relation, P1= P2V2, we have
760(100- V) = 1000(80- V) or, V = 16.7 cc
Therefore, the volume of the solid is 16.7 cc
Charles’ law: Relation between volume and temperature
In 1787, French scientist Jacques Charles suggested a law describing the effect of temperature on the volume of a given amount of a gas at a fixed pressure. This law is known as Charles’ law.
At constant pressure, the volume of a given mass of gas is increased or decreased by 1/273 part of its volume at 0°C for every 1-degree rise or fall in temperature. The fraction, 1/273 is the coefficient of volume expansion. The exact value of this fraction is 1/273.15 per °C, but it is generally taken as 1/273. The value of the coefficient of volume i.e., 1/273 per °C is the same for all gases.
Mathematical Explanation:
Let at constant pressure the volumes of a given mass of gas are V0, V1, Vt, and vt at 0°C, 1°C, t°C and -t°C respectively. According to Charles’ Law, increase in volume for a 1°C rise in temperature
⇒ \(\frac {V_0}{273}\) and increase in volume for a t°C temperature rise
⇒ \(t \times \frac{V_0}{273}\). Therefore the volume of the gas at 1°C,
⇒ \(V_1=V_0+\frac{V_0}{273}=V_0 \times\left(1+\frac{1}{273}\right)\) and that at t°C \(V_t=V_0+\frac{t \times V_0}{273}=V_0 \times\left(1+\frac{t}{273}\right)\)
Similarly, decrease in volume for t°C fall in temperature \(=t \times \frac{V_0}{273}\)
Hence, the volume ofthe gas at t°C
⇒ \(V_t^{\prime}=V_0-\frac{t \times V_0}{273}=V_0 \times\left(1-\frac{t}{273}\right)\)
Celsius temperature vs volume of a given amount of gas at a fined pressure:
At a fixed pressure, the volume of a definite mass of a gas increases with the rise in temperature on the Celsius scale (t°C), but the variation of volume with Celsius temperature does not occur in direct proportion.
For example, at a fixed pressure if the temperature (on the Celsius scale) of a definite mass of a gas is doubled, its volume is not twice its initial volume.
Plotting the volume (V) of a definite mass of gas against Celsius temperatures (t°C) at different constant pressures gives a straight line for each constant pressure. Each of these lines does not pass through the origin, as indicated by the relation
⇒ \(V_t=V_0\left(1+\frac{t}{273}\right)\)

This means that the volume of the gas is not directly proportional to the Celsius temperature. If the straight lines are extrapolated backwards, then all these lines meet the temperature axis at -273°C.
Therefore, at constant pressure and at a temperature of -273°C, the volume of a gas becomes zero. Below this temperature, the volume of the gas becomes negative, which is absurd. Thus -273°C is the lowest possible temperature and is called absolute zero.
Definition of absolute zero and absolute temperature:
Absolute zero:
The lowest possible temperature at which the volume of any gas is zero.
The temperature -273°C is called absolute zero because at -273°C the volume of any gas would become zero and any temperature below this would correspond to a negative volume of gas.
In practice, absolute zero temperature can never be reached; however, a temperature very close to absolute zero can be attained.
Reason for using the term absolute in absolute zero:
The absolute zero of temperature is independent of the nature, amount, pressure, or volume ofthe gas and it is not possible to get any temperature below absolute zero. Hence, the term ‘absolute’ is used in absolute zero.
The volume of a gas does not become zero at -273-C because the gas becomes liquid or solid before attaining this temperature.
So, at this temperature, Charles’ law is not applicable. Moreover, a gas is a substance of definite mass, and any mass will always occupy some volume. So, even if a substance exists in a gaseous state at – 273 °C, it must have a certain volume.
Absolute temperature:
The temperature scale which has been set up by taking -273°C as zero point and each degree is equal in magnitude to the one degree in Celsius scale is termed as the absolute scale of temperature. The values of temperature obtained from this absolute scale are called absolute temperatures.
Relation between volume & absolute temperature of a gas:
At constant pressure if the volumes of a certain amount of gas at 0°C and t°C are V0 and V, respectively, then,
⇒ \(V=V_0\left(1+\frac{t}{273}\right)=V_0\left(\frac{t+273}{273}\right)=\frac{V_0}{273} \times T\)
The quantity V0/273 for a definite mass of a gas is constant. So, V∝T. Thus at constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature.
For a given mass of gas at constant pressure, if we measure the volumes (V) of the gas at different absolute temperatures (T) and then draw a graph by plotting V against T, a straight line passing through the origin is obtained. The nature of the line implies that the volume of a given mass of gas at constant pressure is related to absolute temperature in the form of an equation as V = constant x T.
This means that the volume of a definite amount of a gas is directly proportional to its absolute temperature at constant pressure.
Alternative form of Charles’ law:
At constant pressure, the volume of a given mass of gas is directly proportional to the absolute temperature ofthe gas.
Mathematical form:
If at constant pressure, the volume of a given mass of gas at absolute temperature T is V, then according to Charles’ law V∝T or v=kt
Where K is a constant whose value depends on the mass and pressure of the gas.
Corollary-1:
If at constant pressure, a certain amount of gas occupies the volumes V1 and V2 at temperatures T1 and T2, respectively, then according to Charles’ law V1∝ T1 and V2 ∝ T2.
Therefore, V1/V2 =T1/T2
Corollary-2:
If T2 = T1/2 then according to equation V2 = V1/2 and if T2 = 27, then V2 = 2V1.
So, at constant pressure, if the absolute temperature ofa definite mass of gas is reduced by half, the volume of the gas will be halved, and if the absolute temperature is doubled, the volume will become double.

Graphical representation of Charles’ law:

Applicability of Charles’ law:
Only a few gases approximately follow Charles’ law at ordinary temperature and pressure. For foremost gases, this law is best followed at low pressures or at very high temperatures.
Molecular interpretation of Charles law:
The velocity of gas molecules depends upon temperature. If the temperature of a gas is increased, the average velocity as well as the average kinetic energy of gas molecules increases.
Consequently, molecules collide with the walls of the container more frequently with greater force. This would increase the pressure ofthe gas if the volume ofthe gas remains constant. If the pressure of the gas is kept constant during the increase of temperature, then the volume ofthe gas must increase.
Corollary of Charles law—relation between density and temperature:
Let at constant pressure and absolute temperature T, the volume and density of a definite amount (m) of gas are V and d, respectively.
Therefore, \(K T=\frac{m}{d} \text { or, } d=\left(\frac{m}{K}\right) \times \frac{1}{T}\)
Since m is constant and K is fixed for a given mass of gas at constant pressure \(\left(\frac{m}{K}\right)\) is also a constant. Therefore \(d \propto \frac{1}{T}\)
Thus, at constant pressure, the density of a definite amount of gas is inversely proportional to the absolute temperature of the gas. If the absolute temperature of a gas is increased, its density decreases and vice-versa.
If at constant pressure, the densities of a definite amount of gas at absolute temperatures T1 and T2 are dx and d2 respectively, then,
⇒ \(d_1 \propto \frac{1}{T_1} \text { and } d_2 \propto \frac{1}{T_2}\)
Therefore,d1/d2 =t2/t1
Numerical Examples
Question 1. At constant pressure, the temperature of a definite amount of a gas is increased from 0°C to t°C. As a result, the volume of the gas is increased by a factor of three. Calculate the value of t.
Answer:
We know, V1/V2 = T1/T2
Given, T1 = 273 K, T2 = (273 + t)K and V2 = 3V1
Where V1 is the volume ofthe gas at 0°C
∴ \(\frac{V_1}{3 V_1}=\frac{273}{273+t} \text { or, } 273+t=819\)
∴ t= 546°C.
Question 2. A 16 sample of oxygen at 760 mm Hg pressure and 27°C is kept In a container of 12.30 L capacity. What will be the temperature of the gas If the entire gas is transferred to a container of 24.6 L capacity, keeping the pressure constant?
Answer:
In this process, the mass and pressure of the gas remain fixed.
Hence according to Charles’ law, V1/V2 = T1/T2
Given, Vx = 12.30 L, V2 = 24.60 L,
T1 = 273 + 27 = 300 K and T2 = ?
∴ \(T_2=T_1 \times \frac{V_2}{V_1}=300 \times \frac{24.60}{12.30}=600 \mathrm{~K}\)
Therefore, the temperature of the gas in the container of 24.6L will be =(600- 273)°C = 327°C.
Question 3. When ice and sample water, of hydrogen, occupies is an immerse exact volume of mixture 69.37 cc at 1 atm. At the same pressure, if the gas is immersed in boiling benzene, then its volume expands to 89.71 cc. What is the boiling point of benzene?
Answer:
As the pressure and amount of the H2 gas are fixed, according to Charles’ law, V1/V2 = T1/T2
Temperature of the mixture of ice and water = 0°C = 273 K.
Given, Vx = 69.37cc and V2 = 89.71cc.
∴ \(T_2=T_1 \times \frac{V_2}{V_1}=273 \times \frac{89.71}{69.37}=353.05 \mathrm{~K}\)
∴ The boiling point of benzene = (353.05- 273)°C = 80.05 °C
CBSE Class 11 Chemistry Liquids and Gases Notes
Gay-Lussac’s law of pressure: Relation between the pressure and temperature of a gas
Gay-Lussac’s law of pressure:
At constant volume, the pressure of a given mass of gas is directly proportional to its absolute temperature.
Mathematical form of Gay-Lussac’s law:
Let us assume, the pressure of a certain amount of gas at constant volume is P at absolute temperature T. According to Gay-Lussac’s law, P∝T is when the volume and mass of the gas are fixed.
Therefore, P = KT ……………………..(1)
Where K is a constant whose value depends on the mass and volume of the gas.
Corollary:
At fixed volume, if die pressures of a certain amount of gas are P1 and P2 at absolute temperatures T1 and T2 respectively, then according to Gay-Lussac’s law.
P1 = KT1 and P2 = KT, ……………………..(2)
Therefore, p1/p2 = T1/T2 From this equation [2], the value of any of the four quantities can be calculated If the other three quantities are known.
Graphical representation of Gay-Luseac’s law of pressure:

Molecular Interpretation of Gay-Lusm’s Law:
With an increase in the temperature of a gas, the average velocity, as well as the average kinetic energy of the gas molecules, increases, thereby making the molecules strike the walls of the container more frequently with greater force.
As a result, the force applied on the walls per unit area by the gas molecules increases with an increase in temperature, leading to the pressure of the gas.
Numerical Examples
Question 1. An iron cylinder contains He gas at a pressure of 250 kPa at 300K. The cylinder can withstand a pressure of 1 x 106 Pa. The room in which the cylinder is placed catches fire. Predict whether the cylinder will blow up before it melts or not. [M.P. ofthe cylinder = 1800K]
Answer:
We know, P1/P2 = T1/T2.
Given, P1 = 250 kPa = 25 x 104 Pa,
T1 = 300 K,P2 = 106 pa and T2 = ?
⇒ \(T_2=\frac{P_2}{P_1} \times T_1=\frac{10^6}{25 \times 10^4} \times 300=1200 \mathrm{~K}\)
As the final temperature is less than the melting point of iron, the cylinder would explode, instead of melting.
Question 2. A cylinder of cooking gas can withstand a pressure of 14.9 atm. At 27°C, the pressure gauge of the cylinder records a pressure of 12 atm. Due to a sudden fire in the building, the temperature starts rising. At what temperature, will the cylinder explode?
Answer:
We know, \(\frac{P_1}{P_2}=\frac{T_1}{T_2} \text {, }\)
The cylinder can withstand a pressure of 14.9 atm If the pressure ofthe cylinder at T2K is 14.9 atm then \(T_2=\frac{P_2}{P_1} \times T_1=\frac{14.9}{12} \times 300=372.5 \mathrm{~K}\)
Since P1 = 12 atm and T1 = (273 + 27) = 300 K]
As the cylinder can withstand pressure upto 14.9 atm, it will explode at a pressure above 14.9 atm. The pressure of the cylinder becomes greater than 14.9 atm when the temperature is greater than 372.5 K or 99.5°C. Hence the cylinder will explode at a temperature greater than 99.5°C.
Avogadro’s law: Relation between the volume and amount (number of moles) of a gas.
Avogadro’s law:
At a constant temperature and pressure, the volume of a gas is directly proportional to the number of moles ofthe gas.
Explanation:
At constant temperature and pressure, if the volume of n mole of a gas is V, then according to Avogadro’s law, V∝n or, V = Kx n where K= proportionality constant. The value of K depends on temperature and pressure.
At constant temperature and pressure, if the volumes of nl and n2 moles of a gas are V1 and V2, respectively, then according to Avogadro’s law, V1ocn1 and V2 oc n2.
Therefore \(\frac{V_1}{V_2}=\frac{n_1}{n_2}\)
This equation tells us that at constant temperature and pressure, if the number of moles of a gas is halved, the volume of the gas will become half of its initial volume, and, the volume of the gas becomes twice its initial value when the number of moles of the gas is doubled.
Suppose, two gases A and B with the number of moles n1 and n2, respectively, have volumes V1 and V2, at a given temperature and pressure. If:
n1 = n2, then according to equation (1), V1 V2 i.e., at the same temperature and pressure equal volumes of two gases contain the same number of molecules. This deduction is commonly known as Avogadro’s hypothesis.
Graphical representation of Avogadro’s law:

Molecular interpretation of Avogadro’s law:
At constant temperature, increasing the number of moles of the gas does not alter the average kinetic energy of the gas molecules. But the frequency of collisions ofthe molecules with the walls of the container increases as the number of molecules of the gas increases. This would increase the pressure of the gas if the volume of the gas is held constant. If the pressure of the gas remains constant, then the volume of the gas must increase.
A combination of Boyle’s law and Charles’ law
Suppose, the pressure, temperature, and volume of a given mass of gas are P, T, and V respectively.
According to Boyle’s law \(V \propto \frac{1}{p}\) [at constant temperature and for a given mass of gas] According to
Charles’ law, Foe T [at constant pressure and for a given mass of gas] Combining these two relations gives a relation that shows the variation of the volume of a given mass of gas with pressure
and temperature. Doing the combination, we have
⇒ \(V \propto \frac{T}{P}\) ………………………..(1)
⇒ \(\text { or, } \boldsymbol{V}=\boldsymbol{K} \times \frac{\boldsymbol{T}}{\boldsymbol{P}} \quad \text { or, } \frac{\boldsymbol{P V}}{\boldsymbol{T}}=\boldsymbol{K} \text { or, } \boldsymbol{P V}=\boldsymbol{K} \boldsymbol{T}\)
Where K is a proportionality constant whose value depends on the amount of the gas. Equation [1] i.e. PV = KT is the combined form of Boyle’s law and Charles’ law.
This equation explains that the product of pressure and volume of a definite mass of gas is directly proportional to its absolute temperature. If P1 V1, and T1 are the pressure, volume, and temperature, respectively, of a definite mass of gas at a certain state and P2, V2 and T2 are the pressure, volume, and temperature, respectively, for the same gas at another state, then from equation [1], we get
⇒ \(\frac{P_1 V_1}{T_1}=K \text { and } \frac{P_2 V_2}{T_2}=K\)
therefore \(\frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2}\)
Using this ‘equation, we can calculate any one of the quantities if the values ofthe remaining are known ∴
Standard temperature and pressure (STP):
Since the properties of I gases change with a change in pressure and temperature, we must use a common reference state to compare the properties of different gases. For this reason, scientists have set a standard temperature and pressure for comparing the properties of gases.
The standard pressure is taken as 1 atm (or 76 cm Hg or 760 mm Hg) and the standard temperature is taken as 0°C. The conditions 0°C and 1 atm are called Standard Temperature and Pressure (STP).

Numerical Examples
Question 1. At a given temperature and pressure, the volume of 10 g of He gas is 61.6L. How much of He gas has to be taken out at the same temperature and pressure to reduce its volume to 25L?
Answer:
10 g He \(=\frac{10}{4}=2.5 \mathrm{~mol}\) Atomic mass of He = 4 molar mass of He (monatomic gas) = 4 g.mol-1]
We know at constant temperature and pressure \(\frac{V_1}{V_2}=\frac{n_1}{n_2}\)
Given, V1 = 61.6 L, V2 = 25 L, n1 = 2.5 and n2=?
∴ \(n_2=n_1 \times \frac{V_2}{V_1}=2.5 \times \frac{25}{61.6}=1.015 \mathrm{~mol}\)
1.015 mol He = 4 × 1.015 = 4.06 g He
∴ The volume of the gas will be 25L if (10 – 4.06) = 5.94g of He gas is taken out
Gases and Liquids in States of Matter Class 11 Chemistry Notes
Question 2. At a particular temperature and pressure, the volume of 12 g of H2 gas is 134.5 L. What will be the final volume of the gas if 4 g of H2 gas is added to it at the same temperature and pressure?
Answer:
12g H2 \(=\frac{12}{2}=6\) of H2.
Similarly, 4 g H2 = 2 mol of H2.
We know, at constant temperature and pressure \(\frac{V_1}{V_2}=\frac{n_1}{n_2}\)
Given that, n1 = 6, n2 = 6 + 2 = 8, V1 = 134.5 L and V2 = ?
∴ \(V_2=\frac{n_2}{n_1} \times V_1=\frac{8}{6} \times 134.5=179.3 \mathrm{~L}\)
Therefore, at the same temperature and pressure, if 4 g of H2 gas is added to 12 g of H2 gas, then the final volume of the gas will be 179.3 L.
Equation Of State For An Ideal Gas
Four variables, viz. pressure (P), volume (V), temperature (X) and number of moles (n) are generally found to be sufficient to describe the state ofa gas. The relation connecting P, V, and Tandn of a gas is called the equation of state of the gas.
The gas laws, i.e., Boyle’s law, Charles’ law and Avogadro’s law can be combined into an equation that describes the behaviour of an ideal gas. This relation is called the equation of state of an ideal gas.
Derivation of the equation of state of an ideal gas:
According to Boyle’s law, \(V \propto \frac{1}{P}\); [ T & n are constant]
According to Charles’ law, V∝T,[P &t n are constant]
According to Avogadro’s law, V∝ n [P & T are constant]
Combining these three relations, we have \(V \propto \frac{T \times n}{P}\) when P, T and n ofthe gas vary therefore \(V=K \times \frac{n T}{p}\)
K is the proportionality constant. It has been experimentally found that the value of K for 1 mol of any gas is the same.
For 1 mol of a gas, K is denoted by R, which is called the molar gas constant. As the value of R is the same for all gases, ‘JR’ is also called the universal gas constant.
Substituting R for equation [1], we obtain
⇒ \(V=R \times \frac{n T}{P}\) or, PV=nRT
Equation [2] expresses the equation of state for n mol of an ideal gas. In this equation, if the values of the three variables are known, the value ofthe fourth variable can be calculated by this equation.
For 1 mol of an ideal gas i.e., when n = 1, PV = RT— this expresses the equation of state for 1 mol of an ideal gas.
In equation PV = RT, P and V are the pressure and volume, respectively, of 1 mol of an ideal gas at temperature T.
In equation PV = nRT, P and V are the pressure and volume, respectively, of n mol of an ideal gas at temperature T.
The equation PV = NRT does not have any quantity related to the nature of the gas. Thus, this equation applies to an ideal gas.
Ideal gas:
A gas which obeys the equation of state, PV = nRT under all conditions is called an ideal gas. But in reality, there is no such gas which perfectly obeys the ideal gas equation under all conditions.
Therefore, the concept of ideal gas is a hypothetical one. However, it has been found experimentally that gases are nearly deadly at very low pressures and high temperatures.
Real gas:
Gases which do not obey the equation of state, PV = nRT in any condition except at very high temperatures and very low pressures are said to be real gases. All naturally occurring gases are real gases.
Significance of R and values of R in different units
Significance of molar gas constant (R):
For n mol of an ideal gas Pv=nRT or \(\frac{P V}{n T}\). The physical significance of R can be explained from its dimension.
Dimension of R. = \(\frac{\text { pressure } \times \text { volume }}{\text { number of moles } \times \text { temperature }}\)
We know, dimension of pressure = \(\frac{\text { force }}{(\text { length })^2}\)
On the other hand, the dimension ofvolume= (length)³ Temperature is expressed Kelvin scale. Dimension of R =
⇒ \(\frac{\frac{\text { force }}{(\text { length })^2} \times \text { length }^3}{\text { mole } \times K}=\frac{\text { work (or energy) }}{\text { mole } \times \mathrm{K}}\)
Hence, R= work (or energy) per kelvin per mole of gas Therefore, the value of R gives the measure of the work performed by 1 mol of an ideal gas when the temperature of the gas is raised by IK against a fixed pressure. This is the physical significance of R.
Values of R in different units:
We know \(R=\frac{P V}{n T}\) Therefore, the values and units of R depend upon the values and units of P, V, n and T. Temperature is always expressed in kelvin scale and n is expressed in the unit of mole.
Values of R in different units:

Determination of the values of R In different units:

Boltzmann constant:
Dividing the molar gas constant (R) by Avogadro’s number (A) gives a new constant, termed the Boltzmann constant (k). It indicates the universal gas constant for a single molecule.
Therefore, \(k=\frac{R}{N}=\frac{R}{6.022 \times 10^{23} \mathrm{~mol}^{-1}}\)
In CCSA System \(k=\frac{R}{N}=\frac{8.314 \times 10^7 \mathrm{erg} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1}}{6.022 \times 10^{23} \mathrm{~mol}^{-1}}\)
=1.38 × 10-16 erg. K-1
In SI, \(k=\frac{R}{N}=\frac{8.314 \mathrm{~J} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1}}{6.022 \times 10^{23} \mathrm{~mol}^{-1}}\)
= 1.38 × 10-23J . K-1
Application to the Ideal gas equation
Determination of density and molar mass of an ideal gas:
The equation of state for n mol of an ideal gas is PV = nRT, where V is the volume of nmol of the ideal gas at pressure P and temperature T. If m and M are the mass (in gram) and molar mass (in g.mol-1 ) of the gas, respectively, then the number of moles ofthe gas (n) is given by
⇒ \(\frac{m}{M}\)
Substituting \(\frac{m}{M}\) for n in the equation PV = nRT
We have \(P M=\left(\frac{m}{V}\right) R T\)
So, PM = dRT or, \(d=\frac{P M}{R T}\) ………………………….(1)
Equation [1] is the relationship among the density, pressure, absolute temperature and molar mass of an ideal gas. This equation can be used to calculate the molar mass (A) of an ideal gas if the density ofthe gas (d) at a given and T is known or the density of an ideal gas at a given P and T, if the molar mass ofthe gas is known.
Important points related to the equation, d = PM/RT:
1. The relation between the density and molar mass of an ideal gas at a particular temperature and pressure:
According to the equation, d = PM/RT, the density of an ideal gas at a particular temperature and pressure is directly proportional to its molar mass i.e., d∝M.
Therefore, at a given temperature and pressure a heavier gas has higher density than a lighter gas. The average molar mass of air is greater than the molar mass of He gas. So at a particular temperature and pressure, the density of He gas is less than that of air. This is why, a balloon filled with gas floats in the air.
2. The relationship of density with pressure and absolute temperature of a gas:
According to the equation,
⇒ \(d=\frac{P M}{R T}\)
The density of ideal gas is directly proportional to RT to its pressure and inversely proportional to its absolute temperature. Therefore, at a given temperature the density of a gas increases (or decreases) as the pressure of the gas increases (or decreases).
On the other hand, at a given pressure the increase in temperature of a gas results in the lowering of its density and the decrease in temperature increases its density. The density of hot air is less than that of cold air. This is why the balloon filled with hot air easily moves upward in the air.
3. The relation among pressure, temperature and density ofa gas at two different pressures and temperatures:
Let dx be the density of a gas at pressure Px and temperature T1 and d2 be the density of the same gas at pressure P2 and temperature T2. Therefore, according to the equation,
⇒ \(d=\frac{P M}{R T}\)
The densities of the gas at two conditions are d1 =
⇒ \(d_1=\frac{P_1 M}{R T_1} \text { and } d_2=\frac{P_2 M}{R T_2}\)
Thus, \(\frac{d_1}{d_2}=\frac{P_1}{P_2} \times \frac{T_2}{T_1}\) ………………………….(2)
4. This equation expresses the relation between the densities of a gas at two different pressures and temperatures.
If the density of a gas at a particular temperature and pressure, is known, then the density of the gas at another temperature and pressure can be determined from equation [2].
Validity of ideal gas equation
The ideal gas equation is not exactly followed by any gas. However, it has been found that most gases approximately follow this equation when pressure is not too high or temperature is not too low.
Despite its limitations, the ideal gas equation is often used to determine the approximate values of the various properties of real gases under ordinary conditions. But for the estimation of the exact values of the properties, this equation can never be used.
Numerical Examples
Question 1. Find the volume of 2.2g CO2 gas at 25°C & 570 mm Hg pressure. Consider that CO2 behaves ideally.
Answer:
Given, \(P=\frac{570}{760}\) 0.75 atm; T= (273 + 25) = 298 K.
⇒ \(n=\frac{2.2}{44}=0.05 \mathrm{~mol}\)
Molar mass of CO2 = 44g mol-1]
⇒ \(V=\frac{n R T}{P}\)
⇒ \(=\frac{(0.05 \mathrm{~mol}) \times\left(0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1}\right) \times 298 \mathrm{~K}}{0.75 \mathrm{~atm}}\)
= \(1.63 \mathrm{~L}\)
Therefore, at 25°C and 570 mm Hg pressure, the volume of 2.2 g of CO2 gas is 1.63 L.
Question 2. A sample of Ar vapour contains 3 x 104 atoms of a vacuum tube with a volume of 5 mL at -100°C. Calculate the pressure of the vapour in the microtorr unit.
Answer:
Given, V = 5 mL = 5 x 10-3 L; T = 273- 100 = 173 K
⇒ \(\text { and } n=\frac{\text { number of molecules }}{\text { Avogadro’s number }}\)
= \(\frac{3 \times 10^4}{6.022 \times 10^{23}}=4.98 \times 10^{-20} \mathrm{~mol}\)
Argon is monoatomic.
⇒ \(P=\frac{n R T}{V}\)
⇒ \(\frac{4.98 \times 10^{-20} \mathrm{~mol} \times 0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 173 \mathrm{~K}}{5 \times 10^{-3} \mathrm{~L}}\)
= 1.414 × 10-16 atm
As, 1 atm = 760 torr,
P = ( 1.414 × 10-16 × 760) = 1.074 × 10-13 torr
= 1.074 × 10-7 microtorr
Since 1 torr = 106 microtorr
Therefore the pressure of argon vapour = 1.074 × 10-7 micro torr
Question 3. At 273K and 76 pressure, the volume of 0.64 g of gas is 224 mL. At what temperature 1g of this gas will occupy a volume of 1 litre at atmospheric pressure?
Answer:
Given, P = 76 cm Hg = 1 atm, T = 273 K, V = 224 mL = 0.224 L
Number of moles (n) = \(\frac{0.64}{M} \mathrm{~mol}\)
[molar mass of the gas =M .g .mol-1 ]
Putting the values of P, V, n and T into the equation PV = nRT gives
1 atm x 0.224L \(=\frac{0.64}{M} \times 0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 273 \mathrm{~K}\)
Substituting 8 P = 1 atm, V – 1 L,
n = \(\frac{1}{64.04} \mathrm{~mol}\) into equation PV – nRT, we obtain
⇒ \(T=\frac{P V}{n R}\)
= \(\frac{1 \mathrm{~atm} \times 1 \mathrm{~L}}{\frac{1}{64.04} \mathrm{~mol} \times 0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1}}\)
= \(780.02 \mathrm{~K}\)
Therefore, at (780.02-273) = 507.02°C and 1 atm pressure the volume of lg of gas will be 1 L.
Question 4. At 25°C and a certain pressure, 3.7g of a gas occupies the same volume as the volume occupied by 0.184g of H2 gas at 17°C and the same pressure. Calculate the molar mass of the gas.
Answer:
For H2 gas: T=(273 + 17) = 290K and \(n=\frac{0.184}{2}=0.092 \mathrm{~mol}\)
∴ PV= nRT=0.092 mol × 0.0821 L-atm-moH-K-1 × 290K
=2.19 L atm
In case ofthe unknown gas: T = (273 + 25)K = 298 K;
⇒ \(n=\frac{3.7}{M} \mathrm{~mol}\)
∴ PV =nRT = \(=\frac{3.7}{M} \mathrm{~mol} \times 0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 298 \mathrm{~K}\)
= \(\left(\frac{90.52}{M}\right) \mathrm{L} \cdot \mathrm{atm}\)
Since the values of P and V are the same for both gases, the values of PV will also be the same for both gases
∴ \(\frac{90.52}{M}=2.19 \text { or, } M=41.33\)
Therefore, the molar mass of the other gas =41.33 g-mol-1
Question 5. A He balloon is such that it can rise to a maximum height of 50 km above the Earth’s surface. When the balloon rises to this height, its expansion reaches a maximum with a volume of 105 L. If the temperature and pressure of the air at this height are 10 °C and 1.8 mm Hg, respectively, then what mass of He gas will be required for the maximum expansion of the balloon?
Answer:
Given, P = 1.8 mm Hg \(=\frac{1.8}{760}=2.368 \times 10^{-3} \mathrm{~atm} \text {; }\)
T = (273- 10)K = 263 K ; V = 105L and n = ?
∴ \(n=\frac{P V}{R T}=\frac{\left(2.368 \times 10^{-3} \mathrm{~atm}\right) \times\left(10^5 \mathrm{~L}\right)}{\left(0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1}\right) \times 263 \mathrm{~K}}\)
∴ 10.96 mol He = 10.96 × 4 = 43.84 g of He
since 1 mol of = 4 g He] 43.84 g of He will be required for the full expansion of the balloon.
Question 6. At 300K, an evacuated cylinder with a volume of 10L is filled with 2g of H2 and 2g of D2. Find the pressure of the gas mixture in the cylinder. Will the pressure be the same if the container is cubical having the same volume?
Answer:
2g H2 \(=\frac{2}{2}\) H2 and 2g D2= \(=\frac{2}{4}\) 0.5 mol D2
Since molar masses Of H2 And D2 Are 2 and 4 g. mol-1 respectively]
No. of moles of H2 & D2 gas in the cylinder,
n = 1 + 0.5 = 1.5 mol
Given, T = 300 K and V = 10 L
Therefore, \(P=\frac{n R T}{V}=\frac{1.5 \times 0.0821 \times 300}{10}=3.69 \mathrm{~atm} .\)
Thus, the pressure ofthe mixture of H2 and D2 gas in the cylinder will be 3.69 atm.
For a given mass of gas at a fixed temperature, the pressure of the gas depends on the volume ofthe container but not on the shape ofthe container. Thus, the pressure will be the same if the container is cubical with the same capacity.
Question 7. When an open vessel at 27°C was heated, three-fifths of the air escaped from It. If the volume of (the lie vessel remained unchanged, calculate the temperature arc at which the vessel was healed.
Answer:
The amount of air present in the vessel at 27G was nmol, and when it was heated to 7’K \(\frac{3}{5}\) the air was expelled.
So, the amount of air in the vessel after heating
= \(\left(n-\frac{3 n}{5}\right)=\frac{2}{5} n \mathrm{~mol}\)
As the vessel was open and its volume remained unchanged on heating, the pressure (P) and the volume ( V) of the air presenting the vessel would be the same as those at 27°C.
At temperature 300 K: PV = nR × 300
At temperature 7K : \(P V=\frac{2}{5} n R T\)
Thus, no X 300 \(=\frac{2}{5} n R T\) or, T = 750 K
∴ The vessel was heated at (750- 273)°C = 477°C
Question 8. A spherical balloon with a diameter of 21 cm is to be filled with hydrogen gas at STP from a cylinder of H2 gas at 20 atm pressure and 27°C. If the cylinder can hold 2.82 L of water, how many balloons can be filled with the hydrogen gas from the cylinder?
Answer:
Volume ofeach balloon \(=\frac{4}{3} \pi \times\left(\frac{21}{2}\right)^3=4851 \mathrm{~cm}^3=4.851 \mathrm{~L}\)
Since the cylinder can hold 2.82 L of water, the volume of the cylinder is 2.82 L and hence the volume of H2 gas at 20 atm and 27°C is 2.82 L.
Let, volume of H2 gasin the cylinder at STP = V2 L.
We know, \(\frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2}\)
Given, P1 = 20 atm; P2 (STP) = 1 atm;
V1 = 2.82 L; T1 = (273 + 27)K = 300 K ;
T2 (STP) = 273 K and V2 = ?
∴ \(V_2=\frac{T_2}{T_1} \times \frac{P_1}{P_2} \times V_1\)
= \(\frac{273 \mathrm{~K}}{300 \mathrm{~K}} \times \frac{20 \mathrm{~atm}}{1 \mathrm{~atm}} \times 2.82 \mathrm{~L}\)
= \( 51.324 \mathrm{~L}\)
After the balloons are filled with H2 gas, the cylinder will contain H2 gas with a volume equal to its volume, l.e., 2.8.2 Hence, the volume of H2 gas available for filling up the balloons will be = (51.324- 2.82)L =48.504 L Thus, no. of balloons that can be filled up.
⇒ \(\frac{48.504}{4.851}=10\)
Question 9. When 2 g of a gas ‘Af is introduced into an empty flask at 30°C, its pressure becomes 1 atm. If 3g of another gas ‘N’ is introduced in the same flask at the same temperature, the total pressure becomes 1.5 atm. Find the ratio of the molar masses of the two gases
Answer:
Let the molecular masses of gases, M and N be a and b gaol-1 respectively and the volume ofthe flask = VL
In the case of gas Af:
P = 1 atm, T = (273 + 30)K = 303 K number of moles (n) \(=\frac{2}{a} \mathrm{~mol}\)
Therefore, according to the equation
PV = NRT \(1 \mathrm{~atm} \times V \mathrm{~L}\)
= \(\frac{2}{a} \mathrm{~mol} \times 0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 303 \mathrm{~K}\)
∴ \(V=\frac{49.75}{a} \mathrm{~L}\)
The number of moles of N gas \(=\frac{3}{b} \mathrm{~mol}\)
In case of the mixture of gases M and N: Total number of moles in the mixture of gases M and N \(\left(\frac{2}{a}+\frac{3}{b}\right)\) mol.
The total pressure of the mixture = 1.5 atm
By applying the equation, PV = not in the case of the mixture of gases, we obtain
⇒ \(1.5 \times \frac{49.75}{a}=\left(\frac{2}{a}+\frac{3}{b}\right) \times 0.0821 \times 303\)
or, \(\frac{74.62}{a}=\left(\frac{2}{a}+\frac{3}{b}\right) \times 24.87=\frac{49.75}{a}+\frac{74.62}{b}\)
or, \(\frac{24.87}{a}=\frac{74.62}{b}\)
∴ \(\frac{a}{b}=\frac{1}{3}\)
States of Matter: Liquids and Gases Class 11 Chemistry Notes
Question 10. What is nitric oxide’s density (in g-cm-3) (assuming ideal behaviour) at 27 °C and 1 atm pressure?
Answer:
Given, P = 1 atm, T = (273 + 27)K = 300 K
Molar mass (M) of NO2 = 30 g-mol-1
∴ \(d=\frac{P M}{R T}=\frac{1^{\prime} \mathrm{atm} \times 30 \mathrm{~g} \cdot \mathrm{mol}^{-1}}{0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 300 \mathrm{~K}}\)
=1.218 g.L-1
= 1.218 × 10-3g.cm-3
Question 11. The density of CO2 at STP is 1.96 g-L1. A sample of CO2 occupies a volume of 480mL at 17°C and 800mm Hg pressure. What is the mass of the sample?
Answer:
We know, \(\frac{d_1}{d_2}=\frac{P_1}{P_2} \times \frac{T_2}{T_1}\)
STP: d1 = 1.96 g-L-1, P1 =1 atm, =273 K
At 17°C and 800 mm Pressure:
⇒ \(P_2=\frac{800}{760}=1.053 \mathrm{~atm} ; T_2=(273+17) \mathrm{K}=290 \mathrm{~K}, d_2=?\)
∴ \(d_2=d_1 \times \frac{P_2}{P_1} \times \frac{T_1}{T_2}=1.96 \times \frac{1.053}{1} \times \frac{273}{290}=1.94 \mathrm{~g} \cdot \mathrm{L}^{-1}\)
if the mass of 480 mL of CO2 gas be w g, then \(d_2=\frac{w}{0.48} \mathrm{~g} \cdot \mathrm{L}^{-1}\)
∴ \(1.94 \mathrm{~g} \cdot \mathrm{L}^{-1}=\frac{w}{0.48} \mathrm{~g} \cdot \mathrm{L}^{-1}\)
∴ w= 09312g
Therefore, the mass of CO2 gas is = 0.9312 g
Partial Pressure Of A Gas Dalton’s Law
Partial pressure
Let us consider that two non-reacting gases, A and B are kept in a closed vessel (i.e., of fixed volume) at a particular temperature. At this temperature, the mixture of gases (A and B) exerts a definite pressure on the vessel.
If gas B is completely removed from the gas mixture at the same temperature, then the pressure exerted on the vessel by gas A alone is known as the partial pressure of A.
Similarly, if gas A is completely removed from the gas mixture at the same temperature, then the pressure exerted on the vessel by B alone is known as the partial pressure of B.
Partial pressure Definition:
At a given temperature the pressure contributed by a component gas in a mixture of two or more non-reacting gases to the total pressure of the mixture is called the partial pressure of that component.
In 1807, John Dalton proposed a law regarding the partial pressures of two or more non-reacting gases, which is known as Dalton’s law of partial pressures.
Dalton’s law of partial pressures:
At constant temperature, the total pressure exerted by a mixture of two or more non-reacting gases present in a container of definite volume is equal to the sum of the partial pressures of component gases in the mixture.
If P is the total pressure of a gas mixture enclosed in a container of definite volume at constant temperature and p1 p2, p3 etc., are the partial pressures of the component gases at the same temperature, then according to Dalton’s law of partial pressures, p=p1+p2+p3………………….
This law does not work if the component gases react with each other. For example, in the case of a mixture of NH3 and HC1 gases, this law is not applicable because these two gases react to form NH2Cl.
Explanation:
Suppose, two non-reacting gases A and B separately enclosed in two closed containers, each with a volume of V. Let us assume that the temperature of both gases is the same and the pressures of A and B gases are pA and pB, respectively.
If two gases, instead of enclosing separately, are mixed in another closed container with a volume of V, keeping the temperature constant, then the total pressure of the gas mixture will be (pA+pB).

Mathematical form of Dalton’s law of partial pressure
Suppose, a mixture of reacting gases 1, 2, 3.. with the number of moles n1 n2 n3—, n- is enclosed in a closed container with a volume of V at a constant temperature T.
Also suppose, the partial pressure ofthe components 1, 2, 3,… i in the mixture are p1 p2,p3—, , respectively.
If the component gases and the gas mixture obey the ideal gas law, then the total pressure ofthe gas mixture is.
⇒ \(P=\left(n_1+n_2+n_3+\cdots+n_i\right) \frac{R T}{V}\) …………………….(1)
Applying the ideal gas equation separately to each component gas ofthe mixture, we have,
⇒ \(p_1=\frac{n_1 R T}{V}, p_2=\frac{n_2 R T}{V}, p_3=\frac{n_3 R T}{V} \text { etc. }\)
So the total pressure ofthe gas mixture,
⇒ \(\left(p_1+p_2+p_3+\cdots+p_i\right)=\left(n_1+n_2+n_3+\cdots+n_i\right) \frac{R T}{V}\) …………………….(2)
Comparing equations (1) and (2) we obtain p = p1+p2+p3+….+ pi
This is the mathematical expression of Dalton’s law of partial pressures.
The relation between the partial pressure and mole fraction
The mole fraction of a component in a gaseous mixture is the ratio of the number of moles of that component to the total number of moles of all components in the mixture. It is a unitless quantity with a value always less than one (1).
If a mixture of the number of moles of a component and the total number of moles of all the components be n. and n, respectively, then the mole fraction ofthe component,\(x_i=\frac{n_i}{n}\) The sum of the mole fractions of all the components in a mixture is always 1.
Relation between partial pressure and mole fraction:
Suppose, at constant temperature T, there is a mixture of some non-reacting gases in a container with a fixed volume of V and the pressure ofthe gas mixture is P.
If the number of moles of different component gases present in the mixture is n1, n2,n3 …respectively, then the total number of moles of all components in the mixture, n = n1 + n2 + n3 + If, the partial pressures of the component gases of the mixture are p1 p2, P3………………etc., then according to Dalton’slaw of partial pressures, die total pressure of the gas mixture,
P = p1 + p2 + p3+……………………(1)
Applying the ideal gas equation to the gas mixture gives PV = nRT
If this equation is applied to each component in the mixture, then
P1V = n1RT…………………………..(2)
P2V = n2RT…………………………..(3)
P3V = n3RT…………………………..(4)
Dividing equation (2) by equation (1) gives \(\frac{p_1}{P}=\frac{n_1}{n}=x_1\)
or, p1 = x1XP [xa = mole fraction ofthe component 1] Dividing equation [3] by equation [1] gives,
⇒ \(\frac{p_2}{P}=\frac{n_2}{n}=x_2 \text { or, } p_2=x_2 \times P\)
Similarly, for the ith component, if the partial pressure and mole fraction are pt and x; respectively, then
Pi=xi × P…………………………..(5)
Where P is the total pressure of the gas mixture. Equation [5] represents the relation between the partial pressure and mole fraction of a component gas in a gas mixture at a constant temperature. So, the partial pressure of a component in a gas mixture = mole-fraction of the component x total pressure of the mixture.
Determination of partial pressure
Suppose, at a given temperature T, a bulb with a fixed volume of VA contains nA moles of an ideal gas with a pressure PA, and another bulb with a fixed volume of VB contains nB moles of another ideal gas with a pressure PB. These two flasks are connected by a stop-cock of negligible volume. Initially, the stop-cock is closed, so all molecules of gas A are in one bulb and that of gas B are in another bulb.

On opening the stop-cock, the two gases will mix, and the total volume ofthe gas mixture becomes (VA + VB). Let the partial pressures of A and B in the gas mixture be pA and pB, respectively, and the total pressure ofthe mix.
Before opening stop-cock: In case of gas A:
PAVA = nART
In case of gas B: PBVB = nBRT
After opening stop-cock:
In case of gas A: PA(VA VB) = nARP
In case of gas B: PB(VA+ VB) = nBRPT
Note that PA PA because the volumes of gas A before and after the opening of stop-cock are VA and VA + VB respectively. For the same reason, PB≠ PB
In case of gas A: PAVA = PA(VA+ VB). Thus,
⇒ \(p_A=\frac{P_A V_A}{V_A+V_B}p_A=\frac{P_A V_A}{V_A+V_B}\) ………………………..(1)
In case ofgas B: PBVB = PB(VA+ VB) and
⇒ \(p_B=\frac{P_B V_B}{V_A+V_B}\) ………………………..(2)
Equations (1) and (2) express the partial pressures of gas A and B, respectively, in the gas mixture. According to Dalton’s law of partial pressures, the total pressure ofthe gas mixture,
⇒ \(\boldsymbol{P}=p_A+p_B=\frac{P_A V_A}{V_A+V_B}+\frac{P_B V_B}{V_A+V_B} \text { or, } \quad \boldsymbol{P}=\frac{\boldsymbol{P}_A V_A+P_B V_B}{V_A+V_B}\)
If the volume of two bulbs is the same, i.e., VA = 1/B, then the total pressure of the gas mixture,
⇒ \(P=\frac{\left(P_A+P_B\right) V_A}{2 V_A}=\frac{1}{2}\left(P_A+P_B\right)\)
Application of Dalton’s law of partial pressures (Determination of actual pressure of a gas collected by the downward displacement of water):
During the laboratory preparation, gases lighter than air and insoluble in water are usually collected in gas jars by downward displacement of water.
So, the gas becomes saturated with water vapour. Thus, the observed pressure (P) of the collected gas is the pressure ofthe moist gas.

The pressure of the moist gas (P) = pressure of the dry gas (Pg) + saturated vapour pressure of water (Pw) at laboratory temperature. Therefore pg=P-PW
The saturated vapour pressure of water (Pw) at laboratory temperature is known from Regnault’s table. So from equation (1), the pressure of the dry gas can easily be calculated.
The vapour pressure of water, present in the gas collected in a gas jar is called aqueous tension.
Validity of Oalton’s law of partial pressure:
Under ordinary conditions, real gases do not obey Dalton’s law of partial pressure. But they approximately obey Dalton’s law at very low pressures and high temperatures as intermolecular attractive forces become negligible at this condition.
Molecular Interpretation of Dalton’s law of partial pressures:
In an ideal gas, molecules do not feel any forces of attraction or repulsion. So in a mixture of non-reacting ideal gases, molecules behave Independently of one another and the pressure exerted by a component gas is not influenced by the presence of other gases.
So, at constant temperature and volume, the pressure exerted by a mixture of two or more non-reacting ideal gases is equal to the sum of the partial pressures of the component gases.
The partial volume of a gas in a mixture of non-reading gases—Amagato law:
According to this law, at constant temperature and pressure, the total volume of a mixture of two or more non-reacting gases is equal to the sum of partial volumes of component gases in the mixture.
If V is the total volume of a gas mixture at constant temperature and pressure, and v1, v2, v3…..etc., are the partial volumes ofthe component gases ofthe mixture at the same temperature and pressure, then according to Amagat’s law of partial volumes V = v1 + v2 + v3 +…
At constant temperature and pressure, if v-t and xt are the partial volume and mole fraction of the f-th component respectively and V is the total volume of the gas mixture, then it can be shown that vi= xi × v
Therefore, at constant temperature and pressure, the partial volume of a component gas in a gas mixture = mole fraction of that component x total volume of the mixture.
Numerical Example
Question 1. At 27°C, a cylinder of volume 10 L contains a gas mixture consisting of 0.4 g He, 1.6 g O2 & 1.4 g H2. Determine the total pressure of the mixture and the partial pressure of He in the mixture.
Answer:
Total number of moles of He, O2 and N2 gas in the mixture \((n)=\frac{0.4}{4}+\frac{1.6}{32}+\frac{1.4}{28}\) 0.1 + 0.05 + 0.05 = 0.2 mol.
[Molar masses of He, O2 and N2 are 4, 32 and 28gmol-1 respectively)
Given, V = 10 L, T = (273 + 27)K= 300 K
We know, PV= nRTor, P \(=\frac{n R T}{V}\)
∴ \(\mathrm{p}_{=}=\frac{0.2 \mathrm{~mol} \times 0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 300 \mathrm{~K}}{10 \mathrm{~L}}=0.4926 \mathrm{~atm}\)
∴ Total pressure ofthe gas mixture = 0.4926 atm
Number of moles of He gas =0.1 and its mole fraction \(=\frac{0.1}{0.2}=0.5\)
∴ The partial pressure of He gas in mixture = mole-fraction of He in the mixture × total pressure of the mixture = 0.5 × 0.4926
= 0.2463 atm
Question 2. The volume percentages of N2, O2 and He in a gas mixture arc were 25, 35 and 40, respectively. At a given temperature, the pressure of the mixture is 760 mm Hg. Calculate the partial pressure of each gas at the same temperature.
Answer:
According to Amagat’s law, at constant temperature and pressure, the partial volume of a component gas in a gas mixture = mole-fraction of that component x total volume of the mixture.
Therefore, in the given mixture, \(x_{\mathrm{N}_2}=\frac{25}{100}=0.25\)
⇒ \(x_{\mathrm{O}_2}=\frac{35}{100}=0.35 \text { and } x_{\mathrm{He}}=\frac{40}{100}=0.40\)
∴ In the mixture,
Partial pressure of N2 = xN2 ×P =0.25 × 760 =190 mm Hg
Partial pressure of O2 =xO2 × P= 0.35 × 760 = 266 mm Hg and
Partial pressure of He = xHe × P= 0.40 × 760 = 304 mm Hg
Question 3. A mixture of N2 and O2 at 1 bar pressure contains 80% N2 by weight. Calculate the partial pressure of N2 in the mixture.
Answer:
As 80 g N2 is present in 100 g of mixture, the amount of O2 in the mixture is 20 g.
Therefore, the number of moles of N2 in the mixture
⇒ \(\left(n_{N_2}\right) \frac{80}{28}=2.857 \mathrm{~mol}\)
And the number of moles of O2 in the mixture
⇒ \(\left(\mathrm{n}_{\mathrm{O}_2}\right)=\frac{20}{32}=0.625 \mathrm{~mol} .\)
∴ Total moles of the mixture (total)= nN2 + NO2 = 2.857 + 0.625
= 3.482mol
∴ Mole fraction of N2 gas (xN2)
⇒ \(\frac{n_1}{n}=\frac{2.857}{3.482}=0.82\)
And that of O2 gas (xO2) \(=\frac{n_2}{n}=\frac{0.625}{3.482}=0.18\)
In the mixture, the partial pressure of N2 gas =rN2
Total pressure of the mixture, = 0.82 ×1 bar
= 0.82 bar and that of O2 gas = xO2 × total pressure of the mixture
= 0.18× 1 bar
= 0.18 bar.
NCERT Solutions Class 11 Chemistry Liquids and Gases
Question 4. At a given temperature, the pressure in an oxygen cylinder is 10.3 atm. At the same temperature, the pressure in another oxygen cylinder of volume 1/3 rd of the first cylinder is 1.1 atm. Keeping temperature constant, if the two cylinders are connected, then what will be the pressure of O2 gas in the system?
Answer:
Suppose the volume of the first cylinder = VL.
So, the volume of another cylinder \(\frac{V}{3} L.\)
In the case of the first cylinder: If the number of moles of O2 gas
be n1, then according to the equation PV = nRT
10.3 atm × VL = n1 × 0.0821 L.atm. moH-1. K-1 × TK
∴ \(n_1=\frac{125.45 \times V}{T} \mathrm{~mol} .\)
In the case of the second cylinder: If the number of moles of O2 gas is n2, then according to the equation PV = nRT
⇒ \(1.1 \mathrm{~atm} \times \frac{V}{3} \mathrm{~L}=n_2 \times 0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times T \mathrm{~K}\)
∴ \(n_2=\frac{4.46}{T} \times V \mathrm{~mol}\)
If two cylinders are connected, then die total volume of the system
⇒ \(=\left(V+\frac{V}{3}\right) \mathrm{L}=\frac{4}{3} V \mathrm{~L}\)
And the total number of moles of O2 gas = n1 + n2
Applying equation PV = nRT, we obtain
⇒ \(P \times \frac{4}{3} V \mathrm{~L}=\left(n_1+n_2\right) \mathrm{RT}=\left(\frac{125.45 \times \mathrm{V}}{T}+\frac{4.46 \times \mathrm{V}}{T}\right) \times 0.0821 \times \mathrm{T}\)
or, P= \(\frac{3}{4} \times \frac{(125.45+4.46)}{T} \times 0.0821 \times T\)
= \(\frac{3}{4} \times 129.91 \times 0.0821\mathrm{~atm}=7.99 \mathrm{~atm}\)
∴ The pressure of the O2 gas in the system will be 7.99 atm.
Question 5. Thermal decomposition of x g of KClO3 produces 760 mL O2, which is collected over water at 27°C and 714 mm Hg pressure. Find the value of x. [Given that at 27°C, aqueous tension = 26 mm Hg and atomic weights of K = 39, Cl = 35.5, 0=16
Answer:
Actual pressure of O2 collected over water = atmospheric pressure- aqueous tension
⇒ \(=(714-26)=688 \mathrm{~mm} \mathrm{Hg}=\frac{688}{760}=0.9052 \mathrm{~atm}\)
According to the given conditions volume of O2 = 760 mL = 0.76 L and T = (273 + 27)K = 300 K If the number of moles of O2 gas is n then,
⇒ \(n=\frac{P V}{R T}\)
= \(\frac{0.9052 \mathrm{~atm} \times 0.76 \mathrm{~L}}{0.0821 \mathrm{~L} \cdot \mathrm{atm} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 300 \mathrm{~K}}\)
= \(0.028 \mathrm{~mol}.\)
The equation for the thermal decomposition of KClO3 is
⇒ \(2 \mathrm{KClO}_3(s) \rightarrow 2 \mathrm{KCl}(s)+3 \mathrm{O}_2(g)\)
Therefore, 3 mol O2(g) = 2 mol KClO3 = 2 × 12250 g
=245g KCIO3
∴ \(0.028 \mathrm{~mol} \mathrm{O}_2(\mathrm{~g}) \equiv \frac{245}{3} \times 0.028 \mathrm{~g}=2.286 \mathrm{~g} \mathrm{KClO}_3\)
∴ x = 2.286 g
Diffusion And Effusion Of A Gas
Diffusion of a gas:
Due to rapid and random molecular motions and large average intermolecular distance, when two or more non-reacting gases come in contact with each other, they spontaneously mix to form a homogeneous mixture.

For instance, when a bottle of perfume is opened at one corner of a room, a person at the other end of the room smells the pleasant odour of the perfume after some time.
Similarly, when a bottle containing a concentrated solution of ammonia is opened at one end of a laboratory desk, a person at the other end of the desk smells the odour of ammonia. This is because the molecules of the perfume or ammonia Intermix with air rapidly and spread. Such a phenomenon of spontaneous Inlet mixing of gases In called diffusion.
Diffusion of a gas Definition:
The process by which two or more non-reacting gases without any external help Intermix with one another spontaneously, Irrespective of their densities or molar masses to form a homogeneous gas mixture Is termed as diffusion.
Efusion of a gas:
Like diffusion, a process involving the flow of gas molecules IN effusion. If a container holding some gas is kepi In a vacuum and a tiny hole (or pinhole) is made in the container, the enclosed gas flows through the tiny hole Into the vacuum.
This process is called effusion, Effusion Is also found to occur when the pressure of a gas enclosed In a porous container (like a rubber balloon) is higher than that of the outside pressure. Due to higher pressure Inside the container, gas flows out through the pores of the container.

Efusion of a gas Definition:
The process by which a gas escapes its container through a tiny hole (or pinhole) into a vacuum or a region of lower pressure Is called an effusion.
Graham’s law of diffusion or effusion Law of diffusion:
At constant temperature and pressure, the rate of diffusion of a gas is inversely proportional to the square root of the density of the gas.
This law applies to an effusion process also. For an effusion process, according to Graham’s law, the rate of effusion of a gas at constant temperature and pressure is inversely proportional to the square root of its density.
Mathematical form:
If at a particular temperature and pressure, the rate of diffusion (or effusion) of a gas =r and the density of that gas = d, then according to Graham’s law
⇒ \(r \in \frac{1}{\sqrt{d}} \text { or, } r=\frac{K}{\sqrt{d}}\) [k= proportionality constant]………………. (1)
Equation (1) is the mathematical form of Graham’s law. If under the same conditions of temperature and pressure, the rates of diffusion (or effusion) of two gases A and B be r A and B respectively, then according to Graham’s law,
⇒ \(r_A \propto \frac{1}{\sqrt{d_A}} \text { and } r_B \propto \frac{1}{\sqrt{d_B}}\)
∴ \(\frac{r_A}{r_B}=\sqrt{\frac{d_B}{d_A}}\) ……………… (2)
dA and dB are the densities of A and B respectively. Relation between the rate of diffusion (or effusion) and the vapour density of a gas:
At a particular temperature, the density (d) of a gas is proportional to its vapour density (D); i.e., d oc D Therefore, equation (2) can be expressed as
⇒ \(\frac{r_A}{r_B}=\sqrt{\frac{d_B}{d_A}}=\sqrt{\frac{D_B}{D_A}} \text { or, } \frac{r_A}{r_B}=\sqrt{\frac{D_B}{D_A}}\) ……………… (3)
DA and DB are the vapour densities of A and B, respectively.
Hence, at constant temperature and pressure, the rate of diffusion (or effusion) of a gas is inversely proportional to the square root of its vapour density.
Relation between the rate of diffusion or effusion and the molar mass of a gas:
Vapour density \((D)=\frac{\text { molar mass }(M)}{2}\)
⇒ \(\frac{r_A}{r_B}=\sqrt{\frac{D_B}{D_A}}=\sqrt{\frac{M_B / 2}{M_A / 2}}=\sqrt{\frac{M_B}{M_A}} \text { or, } \frac{r_A}{r_B}=\sqrt{\frac{M_B}{M_A}}\)……………… (4)
MA and MB are the molar masses of A and B, respectively. Therefore, at constant temperature and pressure, the rate of diffusion (or effusion) of a gas is inversely proportional to the square root of its molar mass.
Relation between the volume of a gas effused and its molar mass:
At a constant temperature and pressure, if the VA volume of gas A and VB volume of gas B effused through the same porous wall in the same time t, then—
⇒ \(r_A=\frac{V_A}{t} \text { and } r_B=\frac{V_B}{t}\)
∴ \(\frac{r_A}{r_B}=\frac{V_A / t}{V_B / t}=\frac{V_A}{V_B}\)
Substituting this value into equation [4], we obtain
⇒ \(\frac{V_A}{V_B}=\sqrt{\frac{M_B}{M_A}}\)……………… (5)
Thus, under the identical set of conditions, the effused quantities of the two gases have volumes that are inversely proportional to the square roots of their molar masses.
Relation between the time taken for effusion and the molar mass:
Suppose, at constant temperature and pressure, two gases A and B are effusing through a porous wall. If tA and tB are the times required for effusion ofthe same volume ( V) of gases A and B, respectively, then
⇒ \(r_A=\frac{V}{t_A} \text { and } r_B=\frac{V}{t_B}\)
According to Graham’s law of effusion,
⇒ \(\frac{r_A}{r_B}=\sqrt{\frac{M_B}{M_A}} \text { or, } \frac{V / t_A}{V / t_B}=\sqrt{\frac{M_B}{M_A}} \text { or, } \frac{t_B}{t_A}=\sqrt{\frac{M_B}{M_A}} \text { or, } \frac{t_A}{t_B}=\sqrt{\frac{M_A}{M_B}} \cdots[6]\) ……………… (6)
Therefore, under the same conditions of temperature and pressure, the times required for the effusion of the same volume of two gases through a porous wall are directly proportional to the square roots of their molar masses.
At a particular temperature, the rate of diffusion or effusion (r) of any gas is proportional to the pressure (P) of the gas and inversely proportional to the square root of the molar mass (Af) of the gas.
Therefore, If at a constant temperature, two gases A and B diffuse (or effuse) at pressures PA and PB, respectively, and their molar masses are MA and MB, then
⇒ \(r_A \propto P_A / \sqrt{M_A} \text { and } r_B \propto P_B / \sqrt{M_B}\)
Where rA and rB are the rates of diffusion (or effusion) of gases A and B, respectively therefore
⇒ \(\frac{r_A}{r_B}=\frac{P_A}{P_B} \sqrt{\frac{M_B}{M_A}}\)
Applications of diffusion (effusion) of gases:
Determination of the molar mass of a gas:
Under identical conditions of temperature and pressure, if equal volumes of two gases, A and B pass through a porous wall in times tA and tB, respectively, then according to Graham’s law
⇒ \(\frac{t_A}{t_B}=\sqrt{\frac{M_A}{M_B}} \text { or, }\left(\frac{t_A}{t_B}\right)^2=\frac{M_A}{M_B}\)
[MA and MB are the molar masses of A and B respectively]
If the time taken for the diffusion of the same volume of two gases under the same conditions of temperature and pressure and the molar mass of one of the gases is known, then from equation [1], the molar mass of the other gas can be calculated.
Separation of the component gases from the mixture of two gases:
At constant temperature and pressure, the rate of diffusion or effusion of any gas is inversely proportional to the square root of its molar mass. So, if a mixture of two gases is allowed to pass out through a porous wall, then the quantity ofthe gas diffused out after a definite interval will be enriched by the lighter gas.
In the same manner, two gases can be entirely separated by repeated diffusion or effusion through a porous wall. Such a process of separation of the components of a gaseous mixture based on the diffusive properties of gases is called atmolysis.
Example:
Two common isotopes of hydrogen gas, protium (JH) and deuterium (ÿH)can be easily separated by this method. But in most cases, the separation of isotopes is not easier by this method because ofthe very small ratio of the atomic masses of these isotopes.
Detection of marsh gas in coal mines:
To indicate the presence of marsh gas in coal mines, an electric alarm is used, which works on the principle of diffusion.
An experiment of gaseous diffusion
Diffusion of gases can be understood from the following experiment. A cotton plug soaked in concentrated HC1 solution is inserted into one end of a long tube, and another cotton plug soaked in concentrated NH3 solution is inserted into the other end ofthe tube.
The two ends ofthe tube are then closed with rubber corks so that HC1 and NH3, After some time, a white fume first appears at a point P inside the tube. It is the vapour of NH4 Cl formed by the reaction between NH3 and HCl vapours.
Point P lies near the cotton ball soaked in HC1 solution than that soaked in NH3 solution. This is because the diffusion rate of the lighter NH3 gas is greater than that of the heavier HCl gas and hence NH3 molecules travel a longer distance than HCl molecules in a given time.

Numerical Examples
Question 1. The rate of diffusion of a gas is 2.92 times that of NH3 gas. Determine the molecular weight of that gas.
Answer:
If the rates of diffusion of the unknown gas and NH3 gas at constant temperature and pressure are rx and r2, then according to Graham’s law of diffusion
⇒ \(\frac{r_1}{r_2}=\sqrt{\frac{M_{\mathrm{NH}_3}}{M}}\)
gas and MNH3 = 17
Since, rx = 2.92 × r²
∴ \(2.92=\sqrt{\frac{17}{M}} \quad \text { or, } M=2\)
Therefore, the molecular weight ofthe unknown gas = 2
Question 2. 432 mL of gas A effuses out through a fine orifice in 36 min. 288 mL of another gas B effuses out through the same orifice in 48 min. If the molecular weight of B is 64, what is the molecular weight of A?
Answer:
According to Graham’s law of effusion \(\frac{r_A}{r_B}=\sqrt{\frac{M_B}{M_A}}\)
Given \(r_A=\frac{432 \mathrm{~mL}}{36 \mathrm{~min}}=12 \mathrm{~mL} \cdot \mathrm{min}^{-1},\)
⇒ \(r_B=\frac{288 \mathrm{~mL}}{48 \mathrm{~min}}=6 \cdot \mathrm{mL} \cdot \mathrm{min}^{-1} \text { and } M_B=64\)
∴ \(\frac{12}{6}=\sqrt{\frac{M_B}{M_A}}\)
∴ \(M_B=4 \times M_A \text { or, } M_A=\frac{64}{4}=16\)
Question 3. The molecular weights of the two gases are 64 and 100 respectively. If the rate of diffusion of the first gas is 15 mL.sec-1, then what is the rate of diffusion of the other?
Answer:
According to Graham’slaw ofdiffusion \(\frac{r_A}{r_B}=\sqrt{\frac{M_B}{M_A}}\)
Here, MA = 64, MB = 100, rA = 15 mL.s-1 rAB = ?
∴ \(\frac{15 \mathrm{~mL} \cdot \mathrm{s}^{-1}}{r_B}=\sqrt{\frac{100}{64}}=\frac{10}{8}\)
∴ rB = 12 mL-s-1
∴ The rate of diffusion ofthe other gas = 12 mL.s-1
Question 4. Determine the relative rates of diffusion of 235UF6 And 238UF6 UF6 Gases.
Answer:
Molecular weight of 235UF6 =235 + 6 × 19 = 349
And molecular weight of 238 UF6 = 238 UF6
= 238+6 × 19
= 352
At a given temperature and pressure if r1 and r2 are the rates of diffusion of and 238UF6 respectively,
Then according to Graham’s law,
⇒ \(\frac{r_1}{r_2}=\sqrt{\ \frac {M_2}{M_1}}=\sqrt{\frac{352}{349}}=1.0043\)
∴ r1=1.0043 × r2
∴ The rate of diffusion of 235UF6 is 1.0043 times that of 238UF6
Question 5. In a mixture of O2 and an unknown gas, the percentage of the unknown gas Is 20% by mass. At a given temperature and pressure, the time required to effuse VmL of the gas mixture through an aperture is 234.1 s. Under the same conditions, the time required to effuse the same volume of pure 02 gas is 223.1 s. What is the molar mass of unknown gas?
Answer:
Average molecular mass of the gas mixture
⇒ \(\frac{20 \times M+80 \times 32}{100}\)
Where,
M = molar mass of unknown gas. According to the problem, the rate of effusion of VmL of unknown gas mixed with oxygen,
⇒ \(r_1=\frac{V}{234.1} \mathrm{~mL} \cdot \mathrm{s}^{-1}\)
And the rate of effusion of 7raL of pure oxygen
⇒ \(r_2=\frac{V}{223.1} \mathrm{~mL} \cdot \mathrm{s}^{-1}\)
We know, ,\(\frac{r_1}{r_2}=\sqrt{\frac{M_2}{M_1}}\) and \(\frac{r_1}{r_2}=\sqrt{\frac{M_2}{M_1}}\)
Now, \(\frac{M_2}{M_1}=\frac{32}{\frac{20 M+2560}{100}}=\frac{160}{M+128}\)
∴ \(\frac{223.1}{234.1}=\sqrt{\frac{M_2}{M_1}}=\sqrt{\frac{160}{M+128}} \quad \text { or, } 0.9082=\frac{160}{M+128}\)
∴ M= 48.17
The molar mass of unknown gas = 48.17gmol-1
Question 6. A gas mixture consisting of He and CH4 gases in a mole ratio of 4:1 is present in a vessel at a pressure of 20 bar. Due to a fine hole in the vessel, the gas mixture undergoes effusion. What is the composition (or ratio) of the initial gas mixture that is effused out?
Answer:
If the total number of moles in the mixture is n, then the numbers of moles of He and CH4 are \(\frac{4}{5} n \text { and } \frac{1}{5} n\) or, 0.8n and 0.2 n respectively.
In the mixture, the partial pressure of He (pHe)
⇒ \(=\frac{0.8 n}{n} \times 20=16 \text { bar and that of } \mathrm{CH}_4\left(p_{\mathrm{CH}_4}\right)\)
⇒ \(=\frac{0.2}{n} \times 20=4 \text { bar }\)
Rate of effusion of a gas at a constant temperature, \(r \propto \frac{P}{\sqrt{M}}\)
where P = pressure ofthe gas and M molar mass ofthe gas.
Therefore, the rate of effusion of He gas, \(r_1 \propto \frac{p_{\mathrm{He}}}{\sqrt{M_{\mathrm{He}}}}\)
⇒ \(\text { or, } r_1 \propto \frac{p_{\mathrm{He}}}{\sqrt{4}} \text { and that of } \mathrm{CH}_4 \text { gas, } r_2 \propto \frac{p_{\mathrm{CH}_4}}{\sqrt{M_{\mathrm{CH}_4}}} \text { or, } r_2 \propto \frac{p_{\mathrm{CH}_4}}{\sqrt{16}}\)
∴ \(\frac{r_1}{r_2}=\frac{p_{\mathrm{He}}}{p_{\mathrm{CH}_4}} \times \sqrt{\frac{16}{4}}=\frac{2 p_{\mathrm{He}}}{p_{\mathrm{CH}_4}}=\frac{2 \times 16}{4}=8\)
∴ The ratio of the number of moles of He and CH4 (composition) in the initial gas mixture effused out 8:1
Question 7. ‘X’ and ‘ Y’ are the two open ends of a glass tube with a length of lm. NH3 gas through ‘X’ and HCl gas through ‘Y’ are simultaneously allowed to enter the tube. As NH3 and HCl gases diffuse towards each other inside the tube, they meet and react to form NH4Cl(s), as a result of a white fume appears. Where will the white fume appear first inside the tube?
Answer:
Suppose, NH3 gas first comes in contact with HCl gas after it traverses a distance of f cm from the X-end of the tube. At the same time, HCl gas moves a distance of (100-f) cm from the K-end of the tube. Therefore, the first white fume, appearing due to the formation of NH4Cl(s) from the reaction of NH4 with HCl, will be seen tit u distance of f cm f/otn the X -end.

If r1 and r2 are the rates of diffusion of Nil., and HCI respectively, then
⇒ \(r_1=\frac{l}{t} \text { and } r_2=\frac{(100-t)}{t}\)
∴ \(\frac{r_1}{r_2}=\sqrt{\frac{M_{\mathrm{HCl}}}{M_{\mathrm{NH}_3}}}\)
Or, \(\frac{l}{100-l}=\sqrt{\frac{36.5}{17}} \text { or, } \frac{l}{100-l}=1.465\) or, l = 59.43 cm
So, the white fume first appears at a distance of 59.43 cm from the ‘X’-end ofthe tube
The Kinetic Theory Of Gases
The kinetic theory of gases Is based on some postulates to elucidate the behaviour of Ideal gases, This theory was first proposed by Bernoulli and was considerably extended and elaborated by Clausius, Boltzmann, Maxwell, van der Waals arid Jeans.
Fundamental Postulates Of Kinetic Theory Of Gases
- A gas consists of a large number of tiny discrete particles, called molecules.
- The gas molecules are solid, spherical and perfectly elastic.
- Molecules of a gas are alike In all respects.
- The actual volume occupied by the molecules Is negligible in comparison to the volume of the container.
- Gas molecules are always in ceaseless chaotic motion.
- They constantly collide with each other and with the walls of the container. Collisions of gas molecules with the walls of the container give rise to (lie pressure of a gas.
- All molecular collisions are perfectly elastic i.e., during collisions, molecules do not gain or lose kinetic energy.
- There exist no forces of attraction or repulsion among (lie gas molecules, l, a., molecules in a gas behave Independently of one another.
- The average kinetic energy of the molecules of a gas is directly proportional to the absolute temperature ofthe gas.
Justification for the postulates of the kinetic theory of gases:
- According to the kinetic theory of gases, the total volume of the gas molecules is negligible compared to the volume of the container holding the gas. This means that most of the volume occupied by a gas in a container is empty. The high compressibility of gases justifies this assumption.
- The kinetic theory of gases proposes that the gas molecules are always in ceaseless random motion.
- If the molecules of a gas were not in ceaseless random motion, the gas would have a definite shape and size. But in fact, gases do not have a definite shape and size, which supports the ceaseless random motion of gas molecules.
According to the kinetic theory of gases, there exists no force of attraction or repulsion among gas molecules. The indefinite expansion of a gas supports this postulate.
- The kinetic theory of gases tells us that the pressure of a gas arises due to the collisions of gas molecules with the walls of the container. Molecule In a gas his terms that move randomlyIn straight lines In all directions.
- As a result, they constantly collide with one another and with the walls of the container. The force exerted by the molecules unit area of the wall of the container represents the pressure of the gas.
- The collisions of gas molecules among themselves and also against the walls of the container are perfectly elastic. This means there Is no loss or gain of kinetic energy during collisions, i.e., the total kinetic energy of the gas molecules before and after collisions remains the same.
If the kinetic energy of the gas molecules enclosed in an insulated container were not conserved during collisions, the pressure of the gas In that insulated container would also fluctuate.
- But, the fact is that the pressure of the gas enclosed In an insulated container remains the same. So, this confirms that the collisions of gas molecules among themselves and with the walls of the container are perfectly elastic.
- According to the kinetic theory of gases, the average kinetic energy of gas molecules is proportional to the absolute temperature of the gas. With increasing temperature, the pressure of a gas increases at constant volume.
- Again, the pressure of a gas increases as the number of collisions of the gas molecules with the walls ofthe container increases, which in turn, increases with the increase in average kinetic energy of gas molecules.
- Thus, it can be concluded that the average kinetic energy of the gas molecules is proportional to the absolute temperature of the gas.
Kinetic gas equation
Based on the postulates of the kinetic theory of gases, an equation for the pressure of a gas can be deduced. This equation is known as the kinetic gas equation.
The equation is \(P=\frac{1}{3} \frac{m n c_{r m s}^2}{V}\)
Where P and V are the pressure and volume of tyre gas, arms are the root mean square velocity of the gas molecules, m = mass of each gas molecule, and n – is the number of molecules present in volume V.
Average velocity and mean square velocity
Let, the total number of molecules in a sample of gas be n. Of these molecules, suppose, n, molecules move with velocity c1, c2, molecules move with velocity c2, n3 molecules move with velocity c3, and so on.
Average velocity:
It is the arithmetical mean of the velocities of all the molecules in a gas at a given temperature.
Average velocity, \(\bar{c}\)
= \(\frac{n_1 c_1+n_2 c_2+n_3 c_3+\cdots}{n_1+n_2+n_3+\cdots}\)
= \( \frac{n_1 c_1+n_2 c_2+n_3 c_3+\cdots}{n}\)
‘Bar’(-) over c indicates the average value of c]
If the absolute temperature and the molar mass of a gas are T and M, respectively, then it can be shown that
⇒ \(\bar{c}=\sqrt{\frac{8 R T}{\pi M}}.\)
Mean square velocity:
It is the arithmetical mean of the square velocities of all the molecules in a gas at a given temperature.
Mean square velocity
⇒ \(\overline{c^2}=\frac{n_1 c_1^2+n_2 c_2^2+n_3 c_3^2+\cdots}{n_1+n_2+n_3+\cdots}\)
= \(\frac{n_1 c_1^2+n_2 c_2^2+n_3 c_3^2+\cdots}{n}\)
Root mean square velocity
The square root of the mean square velocity of the molecules in a gas at a given temperature is called the root mean square (RMS) velocity. Root mean square velocity
⇒ \(c_{r m s}=\sqrt{\overline{c^2}}=\sqrt{\frac{n_1 c_1^2+n_2 c_2^2+n_3 c_3^2+\cdots}{n}}\)
The average velocity (c) and the root mean square velocity of the molecules of a gas are not the Suppose, we have 2 molecules with velocities 2 m-s-1 and 4 m-s-1, respectively. The average velocity of the two molecules,
⇒ \(c=\frac{(2+4) \mathrm{m} \cdot \mathrm{s}^{-1}}{2}=3 \mathrm{~m} \cdot \mathrm{s}^{-1}\)
And root mean square velocity
⇒ \(c_{t \mathrm{~ms}}=\sqrt{\frac{2^2+4^2}{2}}=3.16 \mathrm{~m} \cdot 5^{-1}\)
To determine the tbs average kinetic energy of gas molecules, the root mean square velocity is used instead of the average velocity
Gases are isotropic i.e. the properties of gases are Independent of direction. So, gas molecules can move in all directions with equal probability.
Therefore, the average velocity of the gas molecules along a particular axis (x or y or z )is zero because at any instant the probability ofthe number of molecules moving along the positive x-axis and the negative x-axis is equal.
- As a result, the average velocity along the x-axis (similarly y-axis or z-axis) becomes zero.
- For this reason, the average velocity is not used to determine the average kinetic energy of the gas molecules.
- On the other hand, the root mean square velocity of the gas molecules is determined from their mean square velocity.
- As the square of a positive or negative quantity Is always a positive quantity, the root mean square velocity is always positive.
- Hence, the root-mean-square velocity is used to determine the average kinetic energy of the gas molecules.
Determination of Crms from the kinetic gas equation:
From the kinetic gas equation we have \(P V=\frac{1}{3} m n c_{r m s}^2\)
P and V are the pressure and volume of gas respectively, at a particular temperature, arms are the root mean square velocity of molecules in the gas at the same temperature, m is the mass of the gas molecule and n is the number of molecules present in volume V. For 1 mol gas, n = N (Avogadro’s number)
Therofore P V=\frac{1}{3} m N c_{r m s}^2 \text { (for } 1 \mathrm{~mol} \text { gas). }\(\)
Molar mass (M) of a gas = mass of each molecule of the gas x Avogadro’s number =mN and for 1 mole of gas, PV = RT.
Therefore. \(R T=\frac{1}{3} M c_{r m s}^2\) or, \(c_{r m s}=\sqrt{\frac{3 R T}{M}}\)
This equation represents the root mean square velocity at a given temperature T of the molecules in a gas with a molar mass of M.
It is evident from this equation that the root mean square velocity of the molecules of a gas is directly proportional to the square root of absolute temperature and inversely proportional to the square root of the molar mass of the gas.
If the molar masses of A and B gases are MA and MB respectively and the root mean square velocities of the molecules of A and B and (arms), respectively, then at a particular temperature (T),
⇒ \(\left(c_{r m s}\right)_A=\sqrt{\frac{3 R T}{M_A}} \text { and }\left(c_{r m s}\right)_B=\sqrt{\frac{3 R T}{M_B}}\)
So, \(\frac{\left(c_{r m s}\right)_A}{\left(c_{r m s}\right)_B}=\sqrt{\frac{M_B}{M_A}}\)
Therefore, at a particular temperature is greater for a lighter gas. For example, at a given temperature c value for H2 molecules is 4 times as high as that of O2 molecules
⇒ \(\frac{\left(c_{r m s}\right)_{\mathrm{H}_2}}{\left(c_{r m s}\right)_{\mathrm{O}_2}}=\sqrt{\frac{32}{2}}=\sqrt{16}=4\)
Or, \(\left(c_{r m s}\right)_{\mathrm{H}_2}=4 \times\left(c_{r m s}\right)_{\mathrm{O}_2}\)
Most probable velocity (cm)
Gas molecules move randomly in all directions, collide with each other and also against the walls oftheir container. Due to such frequent collisions, there is always a redistribution of velocities among the molecules.
At any instant in time, the gas contains molecules moving with very high to very low velocities. Maxwell, by extensive mathematical calculation of the distribution of molecular velocities, has shown that at a particular temperature, the velocity with which the maximum fraction of molecules in a sample of gas move has a constant value. This velocity is known as the most probable velocity and it is represented by the equation.
⇒ \(c_m=\sqrt{\frac{2 R T}{M}}\)
Where T and M are the absolute temperature and molecular mass ofthe gas respectively.
Relation among different molecular velocities: At a given temperature T the average velocity (c), root mean square velocity (arms) and most probable velocity (cm) of the molecules ofa gas with molar mass M are-
⇒ \(\bar{c}=\sqrt{\frac{8 R T}{\pi M}}, c_{r m s}=\sqrt{\frac{3 R T}{M}} \text { and } c_m=\sqrt{\frac{2 R T}{M}}\)
∴ \(\left[c_m: \overline{\boldsymbol{c}}: c_{r m s}\right]=\sqrt{\frac{2 R T}{M}}: \sqrt{\frac{8 R T}{\pi M}}: \sqrt{\frac{3 R T}{M}}\)
⇒ \(=\sqrt{2}: \sqrt{\frac{8}{\pi}}: \sqrt{3}=1: 1.128: 1.224\)
Relation between c and Crms:
At a definite temperature, for a particular gas cm =crms = 0.921
Average velocity = 0.921 × root mean square velocity.
The relation between cm and arms is cm / arms = 0.816
Most probable -velocity = 0.816 × root mean square velocity
For the gas molecules of a given gas at a definite temperature,
crms>c‾>cm
Average kinetic energy of gas molecules
Since all the molecules in a gas do not move at the same velocity, they do not have the same kinetic energy. Thus, the kinetic energy of the molecules in ofa gas is always expressed in terms of average kinetic energy.
Let n be the total number of 6 molecules in a sample of gas, and m be the mass of each molecule. If at a given temperature, molecules move with velocity c1, n2 molecules with velocity c2, n3 molecules with velocity c3— etc., and the respective kinetic energies of these molecules are \(\epsilon_1, \epsilon_2, \epsilon_3 \ldots\) etc., then
⇒ \(\epsilon_1=\frac{1}{2} m c_1^2, \epsilon_2=\frac{1}{2} m c_2^2, \epsilon_3=\frac{1}{2} m c_3^2 \cdots e t c .\)
Therefore, the average kinetic energy of the molecules,
⇒ \(\bar{\epsilon}=\frac{n_1 \epsilon_1+n_2 \epsilon_2+n_3 \epsilon_3+\cdots}{n}\)
⇒ \(=\frac{1}{2} m\left[\frac{n_1 c_1^2+n_2 c_2^2+n_3 c_3^2+\cdots}{n}\right]=\frac{1}{2} m \overline{c^2}\)
Also, mean square velocity = (root mean square velocity)2 i.e., \(\overline{c^2}=c_{r m s}^2\)
∴ Average kinetic energy of a gas molecule \(=\frac{1}{2} m c_{r m s}^2\)
Equation [1] expresses the relation between the average kinetic energy and the root mean square velocity of the molecules in a gas.
Value of average kinetic energy:
The average kinetic energy of the molecules in a gas at a particular temperature,
⇒ \(\bar{\epsilon}=\frac{1}{2} m c_{r m s}^2\) where m is the mass of a gas molecule, cms is its root mean square velocity At a given temperature for the molecules in a gas,
⇒ \(c_{r m s}=\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 k \times N \times T}{m \times N}}=\sqrt{\frac{3 k T}{m}}\)
Since Molar mass (M) of a gas = mass of a molecule (m) of the gas Avogadro’s’ number (N) and R = k (Boltzmann constant) × N]
∴ \(\bar{\epsilon}=\frac{1}{2} m c_{r m s}^2=\frac{1}{2} \times m \times \frac{3 k T}{m}=\frac{3}{2} k T\)
It is evident from this equation that the average kinetic energy of the gas molecules is proportional to the -absolute temperature. Therefore, at TK, the total kinetic energy of the molecules of1 mol ofa gas.
⇒ \(=\epsilon \times N=\frac{3}{2} k T \times N=\frac{3}{2} R T\)
[since r = k × N]
CBSE Class 11 Chemistry States of Matter Liquids Gases Summary
Numerical Examples
Question 1. Determine the ratio of root mean square velocity and average velocity of the molecules in a gas at a given temperature
Answer:
If M and T are the molar mass and temperature ofthe gas, then the root mean square velocity \(c_{r m s}=\sqrt{\frac{3 R T}{M}}\) and the average velocity, \((\bar{c})=\sqrt{\frac{8 R T}{\pi M}}\)
∴ \(\frac{c_{r m s}}{c}=\sqrt{\frac{3 \pi}{8}}=1.085\)
Question 2. Show that the root mean square velocity of an O2 molecule at 54°C is not twice its root mean square velocity at 27°C
Answer:
At 54°C, i.e., at (273 + 54)K = 327K the root mean square velocity of O2 molecules,
⇒ \(\left(c_{r m s}\right)_1=\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 \times 8.314 \times 10^7 \times 327}{32}}\)
= 5.048 × 104 cm-s-1
And at 27°C i.e., at (273 + 27)K = 300K
⇒ \(\left(c_{r m s}\right)_2=\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 \times 8.314 \times 10^7 \times 300}{32}}\)
=4.835 × 104 cm.s-1
∴ (arms)1≠2(crms)2.
Question 3. Determine the rms velocity, average velocity and most probable velocity of H2 molecules at 300 K.
Answer:
Molar mass (M) of H2 gas = 2g.mol-1
∴ Root mean square velocity of H2 molecule
⇒ \(\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 \times 8.314 \times 10^7 \times 300}{2}}=1.934 \times 10^5 \mathrm{~cm} \cdot \mathrm{s}^{-1}\)
Average velocity = \(=\sqrt{\frac{8 R T}{\pi M}}=\sqrt{\frac{8 \times 8.314 \times 10^7 \times 300}{3.14 \times 2}} ;\)
= 1.782×105 cm.5 cm.s-1
Most probable velocity \(\sqrt{\frac{2 R T}{M}}=\frac{2 \times 8.314 \times 10^7 \times 30}{2}\)
= 1.58 × 10 5cm.s-1
Question 4. At what temperature will the most probable velocity of the H2 molecule be equal to the root mean square velocity of O2 molecules at 20°C?
Answer:
At 20°C temperature, the root mean square velocity of the O2 molecule =
⇒ \(\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 \times 8.314 \times 10^7 \times(273+20)}{32}} \mathrm{~cm} \cdot \mathrm{s}^{-1}\)
Let at temperature T1 the most probable velocity of H2 = the rms velocity of O2 at 20°C.
At TK, the most probable velocity of H2
⇒ \(\sqrt{\frac{2 R T}{M}}=\sqrt{\frac{2 \times 8.314 \times 10^7 \times T}{2}} \mathrm{~cm} \cdot \mathrm{s}^{-1}\)
∴ \(\sqrt{\frac{2 \times 8.314 \times 10^7 \times T}{2}}=\sqrt{\frac{3 \times 8.314 \times 10^7 \times 293}{32}}\)
or, \(T=\frac{3 \times 293}{32}=27.50\) Therefore at —245.5°C, the most probable velocity of H2 molecules will be equal to the rms velocity of 02 at 20 °C.
Question 5. The density of O2 gas at 1 atm pressure and 273K is 1.429 g-dm-3. Calculate the root mean square velocity of 02 molecules at 273 K
Answer:
The root mean square velocity,
⇒ \(c_{r m s}=\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 P V}{M}}=\sqrt{\frac{3 P}{d}}\)
Given, P = 1 atm and d = 1.429 g-dm-3
P = latm = 1.013 × 106 dyn-cm-2
= 1.013 × 10-6 g.cm.s-2 .cm-2 = 1.013 × 106 g. cm-1.s-2
and d = 1.429 g.dm-3 = 1.429 × 10-3 g.cm-3
∴ \(c_{r m s}=\sqrt{\frac{3 \times 1.013 \times 10^6 \mathrm{~g} \cdot \mathrm{cm}^{-1} \cdot \mathrm{s}^{-2}}{1.429 \times 10^{-3} \mathrm{~g} \cdot \mathrm{cm}^{-3}}}\)
= 4.611 × 104 cm.s-1
Question 6. Determine the total kinetic energy of the molecules of 1 g CO2 at 27°C in the units of erg and calorie. Assume the ideal behaviour of the gas.
Answer:
1 g \( \mathrm{CO}_2=\frac{1}{44}=2.27 \times 10^{-2} \mathrm{~mol} \mathrm{CO}_2 \text { and }\)
T = (273 + 27)K = 300 K
The total kinetic energy of the molecules in mol of an ideal gas.
⇒ \(\frac{3}{2} R T\)
Therefore, the total kinetic energy of the molecules of
⇒ \(2.27\times 10^{-3} \mathrm{~mol}\)
CO2 gas = \(2.27 \times 10^{-2} \times \frac{3}{2} R T\)
= \(2.27 \times 10^{-2} \times \frac{3}{2} \times 8.314 \times 10^7 \times 300\)
= \(8.5 \times 10^8 \mathrm{erg}\)
= 20.31cal
= 8.5 × 108 erg = 20.31 cal
Since 1 cal = 4.18 × 107erg
Question 7. Determine the total kinetic energy of the molecules of 8.0 g CH4 at 27°C in the unit of joule.
Answer:
8.0 = \(\mathrm{CH}_4=\frac{8}{16}=0.5 \mathrm{molCH}_4\)
since MCH4 = 16g.mol-1]
Total kinetic energy ofthe moleculesin1 mol gas \(=\frac{3}{2} R T\)
∴ Total kinetic energy ofthe molecules of 0.5 mol CH4 gas
⇒ \(=0.5 \times \frac{3}{2} R T=0.5 \times \frac{3}{2} \times 8.314 \times(273+27)=1870.65 \mathrm{~J} .\)
Question 8. At a given temperature, the average kinetic energy of H2 molecules is 3.742 kj. mol-1. Calculate the root mean square velocity of H2 molecules at this temperature
Answer:
Suppose, at TK, the average kinetic energy of H2 gas molecules =3.742 kj.mol-1.
∴ \(\frac{3}{2} R T=3.742 \times 10^3 \mathrm{~J} \cdot \mathrm{mol}^{-1}\)
or, \(\frac{3}{2} \times 8.314 \times T=3.742 \times 10^3\)
∴ t= 300k
Therefore, the root mean square velocity of the H2 molecule
⇒ \(\text { at } 300 \mathrm{~K}=\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{3 \times 8.314 \mathrm{~J} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 300 \mathrm{~K}}{2 \mathrm{~g} \cdot \mathrm{mol}^{-1}}}\)
⇒ \(=\sqrt{\frac{3 \times 8.314 \times 10^3 \mathrm{~g} \cdot \mathrm{m}^2 \cdot \mathrm{s}^{-2} \times 300}{2 \mathrm{~g}}}=1934.2 \mathrm{~m} \cdot \mathrm{s}^{-1}\)
Maxwell’s distribution of velocity
In a collection of gas molecules, there exists a wide distribution of velocity, ranging from very low to very high value. Molecules moving with very low to very high velocities are very small. Most of the molecules move with intermediate velocities.
Maxwell showed that the molecular velocity distribution in a gas depends on the temperature and molar mass of the gas and gave an equation, commonly known as Maxwell’s distribution law of molecular velocity.
According to this law, at a given temperature, the distribution of velocities remains the same although individual velocities of gas molecules may change due to collision. Let the total number of molecules in a gaseous sample be n and at a particular temperature, dn is the number of molecules having velocities in the range c to c + dc \(\left(\frac{d n}{n}\right)\) is a small interval of velocity).
Tints, the fraction of molecules having velocity in the range c to c + dc is \(\left(\frac{d n}{n}\right)\). The value of f changes with a change in the value of c. Maxwell’s distribution is obtained by plotting
⇒ \(\left(\frac{d n}{n}\right)\) as ordinate and c as abscissa.

Description of the graph:
The given graph shows that the fraction of molecules \(\left(\frac{d n}{n}\right)\) increases with c, reaches a maximum, and then decreases rapidly. The velocity corresponding to the maximum in the distribution curve indicates the velocity possessed by most of the molecules.
This velocity is called the most probable velocity (cm). For a gas with molar mass M, the most probable velocity of its molecules at temperature T is:
⇒ \(c_m=\sqrt{\frac{2 R T}{M}}\)
The number of molecules moving with very high or very low velocities is very small.
The average velocity of the molecules is slightly greater than the most probable velocity and the root mean square velocity is still slightly greater than the most probable velocity.
Effect of molar mass on the distribution of molecular velocity at constant temperature:
According to the equation \(c_m=\sqrt{\frac{2 R T}{M}}\) at a given temperature, the most probable velocity of molecules of a gas is inversely proportional to the molar mass of the gas.
Therefore, at a particular temperature, the most probable velocity of a gas with a larger molar mass will be lower than that of a gas with a smaller molar mass, and the maxima in the distribution curve for a lighter gas will occur at a higher velocity than that for a heavier gas. As a result, the curve for a lighter gas becomes more flattened than that for a heavier gas.

Influence of temperature on velocity distribution:
The change in temperature changes the velocity of the molecules. As a result, the distribution of molecular velocities also changes The velocity distribution curves at three different temperatures (T1, T2 and T3 ) are given below.

With increasing temperature, the curve gets flattened and the maximum the curve shifts to the right. This means that with temperature rise, the most probable velocity (cm) of the molecules increases although the number of molecules moving with this velocity decreases. Thus, the velocity distribution curve broadens at higher temperatures.
The middle portion of the curve gets flattened with the increase in temperature. This indicates that with an increase in temperature, the number of molecules with higher velocity increases and that with lower velocity decreases.
If the temperature decreases, the distribution curve becomes gradually narrow and the most probable velocity of the molecules decreases, while the number of molecules with this velocity increases.
Deviation Of Real Gases From Ideal And Behaviour
The equation of state for n mol of an ideal gas is PV = nRT. In practice, there is no such gas which obeys PV = nRT under all conditions of pressure and temperature. Gases which do not obey the equation, PV = nRT, except at very low pressure and high temperature, are said to be real gases. All naturally occurring gases are real gases. Fegnault, Andrews, Amagat, KamerJing and other scientists have studied the deviation of real gases from ideal behaviour.
Amagat curves
Amagat carried out an extensive study on the behaviour of real gases. From the results of his observations, he plotted PV against constant temperature for different gases.
If the gas obeys the equation, PV = NRT (ideal gas), then at a constant temperature, the PV vs P plot will be a straight line parallel to the P-axis and at any temperature, and the value of PV will be equal to that of RT (forI mol of gas). This is shown by a dotted line. In the case of real gases, however, the plots of PV vs P are not a horizontal line Instead they deviate from the line of ideas gas and take a curve-like shape as shown by unbroken lines.

The curves for real gases are of two types. These are—
In the case of gases like N2, CO2 etc., with the increase in pressure, the value of PV initially decreases from the expected value of nRT, passes through a minimum and then goes on increasing, even after exceeding the value of nRT.
Such gases show a negative deviation in the beginning, reach a minimum value and then show a positive deviation after crossing the line for ideal gas. Depression in the curve of gas is characteristic ofthe gas and it also depends on the experimental temperature.
For gases like H2, He etc., the value of PV increases with pressure right from the beginning. No depression appears in their curves. Although at 0°C, the PV vs P curves obtained in the case of H2 and He etc. do not have any depression or concave part, they become identical to those obtained for gases like N2 and O2 etc.
When PV values for H2 and He is measured at temperatures below their respective Boyle temperatures. PV vs P isotherms at different pressures upto 1 atm are found to be straight lines but they are not parallel to the P -axis.
Keeping the temperature fixed at 0°C, when PV vs P curves for mol of different real gases at various pressures upto 1 atm are drawn and extrapolated, it is observed that all the straight lines meet the PV-axis at 22.424 L-atm value at P = 0 J. This value of PV for any real gas is equal to the value of PV for 1 mol ideal gas at STP.
So, at extremely low pressure (P→ 0), real gases exhibit ideal behaviour

Deviations Concerning pressure:
For real gases, the deviations from ideal behaviour concerning pressure can be studied by plotting pressure vs volume at a given temperature. If we plot experimental values of pressure vs volume at constant temperature (i.e., for real gas) and p theoretically calculated values from Boyle’s law (i.e., for ideal gas) the two curves do not coincide.
From this plot, we observe that at very high pressure, the volume of the real gas is more than that of an ideal gas. Pressure, these two volumes approach each other.
Deviation from ideal behaviour and Boyle temperature:
For a given gas, if PV vs P curves at different fixed temperatures are drawn, then it is found that with the increase in temperature, the depths in the curves gradually decrease (curves become more and more flat) along with the simultaneous shifting of the minimum values of PV to the left.
For every gas, there is a certain temperature, characteristic of the gas, at which the PV-P coincides with the line ofthe ideal gas over an appreciable range of pressure. This temperature is called the Boyle temperature of the gas.
This is so called because the PV value for the gas remains constant over the range of pressure indicating that the gas obeys Boyle’s law.

Boyle temperature
For any real gas, there is a certain temperature, characteristic of the gas, at which the gas follows the ideal gas laws over a wide range of pressure.
This temperature is called the Boyle temperature ( TB) or Boyle point of the gas. The Boyle temperature for gas following the van der Waals equation is given by the relation given by, \(T_B=\frac{a}{R b}\)
Alternative definition:
The temperature at which the plot of PV vs P for areal gas results in a line parallel to the P-axis over an appreciable range of pressure, is called the Boyle temperature of the gas.
Alternatively, the particular temperature at which the value of PV for a real gas becomes constant over an appreciable range of pressure is called the Boyle temperature of that gas. -Ideal gas -Real gas V-
The values of Boyle’s temperature are different for different gases. For example—the Boyle temperatures of H2 and N2 are -156°C and 50°C, respectively. Generally, the liquefaction of a gas becomes easier for a gas having a higher Boyle temperature.
Boyle temperatures for most of the gases are found to be higher than the ordinary temperature. For this reason, a concave region is found in the PV vs P plots of N2 O2 CH4 etc. gases at ordinary temperature.
On the other hand, such kind of concave region is absent in the case of the PV vs P plots of H2 and He gases as their Boyle temperatures are much lower than the ordinary temperature. However, such plots for H2 and He gases at temperatures below their respective Boyle temperatures exhibit minima.
Conclusion:
A real gas behaves like an ideal gas at very low and high temperatures. Exhibits a large 10- 10-pressure deviation from ideal behaviour at very high pressures and low temperatures. Behaves like an ideal gas at its Boyle temperature over a wide range of pressure.
Compressibility factor of real gases
Looking at the reveals that in the high-pressure region, for a given value of pressure, the value of PV for a real gas is larger than that for an ideal gas. On the other hand, in the low pressure, region at a given pressure, the value of PV is smaller than that for an ideal gas.
The different values of PV for a real gas and an ideal gas imply that a real gas deviates from the behaviour of an ideal gas. The extent of deviation of a real gas from the ideal behaviour is usually expressed in terms of a quantity, called the compressibility factor.
The equation of state for n mol of an ideal gas, PV = nRT. However, the equality of PV and nRT does not hold well for a real gas so PV nRT. Let, in the case of a real gas PV = Zx nRT; where Z= compressibility factor. Therefore
⇒ \(\mathrm{Z}=\frac{P V}{n R T}\)
For an ideal gas, PV = nRT, hence Z = 1 For areal gas, PVnRT, hence Z ≠1
Let at pressure P and temperature T, the volume for nmol of an ideal gas be V1 and that forn mol of a real gas be V.
For an ideal gas, Z = 1, so, 1 =\(\frac{P V_i}{n R T} \text { or, } n R T=P V_i\)
=\(\frac{P V_i}{n R T} \text { or, } n R T=P V_i\)
∴ \(Z=\frac{P V}{n R T}=\frac{P V}{P V_i}=\frac{V}{V_i}\)
⇒ \(\frac{\text { at a given pressure and temperature the volume of nmol of a real gas}}{\text { the volume of n mol of an ideal gas at the same pressure and temperature } { }}\)
When V = Vi, Z = 1; i.e., the real gas behaves like an ideal gas.
When V> Vi, Z> 1; i.e., under the same conditions of temperature and pressure, the real gas is less compressible than the ideal gas and it deviates from ideal behaviour. This type of deviation is called positive deviation.
When V<Vi, Z <l; i.e., under the same conditions of temperature and pressure, the real gas is more compressible than the ideal gas and it deviates from ideal behaviour. This type of deviation is called negative deviation.
Therefore, when a real gas deviates from the ideal behaviour, its value of Z becomes greater than or less than 1. Z vs P plots for several reed gases at 0°C are given below From the plots, it is found that for N2, CO2, CH2tc., the values of Z at very low pressures are very close to 1 Hence at very low pressures, these gases behave nearly like ideal gas.

Causes of deviation of real gases from ideal behaviour
The Dutch scientist van der Waals explained the causes of the deviation of real gases from ideal behaviour. He mentioned two faulty assumptions ofthe kinetic theory ofgases. These are-
- In the kinetic theory of gases, the gas molecules are considered point particles of very small dimensions, and the total volume occupied by them is negligible compared to the volume of their container.
- But actually, a gas molecule always has a finite volume although it is extremely small. Thus, in a real gas, the actual volume available to the molecules for free movement is somewhat less than the volume of the container in which the gas is kept.
- According to the kinetic theory of gases, the molecules in a gas do not experience any intermolecular forces of attraction. But, this is not true as this is evident from the fact that a real gas always exerts less pressure than that calculated for an Ideal gas under an identical set of conditions.
The gas molecules in a real gas occupy a certain volume and they do feel intermolecular forces of attraction. By applying pressure or decreasing temperature, a gas can be converted into a liquid or a solid. Since a liquid or a solid has molecules in a gas, then it would not be possible to condense a gas into a liquid or a solid.
Difference Between Ideal gas and Real gas:

Explanation of the plot of \(Z\left(=\frac{P V}{n R T}\right)\) vs P:
The reasons for the deviation of real gases from ideal behaviour are— low-pressure region, the effect of intermolecular attractive forces predominates over the effect of the volume of gas molecules der Waals equation van der Waals pointed out two faulty assumptions in the kinetic theory of gases as the cause for the failure of real gases to obey the Ideal gas equation.
These faulty assumptions are -volume assumptions and pressure assumptions. He modified the equation, PV m to rectify those two defects and presented a revised equation which Is widely known as the van Dor Winds equation.
Volume correction:
Let us consider, n moles of a real gas enclosed in a container of volume V at temperature 7’K. As each ofthe gas molecules occupies a definite volume the entire space in the container Is not available to them for free movement. So, the space available for free movement will be somewhat less than (the volume of the container, if the total volume of 1-mole molecules is then the volume for n moles molecules will be’ nb Here the term ‘b’ Is called volume correction term or co-volume or excluded volume.

Therefore, the corrected volume available for the motion ofthe molecules = (V -nb).
The quantity ‘b‘ is related to the actual volume of the gas molecules. It can be shown by calculation that the value of ‘b’ is four times the total volume of mole molecules. If the radius of each gas molecule is r, then, the volume of each molecule \(\frac{4}{3} \pi r^3\).
∴ The total volume of 1 mol molecules
⇒ \(N \times \frac{4}{3} \pi r^3[N=\text { Avogadro no. }]\)
Therefore \(b=4 \times N \times \frac{4}{3} \pi r^3\)
Pressure correction:
According to the kinetic theory of gases, there exist no attractive forces among the molecules in a gas. But, it has been proved by different experiments that intermolecular attractions exist among the gas molecules. A molecule (A) in the bulk of a gas is surrounded by other molecules and attracted equally from all directions.
Consequently, the net force on this. The molecule is zero. However, the situation is different for molecule (B) near the wall of the vessel. Here, the molecule ‘B’ is attracted towards the centre by the molecules inside the vessel. So, the force with which the molecule strikes the wall is less than that with which it would strike if there were no intermolecular forces of attraction. Thus the observed pressure, (P) of the gas will be lower than that calculated for an ideal gas Pi.

Hence, the ideal pressure (Pi) can be obtained if we add a correction term (Pa) to the observed pressure. Therefore, P. = Pi+ Pa Here, P a is the measure of cohesive force due to the intermolecular forces of attraction. It can be shown by calculation, Pa = (n²a)/V²; where n and V are the moles and the volume of the gas respectively and a = constant.
⇒ \(\left(P+\frac{n^2 a}{V^2}\right)\) or Pi and (V- nb)
For the ideal gas equation PiVi = nRT, we get, reduce
⇒ \(\left(P+\frac{n^2 a}{V^2}\right)(V-n b)=n R T\)
This is the van der Waals equation for nmol of real gas
If n=1, then \(\left(P+\frac{a}{V^2}\right)(V-b)=R T\)
This is the van der Waals equation for 1 mol of areal gas. In this equation, P and V are the pressure and volume of 1 mol of a real gas, respectively, at temperature, TK.
The explanation for the deviation of real gases from ideal behaviour by the van der Waals equation
Amagat’s curves indicate the deviation of real gases from ideal behaviour. The nature of Amagat’s curves as well as the behaviour of a real gas at different temperatures and pressures can be explained by the van der Waals equation.
For mol of a real gas, the van der Waals equation is:
⇒ \(\left(P+\frac{a}{V^2}\right)(V-b)=R T\)
let us see the forms of this equation in the following special cases
At very low pressure:
At very low pressure, as the volume (V) of a gas is very large, the value of ‘b’ Is negligible compared to V i.e., {V- b) « V. So, the van der Waals equation becomes-
⇒ \(\left(P+\frac{a}{V^2}\right) V=R T \quad \text { or, }\left(P V+\frac{a}{V}\right)=R T \text { or, } P V=R T-\frac{a}{V}\)
Thus, at a very low pressure, the value of PV is less than that of RT. Also, with pressure increases, the volume (V) of a gas decreases, and hence the value of (a/V) increases. As a result, the value of PV decreases with an increase in pressure. In Amagat’s curves for gases like N2, CO2, CH4 etc., the initial decrease in the value of PV with an increase in pressure can thus be explained.
At very high pressure:
At very high pressure, the volume (V) of a gas is very small, and the value of the gas cannot be neglected in comparison to V. But the value of a/V2 is very small in comparison to P, and hence \(\left(P+\frac{a}{V^2}\right) \approx P\)
Therefore, the van der Waals equation reduces to P(V- b) = RT or, PV = RT+Pb From this equation, it is seen that the value of PV is greater than RT. The value of Pb increases with an increase in pressure. Consequently, the value of PV goes on increasing. This explains the continuous increase of PV with pressure in the Amagat’s curves, for gases like N2, CO2, CH4 etc.
Exceptional behaviour of H2 and He gases:
Because of the very small molar masses of H2 and He, the strength of molecular forces attracting these gases is very weak, and hence the value of V2 at any value of P can be neglected. So, the van der Waals equation for these gases can be expressed as P(V-b) = RT or, PV = RT+Pb. This equation shows that the value of PV is always greater than RT. Thus, PV for H2 and He increases linearly with pressure from the very beginning.
Causes (or ideal, behaviour of real gases at very low pressure and high temperature:
At a very low pressure, the volume ( V) as well as the intermolecular distances among the gas molecules is very large. As a result, the intermolecular forces of attraction become very weak, making the value of ‘a’ negligible.
Thus, the value of the term a/V2 becomes negligible at very low pressure because of the very large value of V and the very small value of a So, the term a V2 can be ignored in comparison to P. The term b can be neglected in comparison to V because of the large value of V.
Therefore, at very low pressure \(P+\frac{a}{V^2} \approx P \text { and } V-b \approx V \text {. }\) In this condition, the van der V2 Waals equation reduces to PV = RT Thus at very low pressure, areal gas behaves like an ideal gas.
At a very high temperature, the volume of a gas becomes very large and the average kinetic energy of the molecules of the gas becomes very high. Hence the intermolecular forces of attraction become insignificant. As a result, the term \(\frac{a}{V^2}\) in comparison to very high temperature therefore \(P+\frac{a}{V^2} \approx P \text { and } V-b \approx V\) So, at a very high temperature, van der Waals equation reduces to PV = RT and a real gas exhibits ideal behaviour.
van der Waals constants
The terms ‘a ‘ and ‘b’ in the van der Waals equation are called van der Waals constants. The values of these constants depend on the nature of a gas.
Significance of van der Waals constants: Significance of a: ‘a’ measures the magnitude of intermolecular forces of attraction in a gas. The stronger the intermolecular forces of attraction in a gas, the larger the value of the gas.
A gas with a larger value can be liquefied easily. Hence, the larger the value of a gas, the greater the ease of its liquefaction.
Significance of a:
The term ‘b’ for a gas gives us an idea of the size of the molecules ofthe gas. The larger the value of ‘b’ the larger the size of the gas molecules, and hence lower the compressibility ofthe gas.
Units of and ‘h’: The units of and are as follows—
Unit of a:
van der Waals equation for moles of a real gas,
⇒ \(\left(P+\frac{n^2 a}{V^2}\right)(V-n b)=n R T\)
As the term (n2a)/v2 is added to the pressure term, its unit will be the same as that ofthe of the pressure. Therefore, the unit of \(\frac{n^2 a}{V^2}\)\frac{n^2 a}{V^2} = unit of pressure
∴ Unit of ‘a’ = unit of pressure \(\text { unit of pressure } \times \frac{(\text { unit of volume })^2}{(\text { number of mole })^2}\)
Unit of b: As nb is subtracted from the volume, its unit will be the same as that ofthe volume. Therefore, Unit of nb = unit of volume
∴ \(\text { Unit of } b=\frac{\text { unit of volume }}{\text { number of mole }}=\mathrm{L} \cdot \mathrm{mol}^{-1}\)
Unit of ‘a’ atm- L² mol-2 Unit of ‘b’ : L.mol-1
Van der Waals constant of some common gases

Liquefaction Of Gases
Liquefaction of a gas is the physical transformation of the gas into its liquid state. By increasing pressure or decreasing temperature, a gas can be converted into its liquid state.
Fundamentally, the gaseous state and liquid state are different for their intermolecular distances.
- Let the molecules gaseous state be widely separated from each other but in a liquid state, they are comparatively close to each other. So, to liquefy a gas, the molecules must be brought closer to each other.
- It is possible to bring the molecules closer the pressure ofthe gases increases or their temperature decreases.
- Due to an increase in pressure, the gas molecules come closer to each other and they come under the influence of strong attractive forces.
- As a result, the gas changes into a liquid state.
On the other hand, due to a decrease in temperature, the average kinetic energy of the gas molecules decreases, which, in turn, increases the strength of intermolecular forces of attraction.
- This causes, the gas molecules to get closer to each other, and ultimately the gas changes into the liquid state.
- Andrews discovered the conditions necessary for the liquefaction of a gas. He stated that there is a particular temperature for every gas above which it cannot be liquefied no matter how high the pressure is.
- This temperature is called the critical temperature ofthe gas.
- The liquefaction ofa gas is possible only when the gas exists at or below its critical temperature.
Critical Temperature:
Every eas has a certain characteristic temperature above which the gas cannot be liquefied even if the pressure is very high. This particular temperature is called the critical temperature of the gas.
It is denoted by Tc. The liquefaction is easier for gases with high critical temperatures.
- Critical pressure: The minimum pressure required to liquefy a gas at its critical temperature is termed the critical pressure (Pc) of the gas.
- Critical volume: The volume occupied by 1 mol of a gas at its critical temperature and critical pressure is termed the critical volume (Vc) of the gas.
- Critical Constants: The values of critical temperature (Tc), critical pressure (Pc) and critical volume (Vc) are constant for a particular gas. Thus, Tc, Vc and Pc are called critical constants.
Critical temperature and pressure of some gases

The critical temperature is low for a gas whose intermolecular forces of attraction are weak. For example, the values of critical temperatures for H2 He, O2 etc. are very low because of their weak intermolecular forces of attraction.
The critical temperature is high for a gas whose intermolecular forces for attraction are strong. For example, the critical temperatures for CH4, NH3, CO, etc. are very high because of their very strong intermolecular forces of attraction.
The behaviour of a gaseous substance at its critical state Andrews was the first to investigate the behaviour of a gas in the neighbourhood of its critical temperature. He carried out his experiment on CO2 gas and studied the variation of volume of CO2 gas with pressure at different constant temperatures. Each curve corresponds to a constant temperature and is called an isotherm. It is evident that some isotherms lie above 31.1 °C, some below it and one at 31.1°C.

Isotherms below 31.1°C:
These isotherms have three parts. Let us consider the isotherm, ABCD corresponding to temperature Tl.
- The first part of this isotherm i.e., line AB indicates the change in volume of CO2 gas with a change in pressure.
- Thus, AB corresponds to the gaseous state of CO2. At point B, CO2 gas begins to liquefy and thereafter the pressure of the system remains the same although the volume of the system goes on decreasing.
- Along line BC, the transition of CO2 gas to liquid CO2 takes place, and as a result, the pressure of the system remains constant.
- The line BC denotes the coexistence of gas & liquid. At point C, CO2 completely convert into a liquid state.
At constant temperature, the liquid does not suffer any significant change in volume with pressure, so the volume change of liquid CO2 with the increased pressure is extremely small, as shown by the die steep line CD.
As the temperature is raised, the horizontal portion is gradually shortened until the temperature of 31.1 °C is reached at which it reduces to a point (C). This state is referred to as the critical state of CO2 gas.
Isotherms at 31.1°C:
In the isotherm obtained at 31.1°C, die horizontal portion vanishes and is reduced to a point. At or below 31.1°C, CPO2 gas can be transformed into liquid by applying pressure. Thus, the liquefaction of CO2 cannot be caused at any temperature above 31.1°C whatever the magnitude of applied pressure may be. Hence, 31.1°C is the critical temperature of CO2.
Isotherms above 31.1°C:
P-V isotherms above 31.1°C are approximately rectangular hyperbolic. In these conditions, CO2 gas behaves more or less as an ideal gas.
Conditions for liquefaction of gases:
Two conditions must be fulfilled for the die condensation of a gas into a liquid.
These are— die temperature of the gas must be brought equal or below the critical temperature and necessary pressure is to be applied on the gas keeping the nature ofthe gas equal or below its critical temperature.
An ideal gas cannot be liquefied due to the absence of intermolecular forces of attraction in it.
Important points related to the critical state of a gas:
Above the critical temperature, due to the very high average kinetic energy of molecules, attractions, between die molecules become negligible. As a result, it is not possible to bring the molecules closer to each other, which is necessary for the condensation of a gas.
Hence, a gaseous substance cannot be liquefied above its critical temperature even by applying very high pressure.
At the critical point, die densities of both the gaseous and liquid states of a substance become equal, and the surface of separation between these two phases disappears.
- If a substance remains above its critical temperature then the substance is called a gas. And if a substance remains below its critical temperature, then the dead substance is called a vapour.
- Thus, vapours can be liquefied only by applying pressure, but not a gas.
- To liquefy a gas, at first, the temperature ofthe gas should be brought below its critical temperature.
- The gas can then be converted into a liquid by applying the necessary pressure.
- Critical temperatures of some permanent gases such as N2, O2, He etc. are much lower than ordinary temperatures.
- Therefore, at ordinary temperatures, these gases cannot be liquefied by applying pressure.
- On the other hand, as the critical temperatures of gases like CO2, CH4 etc. are higher than ordinary temperatures, these gases can be liquefied easily at ordinary temperatures by applying pressure
The expressions of critical temperature (Tc), critical pressure (Pc) and critical volume ( Vc) for a gas obeying van der Waals equation are as follows—
⇒ \(\begin{array}{|c|c|c|}
\hline V_c=3 b & P_c=\frac{a}{27 b^2} & T_c=\frac{8 a}{27 R b} \\
\hline
\end{array}\)
∴ \(\frac{R T_c}{P_c V_c}=\frac{8}{3} \approx 2.66\)
⇒ \(\frac{R T_c}{P_c V_c}\) is called coeffiecient.
Continuity of state
We have already seen that at the critical temperature, there is no difference between the gas and the liquid phases in the P vs V diagram of CO2 because the surface of separation between these two phases disappears at the critical temperature.
However, an isotherm at a temperature below critical temperature has a horizontal portion along which the gas and the liquid phases coexist. This indicates that the transformation of gas into liquid is a discontinuous process.
However, a close examination of an isotherm below the critical temperature reveals that it is possible to convert the gas into liquid or vice versa without any discontinuity. The phenomenon of continuous transition from gas to liquid or liquid to gas phase is called continuity of state.
The following illustrates the continuous transition from gas to liquid or liquid to gas phase. The linesEGF and ABCD indicate the respective P vs V isotherms at critical temperature (Tc) and a temperature below the critical temperature (< Tc). Any point inside the parabolic portion indicates the coexistence of gas and liquid of a substance.
On the other hand, any point outside the parabolic portion represents either the gaseous or liquid state of the substance. Initially, CO2 is present at the gaseous state indicated by point A. The gas is then heated at constant volume till its pressure increases to a value corresponding to the point H. It is then the point H.

It is then cooled at the same pressure until its volume decreases to a value corresponding to point D. But at point D, CO2 exists in its liquid state. Thus, gaseous CO2 is converted into liquid CO2 without any discontinuity because during this transition co-existence of gas and liquid phases does not occur. Hence, there exists a continuity of state during the transition from gas to liquid. The continuity of state is also found to exist during the transition from liquid to gas.
Numerical Examples
Question 1. 2 mol of a van der Waals gas at 27 °C occupies a volume of 20 L. What is the pressure of the gas? [a = 6.5 atm-L²-mol-2; b = 0.056 L-mol-1]
Answer:
Van der Waals equation for mol of a real gas: \(\left(P+\frac{n^2 a}{V^2}\right)(V-n b)=n R T\)
Given, n = 2 mol, V = 20L, T = (273 + 27) = 300 K, a=6.5 atm-L-2mol-2 and b = 0.056 L-mol-1
∴ \(P=\frac{n R T}{V-n b}-\frac{n^2 a}{V^2}=\frac{2 \times 0.0821 \times 300}{(20-2 \times 0.056)}-\frac{(2)^2 \times 6.5}{(20)^2}
\)
= (2.477 -0.065)atm
= 2.412 atm
Question 2. A container with a volume of 5 L holds lOOg of CO at40°C. For CO2 gas, a =3.59 L2 -atm-mol2 and b = 4.27× 10-2 L mol-1 . Determine the pressure of CO2 gas. How much does this value differ from that calculated by using the ideal gas equation?
Answer:
Van der Waals equation for mol of a real gas:
⇒ \(\left(P+\frac{n^2 a}{V^2}\right)(V-n b)=n R T\)
Given, V=5L, T= (273 + 40) =313K \(n=\frac{100}{44}=2.27 \mathrm{~mol}\)
a = 3.59 L2-atm-mol-2 & b = 4.27 x 10-2 L.mol-1
∴ \(P=\frac{n R T}{V-n b}-\frac{n^2 a}{V^2}\)
⇒ \(=\frac{2.27 \times 0.0821 \times 313}{\left(5-2.27 \times 4.27 \times 10^{-2}\right)}-\frac{(2.27)^2 \times 3.59}{(5)^2}\)
=(11.9-0.74)atm = 11.16 atm
Substituting the given values of V, T and n in the ideal
gas equation PV = nRT we get,
⇒ \(P=\frac{n R T}{V}=\frac{2.27 \times 0.0821 \times 313}{5}=11.66 \mathrm{~atm}\)
∴ The pressure of CO2 obtained from the van der Waals equation is less than that calculated from the ideal gas equation by (11.66-11.16) = 0.50 atm.
Question 3. 2 mol of gas is kept in a 4-litre flask. The pressure of the gas at 300K is 11 atm. If the value of the gas is 0.05 L-mol_1, then determine the value by using the van der Waals equation
Answer:
Van derWaals equation for’n’ mol of areal gas:
⇒ \(\left(P+\frac{n^2 a}{V^2}\right)(V-n b)=n R T\)
Given, P = 11 atm, V = 4 L, T = 300 K, n = 2 mol, b = 0.05 L.mol-1
∴ \(\left[11+\frac{(2)^2 \times a}{(4 \mathrm{~L})^2}\right][4-2 \times 0.05]=2 \times 0.0821 \times 300\)
or, (11 + 0.25 × a)(3.9) = 49.26 or, 11 + 0.25 × a = 12.63
∴ a= 6.52 L²-atm.mol-2
Liquid State
The liquid state is the intermediate state between the solid and gaseous states. Liquids have several properties similar to solids and gases. Like gases, liquids are isotropic. The molecules in a liquid are in continuous random motion.
So a liquid does not have a definite shape and flows like a gas. However, liquids maintain a fairly constant density like solids. Hence, liquids are incompressible like solids.
The molecules in a liquid remain ordered, somewhere between the molecular order of a solid and the molecular randomness of a gas. In liquids, the short-range ordered state extends up to a few molecular distances, whereas in solids, it extends to long-range order.
Properties Of Liquids
Shape Of A Liquid
Liquids do not have a definite shape; they take up the shape ofthe container in which they are kept.
Reason:
In liquids, the intermolecular forces of attraction are not strong enough to hold the molecules at fixed positions and thus allow them to move past one another. Hence, liquids do not have a definite shape.
Volume Of A liquid
Liquids have a definite volume. If some of the liquid is placed in a container of any shape (like a beaker, conical flask, test tube etc.), the volume of the liquid in the container will be exactly 10 mL. Like gases, liquids do not occupy the entire volume ofthe container.
Reason:
In liquids, molecules are always in motion but the movements are not as random as gas molecules. There exist stronger intermolecular forces of attraction in liquids compared to gases.
Due to this, molecules in a liquid are not widely separated from each other. Hence, liquid molecules are bound firmly but not rigidly and do not occupy the total volume ofthe container.
Density of a liquid
At ordinary temperature and pressure, the density of a liquid is much higher than that of a gas. On the other hand, the density of a substance in the liquid state is only about 10% lower than the tilting solid state.
Reason:
As the intermolecular forces of attraction are stronger in liquids than in gases the molecules are more closer to each other than the molecules in gases. This results in a little space between the molecules in liquids. This is why liquids are denser than gases.
Compressibility Of A Liquid
Liquids are much less compressible than gases. The change in the volume of liquid due to the application of pressure on it is found to be negligible.
Reason:
As the space between the molecules of liquid is very small, sufficient free space is not available for its compression. This is why the application of pressure on a liquid cannot reduce its volume appreciably.
Diffusion of a liquid
Molecules of both gases and liquids are always in random motion. Consequently, they move from one place to another. Hence, diffusion properties are shown in both gases and liquids. However, liquids diffuse very slowly compared to gases.
Reason:
As the intermolecular distances in liquids are smaller than those in gases, the molecules in a liquid suffer a large number of collisions when they move about, and hence take a longer time to move from one place to another. Hence, the diffusion of liquids occurs slowly.
Evaporation of a liquid
The gaseous state of a liquid is called its vapour. Evaporation is a process by which molecules of a liquid escape from the surface of the liquid into the gas phase. Drying of a floor after it is mopped, the gradual drying of damp clothes, the drying of water from the pond or lake in summer etc. are examples of evaporation.
Although evaporation is a slow process, some liquids have a higher rate of evaporation at ordinary temperatures. They are called volatile liquids. Ether, chloroform, carbon tetrachloride, acetone etc. These are examples of volatile liquids. On the other hand, liquids that have a very low rate of evaporation are called non-volatile liquids. Glycerol, mercury etc. are some examples of volatile liquids.
Reason for evaporation:
During evaporation, the liquid molecules at the surface of a liquid leave the liquid and go into the gaseous phase.
- The molecular interpretation of this phenomenon is as follows. Like gases, all the molecules in a liquid do not have the same kinetic energy.
- In a liquid, there is a distribution of kinetic energies of the molecules as there is a gas, A molecule at the surface of a liquid leaves the liquid when it overcomes the forces of attraction of its neighbour.
- To overcome the forces of attraction, the molecule needs to have a kinetic energy equal to or greater than a minimum kinetic energy.
- Only those molecules at the surface of the liquid will leave the liquid and go to the vapour phase, which has kinetic energies greater than or equal to the minimum kinetic energy necessary for overcoming forces of attraction.

This process in which molecules at the surface of a liquid escape and go to the vapour phase by overcoming the forces of attraction oftheir neighbours is called evaporation of liquid.
Evaporation causes cooling:
- During evaporation, only the molecules having kinetic energies greater than the characteristic kinetic energy (£) escape from the liquid surface.
- Therefore, the average kinetic energy of molecules left in the liquid decreases. As a result, the temperature of the liquid decreases.
- Thus liquid cools down during its evaporation.
Factors affecting the rate of evaporation: Factors on which the evaporation of a liquid depends are as follows:
Nature of liquid:
The rate of evaporation of a liquid depends on intermolecular forces of attraction in the liquid. This rate is slow for a liquid with a strong liquid with a weak force The strength of the intermolecular force of attraction In ethyl alcohol is weaker than that In water, but It is stronger than that in ether.
Consequently, at a given temperature, the evaporation of ethyl alcohol Is faster than that of water but slower than that of ether.
Temperature:
The rate of evaporation of a liquid Increases as temperature rises. With the temperature rise, the fraction of molecules having kinetic energy greater than characteristics kinetic energy (above which the liquid molecules can escape from the liquid surface) Increases. Therefore, the rate of evaporation increases with the temperature rise.
The surface area of the liquid:
The evaporation of a liquid occurs only from its surface. Therefore, the larger the surface area of a liquid, the greater the rate of Its evaporation.
The flow of air:
The rate of evaporation of a liquid increases when a current of air is blown across die surface of the liquid. The current of air carries away the molecules in the vapour phase of the liquid and prevents them from going back to a liquid state. This leads to faster evaporation ofthe liquid. This is why, wet clothes dry up faster when air blows rapidly.
Vapour pressure of a liquid
The evaporation of a liquid occurs from its surface at all temperatures. If a liquid is kept in a closed vessel, the vapours cannot escape from the vessel, and the vapour molecules are trapped in the space over the liquid surface.

The vapour molecules in the space of the vessel are always in random motion, so they collide with one another, with the walls ofthe container and with the liquid surface. Of the vapour molecules striking the liquid surface, those which have lower kinetic energies are recaptured by intermolecular forces of attraction in liquid.
This transfer of molecules from the vapour to the liquid phase is called condensation. Initially, the rate of condensation is slower than the rate of evaporation as the amount of vapour over a liquid surface is very small.

Gradually, the amount of vapour increases over the surface of the liquid. With increasing the amount of vapour, the rate of condensation also increases as the number of gas molecules colliding with the surface of the liquid increases.
After a certain time, the rate of evaporation and the rate of condensation become equal and an equilibrium is established between the two opposite processes, viz., evaporation and condensation. At equilibrium, the amount of liquid and that of vapour become invariant with time.
The vapour that exists over the liquid surface at equilibrium is called saturated vapour because this is the maximum amount of vapour that can be present in the space over the liquid surface at the experimental temperature. The pressure exerted by the saturated vapour is called the saturated vapour pressure or simply the vapour pressure at that experimental temperature.
Vapour Pressure
At a given temperature, the pressure exerted by a vapour in equilibrium with its liquid is called the vapour pressure of the liquid at that temperature
At a definite temperature, the vapour pressure of a liquid is always constant. Factors affecting the vapour pressure of a liquid:
Nature of the liquid:
The vapour pressure of a liquid depends on its volatility.
- The more volatile a liquid is, the higher the vapour pressure of the liquid. The volatility of a liquid depends on its rate of evaporation, which in turn depends on the intermolecular forces of attraction in the liquid.
- A volatile liquid possesses weak intermolecular attractions and has a high rate of evaporation, and hence high vapour pressure. Consequently, at a particular temperature, the vapour pressure of a volatile liquid is greater than that of a non-volatile liquid.
- The rate of evaporation of diethyl ether, ethyl alcohol and water at a definite temperature follows the sequence—diethyl ether > ethyl alcohol > water. Thus at a particular temperature, the order of their vapour pressures is—diethyl ether > ethyl alcohol > water.
Temperature:
The vapour pressure of a liquid increases with the increase in temperature. Because, with the increase in temperature, more and more liquid molecules escape into the vapour phase, thereby increasing, the amount of vapour over the liquid surface. As a result, the vapour pressure ofthe liquid increase

Boiling Of A Liquid
A liquid evaporates at any temperature. If the temperature of a liquid is increased gradually by applying heat, the rate of evaporation increases, and consequently the vapour pressure of the liquid also increases.
When the temperature of the liquid reaches a value at which the vapour pressure becomes equal to the external pressure, the bubbles of vapour are formed inside the liquid.
A very small (infinitesimal amount) increase in temperature will be sufficient for the bubbles of vapour to rise freely to the surface and escape into the air. This phenomenon is called the boiling of a liquid.
Boling Of A liquid:
Process vaporisation of a liquid is accompanied by the rapid formation and growth of bubbles of vapour that break outward through the surface of the liquid.
Boiling Point Of Liquid:
The Temperature at which the vsp[our pressure of a liquid becomes equal to the external pressure is termed as the boiling temperature or boiling of that liquid.
The normal Boiling point of a liquid:
The Temperature at which the vapour pressure of a liquid becomes equal to the normal atmospheric pressure (1 atm) is termed the normal boiling point of that liquid.
For example, the normal boiling point of water is 100°C. This means that at 100°C, the vapour pressure of water becomes equal to the atmospheric pressure. Water under atmospheric pressure (1 atm) starts boiling at this temperature.
Effect of external pressure on the boiling point of a liquid:
The boiling point of a liquid can be altered by changing external pressure. The higher the external pressure, under which a liquid exists, the higher the boiling point of the liquid.
Explanation:
The vapour pressure of a liquid becomes equal to the external pressure at its boiling point. If the external pressure is high, then it is necessary to heat a liquid at a higher temperature to make its vapour pressure equal to the external pressure so that it can start boiling.
Conversely, if the external pressure is low, then heating a liquid at a lower temperature will make its vapour pressure equal to the external pressure, thereby causing the liquid to boil.
Difference between boiling and evaporation:
Both boiling and evaporation involve the transformation of liquid into vapour. Although it would seem that evaporation and boiling might be the same process they differ in some respects.
The differences are as follows:
- Evaporation occurs only at the surface of a liquid, whereas boiling involves the formation of bubbles inside the liquid.
- Evaporation occurs slowly, whereas boiling occurs rapidly.
- Evaporation occurs at any temperature, whereas boiling occurs at a specific temperature.
- During evaporation, the temperature of liquid decreases, whereas during boiling the temperature of a liquid remains the same.
Surface Tension Of A Liquid
- Molecular interpretation of surface tension: The molecules at the surface of a liquid are energetically different from the molecules in its bulk. Let us consider a molecule, ‘A’ inside the liquid.
- The molecule ‘A’ is surrounded by other molecules and thus it is attracted equally from all sides by other molecules. So, die resultant force acting on ‘A’ is zero; that is, there is no unbalanced force acting upon’ A ‘.
- Now consider a molecule ‘ B’ at the surface of the liquid. The molecules lying below it are in liquid state and above it are in the vapour state. The molecule ‘B’ is attracted by the molecules of both liquid and vapour.
- However, as the density of vapour is much less than the density of the liquid, the number of molecules per unit volume of the liquid is much greater than that of vapour.
As a result, the molecule ‘B’ will experience a net downward force acting at right angles along the surface of the liquid.
- Similarly, other surface molecules will also be attracted towards the bulk of the liquid.
- Hence, work is to be done to bring a molecule from the bulk to the surface ofthe liquid against the downward force of attraction.
- As a result, the potential energy of the surface molecule is increased.
- Therefore, a molecule in the bulk has a lower potential energy than a molecule on the surface.
- As the energy of the surface tension of the surface tension molecule is higher, the liquid will try to minimise the energy of the surface layer (surface energy) by decreasing its surface area to a minimum.
This tendency to reduce the surface area originates the concept of a new property of liquid known as surface tension.

Due to this property, the surface of a liquid always remains under tension. This is the molecular interpretation of the surface tension of the liquid.
If we imagine a line on the surface of the liquid,l then because of the tendency of ZZ to reduce surface area, the liquid surface on one side of the line tends to move away from the liquid surface on the other side of the line. As a result, tension acts along the imaginary line from both sides. This tension is termed surface tension.

Surface Tension:
The surface tension of a liquid is defined as the force acting along the surface of the liquid at right angles to an imaginary line of unit length, drawn on the surface of the liquid.
The surface tension of a liquid is denoted by the symbol, γ (Gamma)
Units of surface tension:
Surface tension \(\frac{\text { Force }}{\text { Length }} \text {; }\) So the units of surface tension in CGS system: dyn-cm-1 ; and in SI: N-m-
The surface energy of a liquid:
The surface of a liquid always remains under a state of tension. For this reason, the surface of a liquid tends to contract to the smallest possible surface area.
Thus, it is necessary to do some work to increase the surface area against this tension. This work is ultimately stored on the surface of the liquid as potential energy. This potential energy is termed as the surface energy of a liquid.
The surface energy of a liquid Definition:
The Word Needed To Increase The Unit Area Of The Surface Of A Liquid At a Defined Temperature Is termed The Surface Energy Of That liquid.
Units of surface energy:
Surface energy \(=\frac{\text { Work }}{\text { Area }}\)
So, the unit of surface energy in the CGS system: is erg-cm-2 and in SI: J-m-2.
It can be proved that the magnitudes of surface tension and surface energy per unit area of a liquid are the same. Although the units of these two quantities are different, they are the same. The unit of surface tension in the SI system is N-m-1. In this system, the unit of surface energy is J.m-2 But J-m-2 = N.m.m-2 = N-m-1.
∴ 1 N.m-1 = 1 J.m-2
The unit of surface tension in the CGS system is dyn-cm-1. In this system, the unit of surface energy is erg-cm-2. But erg-cm-2 – dyn-cm-cm-2= dyncm-1
∴ 1 dyn-cm-1= lerg-cm-2
Alternative Definition Of Surface Tension:
The surface tension of a liquid is defined as the amount of work necessary to increase the surface area of the liquid by a unit amount at a constant temperature.
The surface tension of water at 20°C is 7.27 × 10-2 N-m-1. This means, that at 20°C, 7.27 × 10-2 work is needed to increase the surface area of water by m2.
Surface tension of a liquid and intermolecular force of attraction:
For a liquid with strong intermolecular forces of attraction, a great deal of work is required to bring molecules from the interior to the surface i.e., in increasing the surface area of the liquid. The potential energy of the surface molecules will thus be high for such type of liquid.
The surface tension of some common liquids at 20°C

Water has a very high surface tension among the commonly known liquids. Molecules in water remain associated through hydrogen bonds:
Because of this, the intermolecular forces of attraction in water are much stronger than in any other commonly known liquids.
The surface tension of mercury is much higher than that of water:
This is because mercury possesses metallic bonds, which are much stronger than hydrogen bonds. Factors affecting the surface tension of a liquid:
Temperature:
The surface tension of a liquid decreases with the increase in temperature. The average kinetic energy of the molecules In liquid Increases with the increase in temperature, causing the weakening of its intramolecular forces of attraction.
Consequently, the surface tension of the liquid decreases. At the critical temperature, the interface between a liquid and its vapour disappears, and the surface tension of the liquid becomes zero.
Presence of soluble substance in liquid:
- The surface tension of a liquid varies according to the nature of the soluble substance present in it.
- For example, soap detergents, methanol, ethanol etc. are the surface active agents. The addition of such substances to water lowers the surface tension of water. These types of substances prefer to concentrate at the surface.
- The concentration of such a substance in its aqueous solution is found to be more at the surface than in the bulk.
- This causes the surface tension of water to decrease to an appreciable extent. The aqueous solution of soap or detergent can spread like a thin layer due to its very surface tension.
Some typical phenomena related to surface tension:
The spherical shape of the free-falling liquid drop:
- Due to the property of surface tension, the exposed liquid surface behaves like a sheet of rubber stretched on the top of a beaker and always tends to contract to the smallest possible surface area.
- Again, we know the sphere has a minimum surface area for a definite volume. So, in the absence of any external influence, a free liquid drop assumes a spherical shape.
Floating a clean oil-free needle on the surface of water:
- When a clean oil-free needle is carefully placed horizontally onto the surface of water, it is found to be floating on the surface. This happens due to the surface tension of water.
- The surface ofa liquid, due to the surface tension, always tends to be in a contracted state with a minimum space between the surface molecules. This makes the surface impervious to other molecules.
- This is why a clean oil-free needle can float on the surface of water without sinking.
A water drop spreads over the clean glass surface i.e., it can wet the surface ofthe glass. But, if a small amount of mercury is placed on the surface of a glass, it does not wet the glass surface; instead, it forms small beads:
The forces of attraction between the molecules of the same substance are called cohesive forces. The attractive forces acting between the molecules in water or mercury atoms in mercury are cohesive.
- When a substance comes in contact with another substance, the molecules of these two substances attract each other at the surface of contact. The attractive forces between the molecules of different substances are called adhesive forces.
- When water is in contact with a glass surface, the attractive forces between the molecules of water and glass are the adhesive forces. The adhesive forces between the molecules of water and glass are stronger compared to the cohesive forces in water.
- Or in glass. For this reason, a water drop spreads over the glass surface i.e., it can wet the surface of the glass. On the other hand, adhesive forces between the molecules of glass and mercury are weaker in comparison to the cohesive forces in mercury or water.
- For this reason, if mercury is placed on the surface of a glass, it forms small beads on the glass surface instead of wetting the glass.
Capillarity or capillary action:
A tube with a very small internal diameter is called a capillary tube.
- If a capillary tube is dipped into a liquid that wets glass, for example, water, the liquid rises spontaneously in the capillary tube to a certain height.
- This phenomenon is known as capillary action. Surface tension is responsible for this phenomenon.
- The surface of any liquid when kept in a container acquires a curved shape called a meniscus.
- When a capillary tube is partially immersed in a liquid-like water (that wets the glass surface), the water level inside the tube is found to be higher than that outside the tube.
- Also, the meniscus of water is concave.
- The opposite phenomenon occurs in the case of liquids (For example: mercury) that do not wet glass surfaces.
The mercury level inside the capillary tube resides at a lower level than the level outside the tube. The meniscus of mercury is of convex nature.

The forces of adhesion between the glass surface and water are stronger than the forces of cohesion in water or glass.
- To put it another way, water molecules are attracted to the glass surface more strongly than they are attracted to one another. As a result, water rises in the capillary tube and the surface becomes concave.
- On the other hand, the forces of adhesion between glass surface and mercury are weaker than the forces of cohesion in mercury or glass.
- In other words, mercury atoms are attracted to the glass surface less strongly than they are attracted to one another. As a result, the mercury surface becomes convex.
Viscosity of a liquid
If a liquid flows in such a manner that its velocity at any point always remains constant in magnitude and direction, then the flow is called streamline flow. Streamline flow generally occurs when the velocity of a liquid is low.
- When a liquid flows slowly and steadily along a tube i.e., when the flow is streamlined, the liquid can be considered to be made up of stratified layers.
- Among the layers, the layer ofthe liquid in immediate contact with the surface is stationary due to adhesion. The highest velocities are observed in the middle of the tube along its axis. The velocity diminishes as we approach the walls.
- Now, consider two adjacent layers B and C. The layer C is moving with a higher velocity than the layer B because the former is above the latter.
- Because of its higher velocity, layer C tends to increase the velocity of the adjacent lower layer B. Similarly, the slower-moving layer B tends to decrease the velocity of the adjacent upper layer C.
These two layers thus tend to destroy their relative emotions. This means that a dragging force acts on the layers in the backward direction. This force is called viscous force, which arises due to cohesion. The viscous force has a similarity with the frictional force so it is also often called the force of internal friction.

Viscous force resists the flow of a liquid and ultimately stops it. An equal and opposite force ofthe viscous force is to be applied to maintain the flow of the liquid with a constant velocity. If the liquid remains stagnant then viscous force does not act upon it.
Viscosity:
- The viscosity of a liquid is the property of the liquid that resists the flow of the liquid. It is a measure of the ease with which a liquid flows.
- Different liquids have different values of viscosity. The higher the viscosity of a liquid, the lower its tendency to flow. For example, the viscosities of liquids like glycerin, honey, castor oil etc. are high, and hence their flow rates are low.
- On the other hand, the lower the viscosity of a liquid, the higher its tendency to flow. For example, the viscosities of liquids like water, alcohol, ether etc. are low, resulting in high flow rates of these liquids.
Coefficient of viscosity:
Consider a liquid which is flowing steadily and slowly over a flat horizontal surface (streamline flow). The layer just next to the solid surface is at rest due to adhesion.
- With increasing the distances of the layers from the solid surface, the velocities of the layers also increase.
- Let us consider, two layers C and D having velocities v and v + dv, respectively. The distances of these two layers

from the solid surface are x and x + dx respectively. So, the velocity gradient i.e., the rate of change in velocity along the x-axis (axis perpendicular to the flow of liquid) is
⇒ \(\left(\frac{d v}{d x}\right)\) direction opposite to the direction of flow. To maintain the flow, an equal and opposite force to the viscous force is to be applied from outside.
According to Newton’s law of viscous flow, the value of the viscous force (F) is proportional to O the area of the surface in contact (A) and the velocity gradient
⇒ \(\left(\frac{d v}{d x}\right)\)
Therefore \(F \propto A \text { and } F \propto \frac{d v}{d x}\)
∴ \(F \propto A \frac{d v}{d x} \text { or, } F=\eta \times A \times \frac{d v}{d x}\)
Where n (eta) is the proportionality constant called the viscosity coefficient. The value of TJ depends upon the temperature and nature of the liquid.
If A =1 and \(\frac{d v}{d x}=1\) Then according to equation F=n
Viscosity Coefficient: It is the tangential force applied per unit area to maintain a unit velocity gradient between two parallel layers at a constant temperature
Liquids with high viscosity have high values of viscosity coefficients (for example, glycerin, oil, honey etc.). Similarly, liquids with low viscosity have low viscosity coefficients (for example, water, alcohol etc.)
Units of viscosity coefficient:
In the CGS system:
The CGS unit of viscosity coefficient is poise (after the name of the French scientist Poiseuille). From equation [1], we get,
⇒ \(\eta=\frac{F}{A \times \frac{d v}{d x}}=\frac{\text { dyn }}{\mathrm{cm}^2 \times \frac{\mathrm{cm} \cdot \mathrm{s}^{-1}}{\mathrm{~cm}}}=\text { dyn } \cdot \mathrm{s} \cdot \mathrm{cm}^{-2}\)
1 poise =1 dyn.s.cm-2
Poise:
The force in dyne required per 1cm2 area to maintain a velocity difference of 1 cm.s-1 between two parallel layers of a liquid, separated by 1 cm is called 1 poise.
The viscosity coefficients are also expressed in some smaller units like centipoise, millipoise, micropoise etc.
1 centipoise = 10-2 poise,1 millipore = 10-3 poise, 1 microphone = 10-6poise
In the SI system:
The SI unit of viscosity coefficient is poiseuille (PI) From equation [1] we get
⇒ \(\eta=\frac{F}{A \times \frac{d v}{d x}}=\frac{\mathrm{N}}{\mathrm{m}^2 \times \frac{\mathrm{m} \cdot \mathrm{s}^{-1}}{\mathrm{~m}}}=\mathrm{N} \cdot \mathrm{s} \cdot \mathrm{m}^{-2}=\mathrm{Pa} \cdot \mathrm{s}\)
Since 1 N-m-2 = 1 Pa
Since 1 poiseuille or 1 PI =1 N s m-2 = 1 Pa s
1 poise = lady-s-cm-2 = 10-5N-s × (10-2m)-2
= 0.1 N-s-m-2 = 0.1 PI
∴ 1 PI = 10 poise
Factors that influence the viscosity of a liquid:
Intermolecular forces of attraction in a liquid:
Viscosity is the property of a liquid that originates from its intermolecular forces of attraction. The stronger the intermolecular forces of attraction, the higher the viscosity of a liquid.
Example:
At a constant temperature, the viscosity coefficient of ethyl alcohol is greater than that of dimethyl ether. In dimethyl ether, molecules experience dipole-dipole attractive forces in addition to London forces.
In ethanol, molecules form hydrogen bonds. London forces also exist between the molecules in ethanol.
Attractive forces due to hydrogen bonding are much stronger than dipole-dipole attractive forces. So, intermolecular forces of attraction are stronger in ethanol than those in dimethyl ether. This results in a higher value of coefficient of viscosity for ethanol compared to dimethyl ether.
Temperature:
With the increase in temperature, the average kinetic energy of the molecules in a liquid increases, this causes the weakening of its intermolecular forces of attraction which are responsible for the viscosity of the liquid.
For this reason, the viscosity of a liquid decreases with the increase in temperature. Experimentally, it has been found that the viscosity of a liquid decreases approximately by 2% for a 1° rise in temperature.
States Of Matter Gases And Liquids Numerical Examples
Question 1. At 10-3 mm pressure and 300K, a 2L flask contains equal numbers of moles of N2 and water vapour, What is the total number of moles of N2 and water vapour in the mixture? What is the total mass ofthe mixture?
Answer:
⇒ \(n=\frac{P V}{R T}=\frac{\left(10^{-3} / 760\right) \times 2}{0.0821 \times 300}=1.06 \times 10^{-7} \mathrm{~mol}\)
⇒ \(n_{\mathrm{N}_2}=n_{\mathrm{H}_2 \mathrm{O}}=\frac{n}{2}=\frac{1.06 \times 10^{-7}}{2}=5.3 \times 10^{-8} \mathrm{~mol}\)
∴ Total Mass \(m_{\mathrm{N}_2}+m_{\mathrm{H}_2 \mathrm{O}}\)
⇒ \(=\left(5.3 \times 10^{-8} \times 28+5.3 \times 10^{-8} \times 18\right) \mathrm{g}=2.44 \times 10^{-6} \mathrm{~g}\)
Question 2. At constant temperature and 1 atm pressure, an ideal gas occupies a volume of 3.25m3. Calculate the final pressure of the gas in the units of atm, torr and Pa if its volume is reduced to 1.25m2 while its temperature is kept constant.
Answer:
⇒ \(P_2=\frac{P_1 V_1}{V_2}=\frac{1 \times 3.25}{1.25}=2.6 \mathrm{~atm}\)
= \(2.6 \times 760 \mathrm{torr}=1.976 \times 10^3 \mathrm{torr}=2.633 \times 10^5 \mathrm{~Pa}\)
Question 3. The density of a gas at 27°C and 1 atm is 15g-mL-1.At what temperature, will the density of that gas be 10g-mL-1, at the same pressure?
Answer:
⇒ \(\frac{d_2}{d_1}=\frac{T_1}{T_2} \text { or, } T_2\)
= \(\frac{d_1}{d_2} \times T_1=\frac{15}{10} \times 300=450 \mathrm{~K} \text {; }\)
∴ t= 177°C
Question 4. The density of a gas at 30°C and 1.3 atm pressure is 0.027 g- mL-1. What is the molar mass ofthe gas?
Answer:
⇒ \(d=\frac{P M}{R T} \text { or, } M=\frac{d R T}{P}\)
=\(\frac{0.027 \times 0.0821 \times 10^3 \times 303}{1.3}\)
= \(516.6 \mathrm{~g} \cdot \mathrm{mol}^{-1}\)
Question 5. 0.0286 g of a gas at 25°C and 76 cm pressure occupies a volume of 50 cm³. What is the molar mass of the gas?
Answer:
M = \(\frac{g R T}{P V}=\frac{0.0286 \times 0.0821 \times 298}{1 \times 50 \times 10^{-3}}\)
= \( 14 \mathrm{~g} \cdot \mathrm{mol}^{-1}\)
Question 6. The density of a gas at -135°C and 50.66 atm pressure is 2g- cm-3. What is the density ofthe gas at STP?
Answer:
⇒ \(\frac{d_2}{d_1}=\left(\frac{P_2}{P_1}\right) \times\left(\frac{T_1}{T_2}\right)\)
⇒ \(\text { or, } d_2=2 \times \frac{1}{50.66} \times \frac{138}{273}=0.02 \mathrm{~g} \cdot \mathrm{cm}^{-3}\)
Question 7. A gaseous mixture contains 336 cm3 of H2 and 224 cm3 of HE at STP. The mixture shows a pressure of 2 atm when it is kept in a container at 27°C. Calculate the volume of the gas.
Answer:
⇒\(\text { At STP, } 336 \mathrm{~cm}^3 \text { of } \mathrm{H}_2 \equiv \frac{336}{22400} \equiv 0.015 \mathrm{~mol} \text { of } \mathrm{H}_2 \text { and }\)
⇒ \(224 \mathrm{~cm}^3 \text { of } \mathrm{He} \equiv \frac{224}{22400} \equiv 0.01 \mathrm{~mol} \text { of } \mathrm{He} \text {. }\)
⇒ \(V=\frac{n R T}{P}=(0.015+0.01) \times \frac{0.0821 \times 300}{2}=307.8 \mathrm{~cm}^3\)
Question 8. At 100°C a 2-litre flask contains 0.4 g of O2 and 0.6 g of H2. What is the total pressure of this gas mixture in the flask?
Answer:
⇒ \(P=\frac{n R T}{V}=\frac{\left(\frac{0.4}{32}+\frac{0.6}{2}\right) \times 0.0821 \times 373}{2}=4.78 \mathrm{~atm}\)
Question 9. 1.0 g of benzene is burnt completely in the presence of 4.0 g O2 in a completely evacuated bomb calorimeter of volume 1L. What is the pressure inside the bomb at 30°C, if the volume and pressure of water vapour produced are neglected?
Answer:
lg of benzene \(=\frac{1}{78}\) 0.0128 mol of benzene
⇒ \(4 \mathrm{~g} \text { of } \mathrm{O}_2\)
=\(\frac{4}{32}=0.125 \mathrm{~mol} \text { of } \mathrm{O}_2\)
⇒ \(\mathrm{C}_6 \mathrm{H}_6(l)+\frac{15}{2} \mathrm{O}_2(g) \longrightarrow 6 \mathrm{CO}_2(g)+3 \mathrm{H}_2 \mathrm{O}(g) 0.0128 \mathrm{~mol} \frac{15}{2} \times 0.0128 6 \times 0.0128\)
= \(0.096 \mathrm{~mol}=0.0768 \mathrm{~mol}\)
Total number of moles of CO2 produced and O2 remained = (0.0768 + (0.125- 0.096)
⇒ \(P=\frac{n R T}{V}=\frac{0.1058 \times 0.0821 \times 303}{1}=2.63 \mathrm{~atm}\)
Question 10. A closed vessel of fixed volume is filled with 3.2 g O2 at a pressure of P atm and a temperature of Tk. The containers were then heated to a temperature of (T+ 30)K. To maintain the pressure of P atm inside the container at (T+30)K, a certain amount of gas is removed from the container. The gas removed is found to have a volume of 246mL at atm and 27°C. Calculate T.
Answer:
Suppose the number of moles Of O2 removed = n1 mol
∴ \(n_1=\frac{P V}{R T}=\frac{1 \times 246 \times 10^{-3}}{0.0821 \times 300}=9.98 \times 10^{-3} \mathrm{~mol}\)
The number of moles of oxygen that remained in the container
⇒ \(\left(n_2\right)=\left(\frac{3.2}{32}-9.98 \times 10^{-3}\right)\mathrm{mol}=0.09 \mathrm{~mol}\)
Initial state: PV = NRT \(=\frac{3.2}{32} R T=0.1 R T\)
Final state: PV = n2RT = 0.09 X R(T+ 30)
∴ 0.17TT = 0.09 x R(T+ 30); hence, T = 270K
Question 11. The temperature of an ideal gas is 340K. The gas is heated to a temperature at constant pressure. As a result, its volume increases by 18%. What is the final temperature of the gas?
Answer:
⇒ \(\frac{V_2}{V_1}=\frac{T_2}{T_1} \text { or, } \frac{(1.18) V_1}{V_1}=\frac{T_2}{340} ; \text { hence, } T_2=401.2 \mathrm{~K}\)
Question 12. What is the density of air at STP? Assume that air contains 78% N2 and 22% O2 by masses.
Answer:
Average molar masses are –
⇒ \(\frac{28 \times 78+32 \times 22}{100}=28.88 \mathrm{~g} \cdot \mathrm{mol}^{-1}\)
d= \(\frac{P M}{R T}=\frac{1 \times 28.88}{0.0821 \times 273} \mathrm{~g} \cdot \mathrm{L}^{-1}=1.288 \mathrm{~g} \cdot \mathrm{L}^{-1}\)
Question 13. At 100°C and 1 atm pressure, the densities of water and water vapour are 1.0 g-mL-1 and 0.0006 g-mL-1 respectively. What is the total volume of water molecules in a litre of steam at 100°C?
Answer:
Mass of1L of steam = 1000 × 0.0006 g = 0.6 g
Amount of water in 1L of steam = 0.6 g
The volume of water in 1 L of steam
Question 14. The volume of 0.44 g of a colourless oxide of N2 is 224 mL at 273°C and 1530 mm pressure. What is the compound?
Answer:
PV = \(\frac{g}{M} R T\)
Or,M =\(\frac{g R T}{P V}\)
= \(=\frac{0.44 \times 0.0821 \times 546}{\left(\frac{1530}{760}\right) \times 224 \times 10^{-3}}\) 44g.mol-1
The expected compound is N2O.
Question 15. A spherical balloon is filled with air at 2 atm pressure. What pressure is to be exerted on the balloon from the outside so that its diameter will be reduced to half of its initial diameter?
Answer:
⇒ \(\text { Initial state, } 2 \times \frac{4}{3} \pi r^3=n R T \text {; }\)
⇒ \(\text { Final state: } P \times \frac{4}{3} \pi\left(\frac{r}{2}\right)^3=n R T[r=\text { radius of the balloon }]\)
∴ \(P V=n R T ; P \times \frac{1}{8}=2 \text { or, } P=16 \text { atm }\)
Question 16. If 3.2 g of sulphur is heated to a temperature, the sulphur vapour produced occupies a volume of 780 mL at 723 mm pressure and 450°C. What is the molecular formula of sulphur at this state?
Answer:
Let the molecular formula of sulphur is SX.
∴ \(3.2 \mathrm{~g} \text { of sulphur }=\frac{3.2}{32 \times x}=\frac{1}{10 x} \mathrm{~mol} \text { of sulphur }\)
PV=nRT
⇒ \(\text { or, } \frac{723}{760} \times 780 \times 10^{-3}=\frac{1}{10 \times x} \times 0.0821 \times(450+273)\)
∴ The molecular formula of sulphur = Sb.
Question 17. Determine partial pressures of O2 and N2 in air at 0°C and 760 mm Hg pressure. Air contains 78% N2 and 22% O2 by volume.
Answer:
Mole fraction of
⇒ \(\mathrm{N}_2\left(x_{\mathrm{N}_2}\right)=\frac{\text { Partial volume of } \mathrm{N}_2}{\text { Total volume }}=\frac{78}{100}\)
= 0.78
Similarly, mole-fraction of O2(XO2 ) \(\frac{22}{100}=0.22\)
PN2 = XN2 × P = 0.78 × 760mm
Hg = 592.8 mm Hg
PO2 = XO2 × P = 0.22 ×760mm
Hg = 167.2 mm Hg
Question 18. 200 cm3 of N2 gas is collected over water at 20°C and 730 mm pressure. At this temperature, aqueous tension is 14.20 mm. What is the mass of N2 gas collected?
Answer:
PO2 = (730- 14.200mm Hg
Hg = 0.9418 atm
⇒ \( p_{\mathrm{N}_2} \times V_{\mathrm{N}_2} \)
= \(n R T \text { or, } 0.9418 \times 0.2=\frac{w}{28} \times 0.0821 \times 293\)
∴ w = 0.219 g
Question 19. 0.5 g O2 gas is collected over water at 20°C and 730 mm pressure. If aqueous tension at that temperature is 14.20 mm, what is the volume of O2 gas collected?
Answer:
⇒ \(p_{\mathrm{O}_2}=(730-14.20) \mathrm{mm} \mathrm{Hg}=0.9418 \mathrm{~atm}\)
⇒\(p_{\mathrm{O}_2} \times V_{\mathrm{O}_2}=n R T\)
Or, \(0.9418 \times V_{\mathrm{O}_2}\)
= \(\frac{0.5}{32} \times 0.0821 \times 293\)
∴ \(V_{\mathrm{O}_2}=0.399 \mathrm{~L}\)
= 399cc
Question 20. A vessel contains equal masses of CH4 and H2 gas at 25°C. What part of the total pressure inside the vessel is equal to the partial pressure of H2 gas?
Answer:⇒
⇒ \(W_{\mathrm{CH}_4}=W_{\mathrm{H}_2}=W ;\)
∴ \(n_{\mathrm{CH}_4}=\frac{W}{16} \mathrm{~mol} \text { and } n_{\mathrm{H}_2}=\frac{W}{2} \mathrm{~mol}\)
⇒ \(\text { Total mol, } n=\left(\frac{W}{16}+\frac{W}{2}\right) \mathrm{mol}=\frac{9 W}{16} \mathrm{~mol}\)
Question 21. 8g O2 and some quantity of CO2 are introduced at 30°C into an empty flask of volume 10L. If the total pressure of the gas, the mixture in the flask is 1520 mm. Find the amount of CO2 gas taken.
Answer:
⇒ \(P V=n R T \text { or, } \frac{1520}{760} \times 10=\left(\frac{8}{32}+\frac{W}{44}\right) \times 0.0821 \times 300\)
∴ W= 24.72 g
Question 22. Partial pressures of the component gases in a gas mixture are H2 = 300 mm; CH4 – 150 mm; N2 = 250 mm. What is the percentage of N2 gas by volume in the mixture?
Answer:
⇒ \(x_{\mathrm{N}_2}=\frac{\text { Partial volume of } \mathrm{N}_2}{\text { Total pressure }}=\frac{250}{300+150+250}=0.3571\)
The volume per cent of N2 in the mixture = Mole-fraction of N2 in the mixture ×100
= 35.71%
Question 23. At constant temperature, 2L of N2 gas at 750 mm Hg pressure is mixed with 3L of O2 gas. As a result, the pressure and volume ofthe gas mixture are found to be 732 mm Hg and 5L respectively. What is the initial pressure of O2 gas?
Answer:
⇒ \(P_{\mathrm{N}_2} \times V=n_{\mathrm{N}_2} R T\) : \(\mathrm{P}_{\mathrm{O}_2} \times V=n_{\mathrm{O}_2} R T\)
⇒ \(750 \times 2=n_{\mathrm{N}_2} R T\) : \(P_{\mathrm{O}_2} \times 3=n_{\mathrm{O}_2} R T\)
In mixture:
⇒ \(P V=n R T=\left(n_{\mathrm{O}_2}+n_{\mathrm{N}_2}\right) R T=P_{\mathrm{O}_2} \times 3+750 \times 2\)
⇒ \(732 \times 5=P_{\mathrm{O}_2} \times 3+1500\)
∴ \(P_{\mathrm{O}_2}=720 \mathrm{~mm} \mathrm{Hg}\)
Question 24. The respective mole fractions of N2 and O2 in dry air are 0.78 and 0.21. If the atmospheric pressure and temperature are 740 torr and 20°C respectively, then what will be the mass of N2 and O2 present in a room of volume 3000ft3? (Assuming the relative humidity of the air as zero)
Answer:
⇒ \(p_{\mathrm{N}_2}=0.78 \times 740 \text { torr }=0.78 \times \frac{740}{760}=0.76 \mathrm{~atm}\)
3000 ft³ = 3000 × (30.48)3 [since ft = 30.48 cm]
= 84.95 ×106 cc = 84.95 × 103L
∴ PN2 × V= nN2 ×RT
or, 0.76 × 84.95 × 10³ = N × 0.0821 × (273 + 20)
Or, nN2= 2.683 × 10³ mol
∴ Mass of N2 = 2.683×10³ × 28g = 75.149 kg In calculation, it can be shown that mass of O2 = 23.107 kg
Question 25. 300 cm3 of H2 gas diffuses through a fine orifice in 1 minute. At the same temperature and pressure, what volume of CO2 gas will diffuse through the same orifice in 1 minute?
Answer:
⇒ \(\frac{V_{\mathrm{H}_2}}{V_{\mathrm{CO}_2}}=\sqrt{\frac{M_{\mathrm{CO}_2}}{M_{\mathrm{H}_2}}}=\sqrt{\frac{44}{2}}\)
⇒ \(\text { or, } V_{\mathrm{CO}_2}=\frac{1}{\sqrt{22}} \times 300 \mathrm{~cm}^3=63.96 \mathrm{~cm}^3\)
Question 26. At constant temperature and pressure, the rates of diffusion of two gases A and B are in the ratio of 1: 2. In a mixture of A and B gases mass ratio of A and B is 2:1, so find the mole fraction ratio of A and B in the mixture.
Answer:
⇒ \(\frac{r_A}{r_B}=\sqrt{\frac{M_B}{M_A}} \text { or, } \frac{1}{2}=\sqrt{\frac{M_B}{M_A}} \text { or, } M_A=4 M_B\)
⇒ \(\frac{\mathrm{W}_A}{\mathrm{~W}_B}=2 \text { or, } \frac{n_A \times M_A}{n_B \times M_B}=2 \text { or, } \frac{n_A}{n_B}=\frac{1}{2} \text {; }\)
∴ \(\frac{x_A}{x_B}=\frac{n_A}{n_B}=\frac{1}{2}\)
Question 27. The average velocity of the molecules of a gas is 400m- s-1. At the same temperature, what will be the rms velocity of the molecules?
Answer:
⇒ \(c_a=\sqrt{\frac{8 R T}{\pi M}} \quad c_{r m s}=\sqrt{\frac{3 R T}{M}}\)
∴ \(c_a=\sqrt{\frac{8}{3 \pi}} \times c_{r m s}\)
⇒ \(\text { or, } c_{r m s}=\sqrt{\frac{3 \pi}{8}} \times 400=434.05 \mathrm{~m} \cdot \mathrm{s}^{-1}\)
Question 28. At a constant temperature and 1 atm pressure, the density of O2 gas is 0.0081 g- mL-1. At the same temperature, calculate the average velocity, root mean square velocity and most probable velocity of O2 molecules.
Answer:
⇒ \(c_{r m s}=\sqrt{\frac{3 P}{d}}=\sqrt{\frac{3 \times 76 \times 13.6 \times .981}{0.0081}}=1.937 \times 10^4 \mathrm{~cm} / \mathrm{s}\)
⇒ \(c_a=\sqrt{\frac{8}{3 \pi}} c_{r m s}\)
= \(\sqrt{\frac{8}{3 \pi}} \times 1.937 \times 10^4 \mathrm{~cm} / \mathrm{s}\)
= \( 1.785 \times 10^4 \mathrm{~cm} / \mathrm{s}\)
⇒ \(c_m=\sqrt{\frac{2 R T}{M}}=\sqrt{\frac{2}{3}} \times \sqrt{\frac{3 R T}{M}}=\sqrt{\frac{2}{3}} \times c_{r m s}\)
⇒ \(\sqrt{\frac{2}{3}} \times 1.937 \times 10^4 \mathrm{~cm} / \mathrm{s}=1.581 \times 10^4 \mathrm{~cm} / \mathrm{s}\)
Question 29. A certain gas of mass 6.431 g occupies a volume of 5 L at a definite temperature and 750 mm pressure. At the temperature, what will be the rms velocity of the molecules of that gas?
Answer:
⇒ \(P V=n R T=\frac{W}{M} R T \text { or, } \frac{R T}{M}=\frac{P V}{\mathrm{~W}}=\frac{750}{760} \times 5 \times \frac{1}{6.431} \mathrm{~g}^{-1}\)
= 0.7672 L-atm.g-1
= 0.7672 ×103 × 1.013 × 106 cm 2.s-2
[since 1 atm = 1.013 × 106g-cm-1-s-2]
∴ \(c_{r m s}=\sqrt{\frac{3 R T}{M}}=\sqrt{3 \times 0.7672 \times 1.013 \times 10^9} \mathrm{~cm} / \mathrm{s}\)
Question 30. What is the ratio of average velocity and root mean square velocity ofthe molecules of a gas?
Answer:
⇒ \(c_e=\sqrt{\frac{B R T}{\pi M}}, c_{m m}=\sqrt{\frac{3 R T}{M}}\)
∴ \(\frac{c_a}{c_{m s}}=\sqrt{\frac{8}{3 \pi}}=0.9215\)
Question 31. At what temperature will the rms velocity of S02 molecules be equal to the rms velocity of O2 molecules at 25°C?
Answer:
⇒ \(c_{\mathrm{rm}}\left(\mathrm{SO}_2\right)=\sqrt{\frac{3 R T}{64}}, c_{\mathrm{mav}}\left(\mathrm{O}_2, 25^{\circ} \mathrm{C}\right)=\sqrt{\frac{3 R \times 298}{32}}\)
∴ 7= 596K ie.,t = 323
=C
Question 32. Show that the rms velocity of O2 molecules at 50°C is not equal to the rms velocity of N2 molecules at 25°C.
Answer:
⇒ \(c_{\mathrm{rms}}\left(\mathrm{O}_2\right)=\sqrt{\frac{3 R \times(273+50)}{32}}\)
⇒ \(c_{r m s}\left(\mathrm{~N}_2\right)=\sqrt{\frac{3 R \times(273 \div 25)}{28}}\)
∴ \(c_{\mathrm{ms}}\left(\mathrm{O}_2 \text { at } 50^{\circ} \mathrm{C}\right)=c_{\mathrm{ms}}\left(\mathrm{N}_2 \text { at } 25^{\circ} \mathrm{C}\right)\)
Question 33. The average kinetic energy of the atom of Hg vapour is 1000 cal-mol-1. What will be the value of its rms velocity? [Hg = 200]
Answer:
E = \(\frac{3}{2} R T\)
=\(1000 \mathrm{cal} \cdot \mathrm{mol}^{-1}\)
=\(1000 \times 4.157 \times 10^7 \mathrm{erg} \cdot \mathrm{mol}^{-1}\)
∴ 3RT = 2000 × 4.157 ×107g.cm2.s-2.mol-1
ns = \(\sqrt{\frac{3 R T}{M}}=\sqrt{\frac{2000 \times 4.157 \times 10^7}{200}} \mathrm{~cm} / \mathrm{s}\)
=2.04 × 104 cm/s
Question 34. In a container of volume 1L, there are 1023 gas molecules, each of which has a mass of 10-22g. At a certain temperature, if the rms velocity of these molecules is 105cm.s-1, then what would be the pressure at the temperature inside the container?
Answer:
P V = \(\frac{1}{3} m n c_{r m s}^2\)
Or, \(P \times 1000\)
=\(\frac{1}{3} \times 10^{-22} \times 10^{23} \times\left(10^5\right)^2\)
∴ P = 3.33× 1010 g.cm-1.s2
= 3.33 × 1010 dyne = 32.87 atm
Question 35. At a constant temperature, a vessel of 1-litre capacity contains 1023 N2 molecules. If the rms velocity of the molecules is 103m/s, then determine the total kinetic energy of the molecules and the temperature ofthe gas.
Answer:
Average kinetic energy per molecule
⇒ \(\frac{1}{2} m c_{r m s}^2=\frac{1}{2} \times \frac{28}{6.023 \times 10^{23}} \times\left(10^3\right)^2 \mathrm{~g} \cdot \mathrm{m}^2 \cdot \mathrm{s}^{-2}\)
= 2.324 × 1017g-m2-s-2
= 2.324 × 1020 J
Average kinetic energy per molecule \(=\frac{3}{2} k T\)
⇒ \(\text { or, } \frac{3}{2} \times \frac{8.314}{6.023 \times 10^{23}} \times T=2.324 \times 10^{-20} ; T=1122.6 \mathrm{~K}\)
Total kinetic energy of 1023 molecules
Question 36. At 0°C the kinetic energy of 102 molecules is 5.62 x 10-14 erg. Determine Avogadro’s number.
Answer:
⇒ \(\bar{\epsilon}=\frac{3}{2} K T=\frac{3}{2} \times \frac{R}{N_A} \times T\)
⇒ \(\text { or, } 5.62 \times 10^{-14}=\frac{3}{2} \times \frac{8.314 \times 10^7}{N_A} \times 273 \text {; }\)
∴ NA= 6.058 ×1023
Question 37. The volume of 2 moles of SO2 at 30°C and 55 atm pressure is 680 mL. What is the value of the compressibility factor of the gas? What is the nature of the deviation of the gas from ideal behaviour?
Answer:
⇒ \(Z=\frac{P V}{n R T}=\frac{55 \times 680 \times 10^{-3}}{2 \times 0.0821 \times 303}\)
= 0.7517
As Z < 1, the gas shows a negative deviation from ideal behaviour.
Question 38. The compressibility factor of a real gas at 0°C and 100 atm pressure is 0.927. At this temperature and pressure, how much of this real gas is required to fill a vessel of 100L [molar mass =40g-mol-1?
Answer:
⇒ \(Z=\frac{P V}{n R T} \text { or, } 0.927=\frac{100 \times 100}{n \times 0.0821 \times 273}\)
∴ n = 481.298mol
∴ Amount of gas required =481.298 × 40g = 19.2519kg
Question 39. For a van der Waals gas, b = 5.0 x 10-2 L-mol-1. What is the diameter of a molecule of this gas?
Answer:
⇒ \(b=4 N_A \times \frac{4}{3} \pi r^3\)
⇒ \(5 \times 10^{-2} \times 1000 \mathrm{~cm}^3 \cdot \mathrm{mol}^{-1}\)
= \(4 \times 6.023 \times 10^{23} \times \frac{4}{3} \pi r^3\)
r = \(1.705 \times 10^{-8} \mathrm{~cm}\)
∴ Diameter of a molecule = 3.41 × 10-8 cm
Question 40. The volume of 2 moles of CO2 gas at 27°C is 0.001 m3. What will be the pressure of this gas According to the van der Waals equation and ideal gas equation? [Given a(CO2 ) =0.364 N.m4.mol-2 and b(CO2) = 4.27 × 10-5 m3.mol-1 ]
Answer:
1. \(\left(P+\frac{n^2 a}{V^2}\right)(V-n \dot{b})=n R T\)
Or,\(\text { or, }\left(P+\frac{2^2 \times 0.364 \mathrm{~N} \cdot \mathrm{m}^4}{10^{-6} \mathrm{~m}^6}\right)\left(10^{-3}-2 \times 4.27 \times 10^{-5}\right) \mathrm{m}^3\)
= 2 × 0.0821 × 300 L-atm
Or, (P + 1.456 × 106N-m-2)(9.146 ×10 m3)
= 2 × 0.0821 × 300 × 10-3 m3-atm
or, P+ 1.456 × 106 × 10-5atm = 53.859 atm
P = 39.299 atm
2. PV = nRT
Or, P = \(\frac{2 \times 0.0821 \times 300}{0.001 \mathrm{~m}^3} \mathrm{~L} \cdot \mathrm{atm}\)
= \(49260 \frac{\mathrm{L} \cdot \mathrm{atm}}{\mathrm{m}^3}=\)
=49.26 atm
Question 41. The equation of state for 1 the mole of a gas is (V-b) = RT. At STP, 1 mole of this gas occupies a volume of 28L. Calculate the compressibility factor ofthe gas at STP.
Answer:
Z= \(\frac{P V}{n R T}=\frac{1 \times 28}{1 \times 0.0821 \times 273}=1.249\)
Question 42. The compressibility factor of 2 mol of NH3 gas at 27°C and 9.18 atm pressure is 0.931. If the volume of the gas molecules is not taken into consideration, then what is the value of van der Waals constant ‘a’ for NH3 gas?
Answer:
⇒ \(\left(P+\frac{n^2 a}{V^2}\right) V=n R T \text { or, } P V=n R T-\frac{n^2 a}{V}\)
⇒ \(\text { or, } \frac{P V}{n R T}=1-\frac{n a}{V R T} \text { or, } Z=1-\frac{n a}{V R T}=1-\frac{2 \times a}{V R T}\)
⇒ \(\text { again, } \quad Z=\frac{P V}{n R T} \quad \text { or, } \quad 0.931=\frac{9.18 \times V}{2 \times 0.0821 \times 300}\)
∴ V= 4.991
∴ 0.931 \(=1-\frac{2 \times a}{4.99 \times 0.0821 \times 300}\)
a= 4.2412.atm.mol¯²
Question 43. The pressure exerted by 12g of an ideal gas at t°C in a vessel of volume VL is late. When the temperature is increased by 10°C at the same volume, the pressure increases by 10%. Calculate the temperature ‘f and volume V (molar mass of gas = 120).
Answer:
⇒ \(12 \mathrm{~g} \text { of the gas }=\frac{12}{120}=0.1 \mathrm{~mol} \text { of the gas }\)
Given that the initial pressure of the gas, P = 1 atm and its initial temperature, T = (273 + t)K
By applying ideal gas equation, PV= nRT, we get 1×V = 1 × 0.0821 × (273 + t)………………….(1)
It is given, the final pressure of the gas, \(P=\left(1+\frac{10}{100} \times 1\right)\)
1.1 atm
and its final temperature = (273 + 1 + 10)K = (283+)K
By applying the gas equation, PV = nRT, we get
1.1 × V = 1 × 0.0821 × (283 + t) ………………….(2)
From the equations (1) and (2), we have
1.1 × 0.0821 × (273)+= 1 × 0.0821 × (283 + t)
or, 1.1(273 + t) = 283 + t
∴ t = -173; So, the value off = -173°C
Putting t = -173 in equation [1] (or in equation (2),
We have, V = 1 × 0.0821(273-173) = 0.821L
Question 44. An open vessel contains air at 27°C. At what temperature should the vessel be heated so that l/4th of air escapes from the vessel? Assume that volume ofthe vessel remains the same on heating.
Answer:
Initial temperature of air = (273 + 27)K = 300K.
Let the amount of air in the vessel at 27°C = x mol.
Suppose, the vessel is heated to a temperature of T1K so that 1 /4 th of air escapes from the vessel.
So, the amount of air in the vessel at,\(T_1 K=x-\frac{x}{4}=\frac{3}{4} x\)
Since the vessel is open, the pressure of air in the vessel either at 300K or at TK is equal to atmospheric pressure, i.e., 1 atm.
Again, the volume of the vessel does not change because of heating. Therefore, at temperature 300K.
⇒ \(x \times 0.0821 \times 300=\frac{3}{4} x \times 0.0821 \times T_1 \text { or, } T_1=400 \mathrm{~K}\)
Question 45. A container of fixed volume 0.4L contains 0.56g of gas at 27°C. The pressure of the gas at this temperature is 936 mmHg. If the amount ofthe gas is increased to 2.1g and its temperature is decreased to 17°C, then what will be the pressure of the gas? Assuming gas behaves ideally.
Answer:
Suppose, the molar mass of the gas =M g- mol-1
So, 0.56g of the gas \(=\frac{0.56}{M}\) mol of the gas
At the initial state
⇒ \(P=\frac{936}{760}=1.23 \mathrm{~atm}, n=\frac{0.56}{M} \mathrm{~mol} \text {, }\)
V=0.41
And T = (273 + 27)K = 300K
Substituting the values of P, n, V and T in the Ideal gas equation, PV= nRT, we have
⇒ \(1.23 \times 0.4=\frac{0.56}{M} \times 0.0821 \times 300\)
∴ molar mass of the gas = 28g. mol-1
Question 46. A cylinder capable of holding 3L water contains H2 gas at 27°C and a pressure of atm. At STP, it is possible to fill up 10 balloons, each of which has a radius of 10cm, with the gas present in the cylinder. Find the value of P.
Answer:
The volume of each balloon
⇒ \(\frac{4}{3} \pi \mathrm{r}^3=\frac{4}{3} \pi(10 \mathrm{~cm})^3=4.19 \mathrm{~L} .\)
As the cylinder can hold 31. of water, the US volume Is 31. The volume of H0 gas required to 1111 up 10 balloons 10 × 4.19 = 41.9L.
At STP, If the volume of H2 gas present in the cylinder is
⇒ \(V ., \text { then } \frac{1 \times V}{273}=\frac{P \times 3}{300} \text { or, } V=\frac{273 \times 3}{300} P=2.73 P\)
Even after the balloons have been filled up with 11, gas, the cylinder, still contains H gas of its volume. So, the volume of H2 gas = (2.73P- 3)L
∴ 2.73p- 3 = 41.9 or, P = 16.44
Question 47. At room temperature, 2NO + O2→2NO2 → N2O4 reaction proceeds near completion. The dimer, N2O4, solidifies at 262K. A 250 mL flask and a 100 mL flask are separated by a stop-cock. At 300K, nitric oxide in the longer flask exerts a pressure of 1.053 atm and the smaller one contains oxygen at 0.789 atm.
The gases are mixed by opening the stopcock and after the end of the reaction, the flasks are cooled to 220K. Neglecting the vapour pressure of the dimer, find out the pressure and composition ofthe gas remaining at 220K (assume that the gases behave ideally).
Answer:
Number of moles of NO2 gas lit 250ml, flask
⇒ \(\frac{P V}{R T}=\frac{1.053 \times 0.25}{0.0821 \times 300}=0.01 \mathrm{~mol}\)
And that of O., in 100ml. flask, \(=\frac{P V}{R T}=\frac{0.789 \times 0.1}{0.0821 \times 300}=3.2 \times 10^{-3} \mathrm{~mol} .\)
Reaction: 2NO + O2→2NO2
Thus 1 mol of O2 gns completely reacts with 2 mol of NO gns.
Therefore, 3.2 × 10-3 mol 0f O2 guns will react with
2 × 3.2 × 10-3 = 6 4 ×10-3mol if NO2
After the completion of the reaction number of moles of NO left the reaction system = (0.01 – 6.4 × 10-3 )
3.6 × 10-3mol
The total volume of the reaction system = (0.25 + 0.25)L = 0.351.
If the pressure of NO gas left in the reaction system is then P × 0.35 m 3.6 × 10-3× 0.0821 × 220 P= 0.185 atm.
Question 48. An LPG cylinder weighs 18.4kg when empty when full, it weighs 29.0 Kg and shows a pressure of 2.5 atm. In the course of 27°C, the mass of the full cylinder is reduced to 23.2 Kg. Find out the volume of the gas in cubic meters used up at the normal usage conditions, and the final pressure inside the cylinder. Assume LPG to be n-butane with a normal boiling point of 0°C.
Answer:
Mass of the gas in full cylinder=(29.0 – 18.4) kg – 10,6 kg Mass of the gas after some of is used up =- (23.2 – 18.4) 4.8kg
Mass ofthe gas used up = (10.6 – 4,8) = 5,8 kg. So, 5.8kg of gas = 5.8 kg of n-butane
⇒ \(\frac{5.8 \times 10^3}{58}=100\)
mol of H-butane [molar mass of-butane 58 g- mol-1]
⇒ \(V=\frac{n R T}{P}=\frac{100 \times 0.0821 \times 300}{1}=24631 .\)
[at normal usage condition, P 1 atm)
Therefore, the volume of used gas at normal usage conditions = 24631. = 2.463m3 since =10-nm³]
In the cylinder, LPG exists In a liquid state which remains in equilibrium with its vapour.
So long as LPG exists in a liquid state in the cylinder, the pressure in the cylinder remains fixed. So, the pressure of the remaining gas in the cylinder will be 2.5 atm.
Question 49. An evacuated vessel weighs 50.0g when empty, 148 when filled with a liquid (d = 0.98 g-mL-1) & 50g when filled with an ideal gas at 760 mmHg at 300K. Determine the molar mass of the gas.
Answer:
Mass of the evacuated vessel = 50.0g.
Mass of the vessel when filled with a liquid of density O.98gml-1= 148g
∴ Volume of 98g of liquid \(=\frac{98}{0.98}=100 \mathrm{~mL}=0.1 \mathrm{~L}\)
Question 50. A gas mixture composed of N2 and O2 gases has a density of 1.17 g-L-1. At 27°C and 1 atm pressure. Calculate the mass percents of N2 and O2 in the mixture. Assume that the gas mixture behaves like an ideal gas.
Answer: Given:
P = 1 atm and T = (273 + 27)K = 300K
∴ \(M=\frac{d R T}{P}=\frac{1.17 \times 0.0821 \times 300}{1}=28.8 \mathrm{~g} \cdot \mathrm{mol}^{-1}\)
The average molar mass of the gas mixture = 28.8 g.mol-1.
In the mixture, if the mole-fraction of N2 is x, then the mole-fraction of O2 is (1-x).
∴ M = 28.8g-mol-1.
= [28 × x+32(1 -x)] g-mol-1
Or, 4x = 32 – 28.8;
Hence, x = 0.8
Mole-fraction of N2 = 0.8
And that of O2 = 1- 0.8
= 0.2.
Mass per cent of
⇒ \(\mathrm{N}_2=\frac{0.8 \times 28}{0.8 \times 28+0.2 \times 32} \times 100=77.77 \%\)
And mass present 6 Of O2 = (100-77.77)%
= 22.23%
Question 51. One mole of nitrogen gas at 0.8 atm takes 38s to diffuse through a pin-hole, whereas one mole of an unknown compound of xenon with fluorine at 1.6 atm takes 57s to diffuse through the same hole. Determine the molecular formula ofthe compound.
Answer:
At a given temperature and a pressure of P, the rate of diffusion of a gas \(r \propto \frac{P}{\sqrt{M}}\)
M= molar mass of the gas] If the rates of diffusion of N2 and the unknown gas are r and r.) respectively, then
⇒ \(r_2 \propto \frac{P_2}{\sqrt{M_{\text {compound }}}} \text { and } r_1 \propto \frac{P_1}{\sqrt{M_{N_2}}}\)
∴ \(\frac{r_1}{r_2}=\frac{p_1}{p_2} \times \sqrt{\frac{M_{\text {compound }}}{M_{N_2}}}\)
⇒ \(\text { Given that } r_1=\frac{1}{38} \mathrm{~mol} \cdot \mathrm{s}^{-1}, r_2=\frac{1}{57} \mathrm{~mol} \cdot \mathrm{s}^{-1} \text {, }\)
p1 = 0.8 atm and p2 = 1.6 atm
∴ \(\frac{\frac{1}{38}}{\frac{1}{57}}=\frac{0.8}{1.6} \sqrt{\frac{M_{\text {compound }}}{28}} \text { or, } \sqrt{\frac{M_{\text {compound }}}{28}}=3\)
∴ M compound = 3 × 28 = 252g. mol-1
Let the molecular formula of the compound = XeFx
∴ 131 + x × 19 = 252 or, x = 6.368 ≈ 6
∴ Molecular formula ofthe compound = XeF6
Question 52. A gas bulb of capacity contains 2.0 x 1021 molecules of nitrogen exerting a pressure of 7.57 x 103 N.m-2. Calculate the rms velocity and temperature of the gas. If the ratio of the most probable speed to the root mean square speed is 0.82, calculate the most probable speed of the molecules at this temperature.
Answer:
2.0 ×1021molecules of nitrogen are contained in
⇒ \(\frac{2 \times 10^{21}}{6.023 \times 10^{23}}=3.32 \times 10^{-3} \mathrm{~mol} \text { of } \mathrm{N}_2 \text { gas. }\)
Given that P = 7.57 × 103 N.m-2and V = 1
∴ P = 7.57× 103N.m-2= 7.57 × 103Pa
⇒ \(\frac{7.57 \times 10^3}{1.013 \times 10^5} \mathrm{~atm}=0.0747 \mathrm{~atm}\)
[since 1N.m-2 = IPa and latm = 1.013 × 105 Pa]
∴ \(T=\frac{P V}{n R}=\frac{0.0747 \times 1}{3.32 \times 10^{-3} \times 0.0821}=274.05 \mathrm{~K}\)
∴ Temperature of the gas = 274.05K
we know \(c_{r m s}=\sqrt{\frac{3 R T}{M}}\)
∴ Root mean square velocity of N2 molecules
⇒ \(=\sqrt{\frac{3 \times 8.314 \times 10^7 \times 274.05}{28}} \mathrm{~cm} \cdot \mathrm{s}^{-1}\)
= 4.94 × 104cm.s-1
⇒ \(\text { Given that } \frac{c_m}{c_{r m s}}=0.82\left[c_m=\text { most probable speed }\right]\)
∴ \(c_m=0.82 \times c_{r m s}\)
=\(0.82 \times 4.94 \times 10^4\)
= \(4.05 \times 10^4 \mathrm{~cm} \cdot \mathrm{s}^{-1}\)
∴ The most probable velocity of the molecules = 4.05×104 cm.s-1
Question 53. The composition of the equilibrium mixture (Cl2 ⇌ 2Cl), which is attained at 1200°C, is determined by measuring the rate of effusion through a pin-hole. It is observed that at 1.80 mm Hg pressure, the mixture effuses 1.16 times as fast as Krypton effuses under the same conditions. Calculate the fraction of chlorine molecules dissociated into atoms. (Relative atomic mass of Kr = 84)
Answer:
If the rates of effusion of the equilibrium mixture and Kr gas are r1 and r2 then
⇒ \(\frac{r_1}{r_2}=\sqrt{\frac{M_{\mathrm{Kr}}}{M}} ;\) where M= average molar mass of the equilibrium mixture.
Given that r1 = r2 ×1.16
∴ \(1.16=\sqrt{\frac{84}{M}}\)
∴ M = 62.42 g.mol-1
Let the initial amount of Cl2 gas be 1 mol and its degree of dissociation = x. Therefore, the number of moles of Cl2 and Cl at equilibrium will be as follows:
⇒ \(\mathrm{Cl}_2 \rightleftharpoons 2 \mathrm{Cl}\)
Initial number of moles:
Number of moles at equilibrium
∴ Total number of moles of Cl2 and Cl in the equilibrium mixture = 1 – x + 2x = 1 + x
Average molar mass of the equilibrium mixture
⇒ \(\frac{(1-x) M_{\mathrm{Cl}_2}+2 x \times M_{\mathrm{Cl}}}{1+x}=\frac{71}{1+x} \mathrm{~g} \cdot \mathrm{mol}^{-1}\)
Now, \(\frac{71}{1+x}=62.42\) or, 62.42 + x × 62.42 = 71
∴ x= 0.1374
Fraction of Cl2 molecules dissociated into atoms = 13.74%
Question 54. The density of the vapour of a substance at 1 atm and 500K is 0.36 Kg-m-3. The vapour effuses through a hole at a rate of 1.13 times faster than O2 under the same condition. Determine, the molar mass, molar volume, and Compression factor of the vapour and which forces among the gas molecules are dominating attractive or repulsive. If vapour behaves ideally at 1000K, find the average translational kinetic energy of a molecule.
Answer:
If the rates of effusion ofthe vapour and O2 gas are r1 and r2 respectively then
⇒ \(\frac{r_1}{r_2}=\sqrt{\frac{M_{\mathrm{O}_2}}{M_{\text {vapour }}}}\)
∴ \(1.33=\sqrt{\frac{32}{M_{\text {vapour }}}}\)
The molar mass of the vapour = 18.09g-mol-1
The molar volume of the vapour
⇒\(\frac{\text { Molar mass of the vapour }}{\text { density of the vapour }}\)
= \(\frac{18.09 \mathrm{~g} \cdot \mathrm{mol}^{-1}}{0.36 \times 1000 \times 10^{-3} \mathrm{~g} \cdot \mathrm{L}^{-1}}=50.25 \mathrm{~L} \cdot \mathrm{mol}^{-1}\)
⇒ \(Z=\frac{P V_m}{R T}=\frac{1 \times 50.25}{0.0821 \times 500}=1.224\)
[Vm = molar volume] Hence, Z >1
Z> 1 indicates that the vapour shows a positive deviation from ideal behaviour. This kind of deviation occurs in gas when the effect of intermolecular repulsive forces dominates over the effect of intermolecular attractive forces.
Average translational kinetic energy of a molecule \(=\frac{3}{2} k T\)
⇒ \(\frac{3}{2} \times 1.32 \times 10^{-23} \mathrm{~J} \cdot \mathrm{K}^{-1} \times 1000 \mathrm{~K}=1.98 \times 10^{-20} \mathrm{~J}\)
Question 55. The compressibility factor for 1 mole of a van der Waals gas at 0°C and 100 atm pressure is 0.5. Assuming that the volume of a gas molecule is negligible, calculate the van der Waals constant, a.
Answer:
Compressibility factor \(Z=\frac{P V}{n R T}\)
Given that Z = 0.5, n = 1 mol, T – 273K and = 100 atm
∴ \(V=\frac{Z \times n R T}{P}=\frac{0.5 \times 1 \times 0.0821 \times 273}{100} \mathrm{~L}=0.112 \mathrm{~L}\)
For 1 mol of a real gas, the van der Waals equation is—
⇒ \(\left(P+\frac{a}{V_m^2}\right)\left(V_m-b\right)=R T\left[V_m=\text { molar volume }\right]\)
As the volume of a gas molecule is assumed to be negligible, but, and hence Vm-b~ Vm
∴ \(\text { or, } \frac{P V_m}{R T}=1-\frac{a}{\mathrm{RT} V_m} \quad \text { or, } Z=1-\frac{a}{R T V_m}\)
⇒ \(\text { or, } \frac{P V_m}{R T}=1-\frac{a}{\mathrm{RT} V_m} \quad \text { or, } Z=1-\frac{a}{R T V_m}\)
⇒ \(\text { or, } 0.5=1-\frac{a}{0.0821 \times 273 \times 0.112} ; \quad \text { hence, } a=1.255\)
Question 56. The pressure in a bulb dropped from 2000 to 1500 mm of Hgin 47 min when the contained oxygen leaked through a small hole. The bulb was then evacuated. A mixture of oxygen and another gas of molar mass 79 in the molar ratio of 1: 1 at a total pressure of 4000 mm of Hg was introduced. Find the molar ratio of two gases remaining in the bulb after 75 min.
Answer:
Decrease in pressure of O2 in 47 min=2000- 1500=500mmHg
Decrease in pressure of O2 in 74 min = \(=\frac{500}{47} \times 74=787.23\) mm kg
In the mixture, the mole ratio of O2 and the other gas =1: 1
So, mole-fraction of each of O2 and other gas in the mixture \(=\frac{1}{2}\)
In the mixture, the partial pressure of \(\mathrm{O}_2=\frac{1}{2} \times 4000=\) 2000 mmHg and that of the other gas
⇒ \(=\frac{1}{2} \times 4000 \mathrm{mmHg}=\) = 2000 mm kg If the rates of effusion of 02 gas and the other gas are r1 and respectively, then
⇒ \(\frac{r_1}{r_2}=\sqrt{\frac{M}{M_{\mathrm{O}_2}}}\) [M= molar mass of other gas]
Now,\(r_1=\frac{787.23}{74} \mathrm{mmHg} \cdot \mathrm{min}^{-1}\)
= 10.63 mm hg. min-1
∴ \(r_2=r_1 \times \sqrt{\frac{M_{\mathrm{O}_2}}{M}}=10.63 \sqrt{\frac{32}{79}}=6.76 \mathrm{mmHg} \cdot \mathrm{min}^{-1}\)
Now, \(r_2=\frac{\begin{array}{l}
\text { the decrease in pressure of the other gas } \\\end{array}}{74}\)
∴ The decrease In pressure of the other gas = r2 × 74 = 6.76 × 74 = 500.24 mmHg
Therefore, In the mixture, partial pressure of O2 gas
= (2000- 787.23) 1212.77mmHg and that of the other gas =(2000- 500.24)=
1499.70 mm g Hence, the ratio of mole-fraction of the other gas to O2 gas
=1499.70: 1212.77 = 1.236: 1 In the mixture, the molar ratio of the two gases = 1.230: 1.









 NaNH2/liq.NH3 gives—
NaNH2/liq.NH3 gives—
 via the benzyne mechanism
via the benzyne mechanism





 Identify the correct method for the synthesis of the compound shown above from the following alternatives—
Identify the correct method for the synthesis of the compound shown above from the following alternatives—

 The major product of the above reaction is—
The major product of the above reaction is—

 Identify’X’ in the following sequence of reactions—
Identify’X’ in the following sequence of reactions—

















 the product C is—
the product C is—














 The major products 1 and 3 are respectively
The major products 1 and 3 are respectively



 is aromatic because it has—
is aromatic because it has—





 does not form because the intermediate carbonation
does not form because the intermediate carbonation  i.e is highly stable, so it does not undergo rearrangement
i.e is highly stable, so it does not undergo rearrangement






 the product P is
the product P is















 Identify X
Identify X 


 ‘A’is________
‘A’is________










 The compounds are— each form and
The compounds are— each form and

 In this reaction a is
In this reaction a is
 react with witting reagents (Ph3P—CHR) to form—
react with witting reagents (Ph3P—CHR) to form—

 Here , X is
Here , X is

 undergoes monobromination(with Fe Br)is
undergoes monobromination(with Fe Br)is 

 compound X is________________
compound X is________________