CBSE Class 11 Chemistry Notes For Chapter 13 Hydrocarbons Introduction
Organic compounds containing only carbon and hydrogen atoms are called hydrocarbons. Their major sources are petroleum, natural gas, and coal. Hydrocarbons are considered to be the parent organic compounds. All other compounds are considered to be derived from hydrocarbons by replacement of one or more of their H-atoms by appropriate functional groups.
Hydrocarbons play a very important role in our daily life. Some of these are used as fuels. The largely used fuels are LPG (liquefied petroleum gas), LNG (liquefied natural gas), CNG (compressed natural gas), gasoline (petrol), diesel, kerosene etc.
These are a mixture of different hydrocarbons. The main constituent of natural gas is methane. Some hydrocarbons are used to manufacture polymers such as polythene, polypropene, polystyrene, nylon, terylene etc. Some hydrocarbons are used as solvents in the paint industry and as the starting material for the manufacturing of many dyes and drugs.
Petroleum Or Crude Oil Commercial Source Of Hydrocarbons
Petroleum is a dark viscous oily liquid that is a mixture of hydrocarbons containing different impurities and found within impenetrable rock structures deep below the earth’s crust In Latin, petroleum means rock oil [Latin: petra = rock, oleum = oil). As it is collected from underneath the earth, its alternative name is mineral oil.It is also called crude oil. Petroleum is also known as liquid gold because of its commercial importance. The colour of petroleum depends on its source and nature.
CBSE Class 11 Chemistry Notes Chapter 13 Hydrocarbons
Natural gas:
The gas mixture found above petroleum at various depths below the earth’s crust is referred to as natural gas. The main constituent of natural gas is methane (90%). It also contains ethane, propane, butane and very small amounts of pentane and hexane vapors.
Composition Of petroleum:
Petroleum mainly consists of three types of hydrocarbons. These are chiefly alkanes
(C1-C40), small amount of cycloalkanes
For example: Methylcyclopentane, cyclohexane, methylcyclohexane) and a very small amount of aromatic hydrocarbons (benzene, toluene, xylene, etc.).
Besides hydrocarbons, it also contains certain organic compounds containing oxygen, sulfur, and nitrogen
Refining of petroleum:
The process of separating crude petroleum into different useful fractions having different boiling ranges with the simultaneous elimination of undesirable impurities is called refining of petroleum. Crude petroleum (mainly a mixture of hydrocarbons with carbon atoms ranging from(C1-C40) is separated into different fractions by fractional distillation. According to the demand and the necessity of different industries, each fraction, obtained by distillation under different boiling ranges is collected.
In addition to the low boiling volatile hydrocarbons, the four main fractions obtained by distillation of crude petroleum are:
- Crude naphtha
- Kerosene or paraffin oil
- Fuel oil or diesel and
- Residual oil.
- Crude naphtha and the residual oil are further fractionated to get fractions within still narrow boiling ranges, suitable for different uses.
Different fractions obtained by fractional distillation and their uses:

Cracking And Reforming
1. Cracking Definition
The process in which high-boiling long-chain hydrocarbons are decomposed to give a mixture of low-boiling smaller hydrocarbons by the action of heat alone or heat in the presence of a catalyst is called cracking.
Cracking involves the breaking of carbon-carbon and carbon-hydrogen bonds. Thermal decomposition of organic com¬pounds is known as pyrolysis and when it is applied to alkanes it is known as cracking. The hydrocarbons that will be formed by cracking depends on the conditions applied for the process
Example:

Thermal cracking:
The type of cracking that involves the conversion of high-boiling long-chain hydrocarbons into a mixture of low-boiling smaller hydrocarbons by the action of heat alone is called thermal cracking.
Due to the random dissociation of C—C bonds in thermal cracking, a complex mixture of a large number of hydrocarbons (both saturated and unsaturated) is obtained. The components may be separated from the mixture so obtained.

Catalytic cracking:
Cracking carried out at a relatively lower temperature (330-380°C) in the presence of a catalyst is called catalytic cracking. The most commonly used catalyst is a 4: 1 mixture of silica (SiO2) and alumina (Al2O3). About 85% of the world’s total production of gasoline is obtained through this method. Kerosene is converted into gasoline applying this process
Oceano number:
All gasoline are not equally effective as fuel. n-heptane ranks as a fuel of inferior quality because its combustion takes place rapidly, producing a knocking in the internal combustion engine. On the other hand, 2,2,4- trimethylpentane or isooctane which burns smoothly, does not produce any significant knocking in the engine. Hence, 2, 2, and 4-trimethylpentane having higher antiknock properties (rated as 100) and n-heptane, with lower antiknock properties (its fuel rating taken to be 0) have been introduced as standards for rating fuel.
Based on these two extreme cases, different fuels are standardized with respect to octane numbers. So, octane number indicates the relative antiknock tendency of a gasoline sample.
- Octane number is defined as the parts by percent of isooctane that must be added to a sample of n-heptane to produce the same fuel efficiency of the fuel whose standardization is to be made.
- The octane number of a fuel is 35’—which means that the efficiency of the fuel is identical to a mixture of 35% isooctane and 65% n-heptane.
- The higher the octane number better is the fuel efficiency.
- The knocking tendency of n -n-isomers is much greater than that of branched-chain alkanes.
- When tetraethyllead [(C2H5)4Pb] is added in small quantities to gasoline, it converts the n -n-alkanes to branched-chain isomers, consequently decreasing the knocking tendency i.e, the octane number of gasoline is increased
- Gasoline obtained by catalytic cracking is more effective for internal combustion engines than that obtained by direct distillation of crude petroleum.
- Because this gasoline contains a large amount of unsaturated hydrocarbons & has a higher octane number
2. Reforming Definition
Reforming It is the method of changing low-grade gasoline to high-grade quality gasoline by changing the structures of constituent hydrocarbons by isomerization and aromatization
Isomerization:
In isomerization, a straight-chain hydrocarbon is heated with Al2Cl3 orPt to give a branched one.
Example:

Aromatisation:
In aromatization, a straight-chain hydro¬ carbon is converted into a cycloalkane by cyclization, which is converted into an aromatic hydrocarbon by dehydrogenation. The process is carried out by heating the alkane in the presence of a catalyst (Pt, Pd or Ni) at 400- 600°C.
Example:
After aromatization, hexane is converted into cyclohexane and heptane into methylcyclohexane.

Platinum is the most effective catalyst used in the reforming process and so the process of reforming is also called platforming.
Importance of reforming:
- The octane number of a fuel can be improved by increasing the percentage of branched-chain alkanes and aromatic hydrocarbons which possess greater efficiency as a fuel.
- A mixture of benzene, toluene, and xylene obtained in the reforming (aromatization) process is known as BTX. Many benzene derivatives may be prepared from these compounds
Classification Of Hydrocarbons
Classification of hydrocarbons may be summarised as:
Based on (their structure, hydrocarbons can be broadly classified
Into two main classes:
- Acyclic or open-chain hydrocarbons and
- Cyclic or closed-chain hydrocarbons.

1. Acyclic or open-chain hydrocarbons
In the molecules of these compounds, the carbon atoms are attached to form open chains, which may be branched or unbranched. They are also called aliphatic hydrocarbons. Depending on the nature of carbon-carbon bonds, these are further classified into the following two categories, these are saturated hydrocarbons (or alkanes) and unsaturated hydrocarbons (alkenes and alkynes)
Saturated hydrocarbons or alkanes:
The hydrocarbons in which the carbon atoms are linked with each other by single covalent bonds are called saturated hydrocarbons.
Example:
1. CH3—CH3 (Etliane)
2. CH3— CH2—CH3 (Propane)
3. CH3—CH2—CH2— CH3 (Butane)
Unsaturated hydrocarbons:
Hydrocarbons in which at least two adjacent carbon atoms are linked by a double bond or triple bond are called unsaturated hydrocarbons.
Compounds containing carbon-carbon double bonds (C=C) are called alkenes and those containing carbon-carbon triple bond (C=C) are called alkynes.
Example: Some alkenes are
1. CH2=CH2 (Ethene)
2. CH3—CH=CH2 (Propene)
3. CH3—CH2—CH=CH2 (But-l-ene) etc.
Example: Some alkynes are
1. CH=CH (Ethyne);
2. CH3—C=CH (Propyne)
3. CH3—CH2—C=CH (But-1-yne)
CBSE Class 11 Chemistry Notes Chapter 13 Hydrocarbons
2. Cuclic or closed-chain hydrocarbons
The hydrocarbons having closed chains or rings of carbon atoms in their molecules are called cyclic or closed-chain hydrocarbons. They are further divided into two classes, alicyclichydrocarbons, and aromatic hydrocarbons.
Alicyclic hydrocarbons:
The cyclic or closed-chain hydrocarbons which have properties similar to those of aliphatic hydrocarbons are called alicyclic hydrocarbons. They can be classified as saturated and unsaturated alicyclic hydrocarbons. Saturated hydrocarbons are cycloalkanes. Unsaturated hydrocarbons are further divided into cycloalkenes and cycloalkynes.
1. Cycloalkanes:
Alicyclic hydrocarbons in which all the ring-forming carbon atoms are joined by single covalent bonds are called cycloalkanes.

2. Cycloalkenes:
Alicyclic hydrocarbons containing one carbon-carbon double bond are called cycloalkenes.
Example:

3. Cycloalkynes:
Alicyclic hydrocarbons containing one carbon-carbon triple bond are called cycloalkynes. Lower cycloalkynes are highly strained and unstable, cyclooctyne is strained but somewhat stable while cyclone yne and higher members are unstrained and stable.
Example:

Aromatic hydrocarbons:
Aromatic hydrocarbons are of two types: benzenoid aromatic hydrocarbons and non benzenoid aromatic hydrocarbons.
1. Benzenoid aromatic hydrocarbons:
Hydrocarbons containing one or more benzene rings (either fused or isolated) are called benzenoid aromatic hydrocarbons.
They are also called arenes.
Example:

2. Non-benzenoid aromatic hydrocarbons:
Aromatic hydrocarbons containing no benzene ring are called non- benzenoid aromatic hydrocarbons.
Examples: Azulene, Pentafulvalenes etc..
CBSE Class 11 Chemistry Notes For Chapter 13 Hydrocarbons Aliphatic Hydrocarbons
Alkanes
Open Chain saturated hydrocarbons are referred to as alkanes. At ordinary temperature and pressure, they generally do not show any affinity towards most of the reagents such as acids, bases, oxidising and reducing agents and because of this inertness, they are called paraffins (Latin: parum = litde, affinis= affinity). Each C-atom presentin an alkane molecule is sp³ -hybridised.Four cr -bonds formed by each sp3 -hybridised carbon are directed towards the comers of a regular tetrahedron. Thus, alkanes have tetrahedral structure around each carbon atom. The molecular formula of alkanes is CnH2n + 2 [wheren 1, 2, ]. Their general formula is RH (R: alkyl group).
Nomenclature of alkanes
The nomenclature of alkanes according to the IUPAC system has been thoroughly discussed. Here, only the trivial names ofthe isomers of butane and pentane and the IUPAC names of some higher alkanes are mentioned


Structure of alkanes
1. Alkanes contain only carbon-carbon and carbonhydrogen single bonds. They have the following structural characteristics:
2. Each C-atom is sp3 -hybridized. Four sp³ -hybrid orbitals are directed towards the comers of a regular tetrahedron. The carbon atom lies at the centre ofthe tetrahedron
3. All C—C and C—H bonds are strong sigma bonds. Each C —C cr -bond is formed as a result o axial overlapping of two sp3 orbitals, one from each carbon atom and each C—H bond is formed by the axial overlapping ofone sp3 orbital ofcarbon with the s -orbital of hydrogen.
C—C and C—H bond lengths are 1.54A & 1.12Arespectively.
4. All bond angles in alkanes (C —C —C, C —C —H and H—C—H) have a value of 109°28′ . Thus, alkanes possess tetrahedral structures

5. Carbon atomsin an alkane molecule having three ormore carbon atoms do not lie along a straight line. Instead they form a zig-zag pattern. Thisis because each carbon atomis sp³ -hybridised and naturally the C—C— C bond angle is 109°28′ instead of 180°. It becomes clear from the structure ofpropane shown

6. C—C and C—H bond dissociation enthalpies are,83kcal -mol-1 and 99 kcal-mol-1 respectively.

Structural isomerism in alkanes
Alkanes (except methane, ethane and propane) exhibit chain isomerism, a type of structural isomerism. This type of isomerism arises due to the difference in the nature of carbon chain or the skeleton ofthe carbon atoms.
Example:
- The two chain isomers having molecular formula (C4H10) are n-butane and isobutane. If a 1° or 2° H atom of a propane molecule is replaced by a methyl group, then these two isomers are formed.
- The two chain isomers having molecular formula (C4H10) are n-butane and isobutane. If a 1° or 2° H atom of a propane molecule is replaced by a methyl group, then these two isomers are formed

2. Three chain isomers of molecular formula C5H12 are n -pentane (CH2CH2CH2CH2CH3), isopentane [(CH3)2CHCH2CH3] and neopentane [(CH3)4C]. These isomers are formed on replacement of different H -atoms ofn-butane and isobutane bymethyl group

There are five chain isomers having molecular formula C6H14 and these are obtained by replacement of different five chain isomers having molecular formula

Hydrocarbons Class 11 NCERT Notes
Conformational isomerism in alkanes Definition
Electron distribution of the sigma molecular orbital of a C—C bond is cylindrically symmetrical around the internuclear axis and as this is not disturbed due to rotation about its axis, free rotation about the C—C single bond is possible. Infinite number of spatial arrangements of atoms which result through rotation about a single bond are called conformations or conformational isomers or rotational isomers or simply conformers or rotamers and the phenomenon is called conformational isomerism.
The difference in potential energy between the most stable conformation and the conformation under consideration is called the conformational energy ofthe given conformation. It is to be noted that the rotation around a C—C single bond is not completely free.It is hindered by a very small energy barrier of 1-20kl-mol-1 due to very weak repulsive interaction between the electron clouds of different cr -bonds. Such repulsive interaction is called torsional strain. Conformations are three-dimensional. These are generally represented in paper by three projection formulae: flying wedge formula, sawhorse projection formula and Newman projection formula
Conformations of ethane
A molecule of ethane (CH3—CH3) contains a carbon-carbon single bond (σ-bond) and each carbon atom is attached to three hydrogen atoms. The two —CH2 groups can rotate freely around the C—C bond axis. Rotation of one carbon atom keeping the other fixed results into infinite number of spatial arrangements of hydrogen atoms attached to the rotating carbon atom with respect to the hydrogen atoms attached to fixed carbon atom.
These are called conformational isomers or conformations or conformers. Thus, there are infinite number of conformations of ethane. However, there are two extreme cases. The conformation in which the hydrogen atoms attached to two carbons are as close together as possible, /.a, in which the dihedral angle between two nearest C —H bonds of two — CH3 groups is zero, is called the eclipsed conformation.
The conformation in which the hydrogen atoms are as far apart as possible, i.e., the dihedral angle between two C —H bonds is 60° is called the staggered conformation. The eclipsed conformation suffers from maximum torsional strain whereas in staggered conformation this strain is minimam.
So, the eclipsed conformation is much less stable than the staggered conformation. Any other intermediate conformation i.e., the conformation in which the dihedral angle is between 0-60°, is called the skew conformation. Its stability is in between the two extreme conformations. Therefore, the order of stability of these three conformations is: staggered > skew > eclipsed. It is to be noted that in all these conformations, the bond angles and the bond lengths remain the same.
Saturated hydrocarbons containing more than two carbon atoms have different conformations. However, as there is only one carbon atomin methane, it does not existin the above-mentioned conformations. The eclipsed and the staggered conformations of ethane can be represented by the flying wedge formula, sawhorse projection formula.
Newman projection formula is as follows
1. Flying wedge formula:
In this representation, the two bonds attached to a carbon atom are shownin the plane of the paper and of the other two, one is shown above the plane and another below the plane. The bonds which are in the plane are shown by normal lines (—) but the bond above the plane is shown by solid wedge ( —) and the bondbelowtheplane isshown by hashed wedge.

2. Sawhorse projection formula:
In this projection, molecule is viewed along the molecular axis. It is then projected on paper by drawing the central C —C bond as a somewhat elongated line. Upper end of the line is slightly tilted towards righthand side. The front carbon is shown at the lower end of the line, whereas the rear carbon is shown at the upper end. Each carbon has three lines attached toit corresponding to three H -atoms. The lines are inclined at 120° angle to each other

3. Newman projection formula:
In this projection, the molecule is viewed along the C —C bond. The C-atom nearer to the eye of the viewer (i.e., the front carbon) is represented by a point and the three H-atoms attached to the front C-atom are shown by the three lines drawn at an angle of 120° to each other. The C-atom situated farther from the eye of the viewer (i.e., the rear carbon) is represented by a circle and the three hydrogen atoms attached to it are represented by three shorter lines drawn at an angle of 120° to each other.
Eclipsed and staggered conformations of ethane in I H H terms of Newman projection formula (along with dihedral angles, 0) are shown below

Energy barrier between two extreme conformations is actually very small and so, rotation of two —CH3 groups takes place extremely rapidly. Due to this, it is not possible to separate the conformations of ethane. However, at any moment, majority of ethane molecules exist in the staggered conformation ofminimum energy {i.e., maximum stability).
The eclipsed conformation is least stable because hydrogens and bondingpairs ofelectrons of eclipsed C —H bonds involving adjacent C-atoms are very close to each other causing maximum repulsion. The staggered conformation is most stable because the hydrogens and bonding pairs of electrons of each pair of C —H bonds involving adjacent C-atoms are at a maximum distance. This causes minimum electronic as well as steric repulsion.The potential energy of the molecule is minimum for staggered conformation.
It increases with rotation and reaches a maximum at eclipsed conformation. Experimentally, it has been found that staggered conformation of ethane is 2.8 kcal-mol_1 more stable than eclipsed conformation. (E eclipsed ~ = 2 8 kcal-moH ). Therefore, rotation about C—C bond is not completely free. However, this energy barrier is not large enough to prevent rotation at room tempe¬ rature as collisions between the molecules supply sufficient kinetic energy to overcome this energy barrier.

Dihedral angle Φ
Dihedral angle (Φ) is the angle between the X—C—C and the C—C—Yplane of X-C-C-Y unit ,it is the angle between the H—1C— 2C plane and 1C—2C—H plane, i.e., it is the angle between the 1C—H bond and the 2C—H bond in the Newman projection formula. It is also called the angle of torsion.

Conformations of propane (1CH3–2CH2–3CH3):
In propane molecule Both C1 —C2 & C2 —C3 bonds are equivalent in propane molecules. An infinite number of conformations of propane can be obtained as a result of rotation about the C1 —C2 (or C2 —C3 ) bond. The two extreme conformations are the eclipsed conformation (I) and the staggered conformation (II).
The staggered conformation is more stable than the eclipsed conformation by 3.4 kcal-mo-1

Conformations of n-butane (CH3-CH2-CH2-CH3): nbutane contains two kinds of C —C bonds. So, conformations likely to be generated depend on that particular C —C bond around which C-atoms are made to rotate.

Rotation about the C1—C2 bond:
Keeping C1 fixed, when C2 is rotated around the C1—C2 bond axis, infinite numbers of conformations are obtained. Among these, twoprincipal conformations are eclipsed (I) and staggered (II) conformations. Their order of stability is: staggered > eclipsed, i.e., molecules of n-butane spend most of their time in staggered conformation (II).

Rotation about the C2-C3 bond:
Infinite number of conformations are possible if C3 is made to rotate around C2—C3 bond axis, keeping C2 fixed. Among these, the four chief conformations are— antistaggered (1), gauche staggered (3), eclipsed (2) and fully eclipsed (IV). In an-staggered conformation, the two —CH3 groups exist anti to each other, i.e., they are oriented at an angle of 180° (<p = 180°).
In the gauc/ie-staggered conformation, the two —CH3 groups make an angle of 60° with each other (Φ = 60°). In the eclipsed conformation, the two pairs of —CH3 and H and one pair of H -atoms are in direct opposition, while in the fully eclipsed conformation, the two pairs of H-atoms and one pair of CH3 groups are in direct opposition.
The order of their stability is:
1 >3 >2 > 4, i.e., the molecules of n -butane pass most of their time in and-staggered conformation (1).
Their Newman projection formulae are shown below:

The most stable and least stable conformations ofn -butane are anti-staggered and fully eclipsed conformations respectively. The angular distance between two similar bonds in the anti-staggered conformation is maximum (180°). Thus, repulsion between electrons of such bond pair is minimum. Again, two —CH3 groups are located farthest from each other and so, no sterlc hindrance or steric strain acts between them. On the other hand, the angular distance between two similar bonds in the fully eclipsed conformation is minimum (0°). Thus, repulsion between electrons ofeach bondpair is maximum.
Again, two —CH3 groups are in direct opposition and hence there occurs severe steric strain involving these two CH3 groups. For this reason, anti-staggered conformation is the most stable while fully eclipsed conformation is the least stable conformation of n -butane

General Methods Of Preparation Of Alkanes
1. From compounds containing the same number of C-atoms
By hydrogenation of unsaturated hydrocarbons (alkenes or alkynes):
Alkanes may be prepared by the reduction of alkenes or alkynes by hydrogen in the presence of finely powdered nickel platinum or palladium catalyst. This process is called catalytic hydrogenation. The pressure and temperature of the reaction depend on the nature of the catalyst used.
When a mixture of the vapors of any unsaturated hydrocarbon and hydrogen is passed over a nickel catalyst heated at 200 – 300°C, alkanes containing the same number of carbon atoms are obtained. This process is known as Sabatier-Senderens reduction.

Reduction of alkenes or alkanes can be carried out at a lower temperature 25°C) by using highly 200°C active Raney nickel as a catalyst
- Each mole of alkene combines with 1 mole of hydrogen while each mole of alkyne combines with 2 moles of hydrogen to yield the corresponding alkane.
- Raney nickel: When an alloy containing equal amounts of Ni and Al is digested with sodium hydroxide solution, aluminum dissolves in alkali and finely divided nickel is obtained as residue. This is called Raney nickel. It is washed with water and stored under water or alcohol

- Hydrogen gas thus produced remains adsorbed and occluded in the finely divided nickel and for this reason, the efficiency of Raney nickel as a catalyst is very high.
Applications of hydrogenation reaction:
- Hydrogenation reaction takes place quantitatively and the volume of hydrogen added can be easily estimated. Therefore, with the help of this reaction, the number of double bonds present in an unsaturated compound can be determined.
- Vanaspati or vegetable ghee,
- For example: Dalda, margarine, etc., (saturated glycerides)
- May be prepared from edible vegetable oils,
- For example: soybean oil, sunflower oil, cotton-seed oil, etc., (unsaturated glycerides) by catalytic hydrogenation.
Hydrocarbons Class 11 NCERT Notes
By reduction of alkyl halides:
Alkanes can be prepared by the reduction of alkyl halides with zinc/hydrochloric acid zinc/acetic acid, zinc/sodium hydroxide, zinc-copper couple/ethanol, aluminum amalgam/ethanol etc.

Mechanism
Zn→ Zn2+- + 2e
R→X + e → R• + X–
•R + e→ :R–
•R– + H+→ R—H
or, :R + C2H5OH→ RH + C2H5O–
Example:

Alkanes may also be obtained by the reduction of alkyl Red P/150°C halides with lithium aluminum hydride (LiAlH4) sodium borohydride(NaBH4) or hydrogen in the presence of palladium (Pd) catalyst.

1. LiAlH4 is not suitable for the reduction of tertiary alkyl halides because in that case alkenes are obtained. However, if NaBH4 is used, the corresponding alkane is obtained

2. Primary, secondary, and tertiary alkyl halides may be reduced to the corresponding alkanes by triphenyltin hydride (Ph3SnH or TPH ).
3. The order of reactivity of alkyl halides (RX) in reduction reaction is: RI > RBr > RC
By Clemmensen, the reduction of aldehydes and ketones:
When aldehydes and ketones are reduced with amalgamated zinc and concentrated hydrochloric acid, the corresponding alkanes are obtained. The reaction is so-called after the name of the discovery

Reduction of alcohol, alkyl iodide, aldehyde, ketone, and carboxylic acid by red P and HI:
When alcohol, alkyl iodide, aldehyde, ketone, and carboxylic acid are reduced by heating with concentrated aqueous solution of hydroiodic acid at 150°C in the presence of a small amount of red phosphorus, the corresponding alkanes are obtained. The reaction is conducted in a closed vessel

Examples:

Red phosphorus reacts with 12 to regenerate HI. Therefore, the backward reaction leading to the formation of the starting compounds does not take place.
3I2 + 2P→2PI3
PI3 + 3H2O→H3PO3 + 3H
By the hydrolysis of Grignard reagents:
When dry and pure metallic magnesium is dissolved in a dry ethereal solution of an alkyl halide, an alkylmagnesium halide (R—MgX) is obtained. This organometallic compound is known as Grignard reagent.
In this compound, the carbon atom is directly attached with the Mg-atom, and the C—Mg bond is a highly polar covalent bond. When Grignard reagents are treated with water or dilute acids, the corresponding alkanes are obtained in this reaction. The alkyl group (R) of the Grignard reagent takes up a proton to generate alkane (RH).

It is to be noted that Grignard reagents may also react with other compounds containing active hydrogen such as alcohols, ammonia, amines etc., to form alkanes
2. From compounds containing a greater number of C-atoms than the corresponding alkanes:
By decarboxylation of carboxylic acids:
When a mixture of anhydrous sodium or potassium salt of a carboxylic acid and soda lime (NaOH+CaO) is strong, a molecule of carbon dioxide is eliminated from the acid (decarboxylation) to produce an alkane

The alkane obtained has one carbon atom less than that of the corresponding carboxylic acid

3. From compounds containing less number of C-atoms than the corresponding alkanes:
1. By Wurtz reaction:
When a dry ethereal solution of an alkyl halide (preferably bromide or iodide) is treated with metallic sodium, the two alkyl groups of two alkyl halide molecules combine to form an alkane. This reaction for the preparation of an alkane is known as the Wurtz reaction. The resulting alkane contains twice the number of carbon atoms present in the molecule of alkyl halide.

Mechanism: Two different mechanisms have been suggested.
1. Through the formation of the organometallic compound as an intermediate
2Na → 2Na+ + 2e
R —X + 2e → R– + X–

2Na+ + 2X→ 2NaX
2. Through the formation of free radicals as intermediates
2Na → 2Na+ + 2e
2R—X + 2e → 2R•+ 2X–
R•+ R• → R—R
2Na+ + 2X–→ 2NaX
Example:

Some important points related to Wurtz reaction:
- Metallic sodium acts as a reducing agent and ether acts as a solvent.1° and 2° alkyl halides participate in the Wurtz reaction while 3° alkyl halides do not participate in this reaction due to steric effect.
- Methane cannot be prepared by this reaction because this reaction always leads to the formation of alkanes containing more than one carbon atom. This processis not suitable for the synthesis of unsymmetrical alkanes.
- This is because in that case, the reaction is to be carried out using an alkyl halide containing an even number of carbon atoms (RX) and an alkyl halide containing an odd number of carbon atoms (R’X).
- RX combines with R’X to yield the desired alkane, R—R’ but at the same time, two molecules of RX combine to form the alkane, R —R and two molecules of R’X combine to form the alkane, R’ —R’. Therefore, a mixture of three alkanes are obtained.
- Although the desired alkane is obtained, its yield is low and it cannot be separated from the mixture easily as the boiling point of the formed alkanes are very close to each other.

Example:
When methyl bromide and ethyl bromide are made to react with each other for the preparation of propane; ethane and butane are also produced along with propane. This results in a very poor yield of propane and it cannot be easily separated from the mixture

Wurtz reaction is applicable for the preparation of symmetrical alkanes containing an even number of Catoms but not for the preparation of unsymmetrical alkanes containing an even or odd number of C-atoms.
Because a symmetrical alkane can be divided into two required equal parts and so two types of alkyl halides are not required for its preparation. However, an unsymmetrical alkane cannot be divided into two equal parts and so two different alkyl halides are required for their preparation.

- The symmetrical alkanes that require tertiary (3°) alkyl halides for their preparation cannot be synthesized by the Wurtz reaction.
- The order of reactivity of various alkyl halides: RI > RBr > RCl
Importance Of Wurtz reaction:
This reaction leads to the formation of C — C bond. The formation of C — C bond is very important in organic synthesis. Also, bicyclic compounds can be prepared by intramolecular Wurtz reaction.
For example:

By Kolbe’s electrolysis method:
When a cold and concentrated aqueous solution of sodium or potassium salt of a carboxylic acid is electrolyzed between platinum electrodes, hydrogen gas and NaOH or KOH are formed at the cathode and at the anode, alkane, and CO2 are obtained.
When the mixture of CO2 and alkane is allowed to pass through a caustic soda solution, CO2 is absorbed and the alkane is obtained: This process for the preparation of alkanes is known as Kolbe’s electrolysis.

Mechanism:
RCOOK ⇌ RCOO- + K+ 2HO⇌ 2H+ + 2OH–
At anode: RCOO– → RCOO•+ e; RCOO• →R•+ CO
R•+ R•→ R —R
At cathode: 2H++ 2e→ [2H•] → H2
Example:
Electrolysis of concentrated and cold aqueous solution of potassium acetate between platinum electrodes produces ethane at the anode

Some important points related to Kolbe’s electrolysis method:
In this method, alkanes with double the number of carbon atoms present in the alkyl group of the carboxylic acid is obtained. Thus,if n is the number of carbon atoms present in the salt of carboxylic acid, the alkane formed must contain 2{n- 1) carbon atoms.
Therefore, methane cannot be prepared by this method This is [fcWI because in this case a mixture of aqueous solution of sodium or potassium salts of two different carboxylic acids is to be subjected to electrolysis and as a result, two more alkanes in addition to the desired alkane will be produced. This reduces the yield of the desired unsymmetrical alkane and it cannot be easily separated from the mixture

Importance of Kolbe’s electrolytic method:
This reaction leads to the formation of C — C bond which is synthetically important. Also, alicyclic compounds can be prepared by intramolecularKolbe’s electrolytic method.

Corey-House synthesis:
An alkyl halide, RX is first treated with lithium metal in dry ether medium to form alkyl lithium (R—Li) which is then treated with iodide to formlithium dialkyl cuprate (R2CuLi). Lithium dialkyl cuprate is finally’ treated with a suitable alkyl halide (R’X or RX) to form desired alkane (R—R’ or R—R)

The third step i.e., the final step is an SN2 reaction, and therefore, no tertiary’ (3°) alkyl halide (R’X) can be used in this step.
Example:

Importance of Corey-House alkane synthesis: This reaction can be used to prepare both symmetrical and Therefore, methane cannot be prepared by this method. unsymmetrical alkanes in good yield.
NCERT Class 11 Chemistry Chapter 13 Hydrocarbons Notes
4. Preparation of alkanes from inorganic compounds
From inorganic carbides:
Some inorganic carbides react with water to liberate saturated hydrocarbons.
For example:
When beryllium carbide and aluminum carbide are heated with water, they get hydrolyzed to form methane. This method gives pure methane

The carbide compounds which react with water to form methane are called methanides.
From alkyl boranes:
1. Alkanes may be prepared by treating trialkyl boranes, obtained by hydroboration of alkenes, with propanoic acid (protonolysis)

2. When a trialkyl borane is heated with a mixture of AgN03 and NaOH at 30-40°C, an alkane of high molecular mass is obtained.
For example: When tripentylborane is heated with a mixture of AgNO3 and NaOH at 30-40°C, decane is formed as the product

General Properties Of Alkanes
1. Alkanes Physical properties
1. With increase In the number of carbon atoms, the physical s states of the alkanes change in the order: gas -> liquid ~> solid. At normal temperature and pressure, straight chain alkanes from C1 to C4 (i.e., methane, ethane, propane, and butane) are colorless gases, C5 to C17 (from pentane to heptadecane) are colorless liquids and from C18 onwards are colorless solids.
2. Alkanes are non-polar, lighter than water and almost insoluble in water but they are soluble in non-polar or less polar solvents like benzene, chloroform, ether, carbon tetrachloride etc
Boiling points of isomeric pentanes:

3. With an increase in molecular mass, boiling points, melting points, and viscosities of straight-chain alkanes increase regularly. Among the Isomeric alkanes, the boiling point decreases with an increase in branching, i.e., a branched chain alkane hasInvariablylower boiling point than the corresponding n -n-alkane,
For example:
In the case of isomeric pentanes, n-pentane has the highest boiling point while neo-pentane has the lowest boiling point.
4. It is also evident that the increase in melting point Is relatively more in moving from an alkane having an odd number of carbon atoms to a higher alkane while it Is relatively less in moving from an alkane with even number of carbon atoms to a higher alkane. As n -n-alkanes with an even number of carbon atoms are more symmetrical than those containing an odd number of carbon atoms, they pack more closely in the crystal lattice involving much stronger intermolecular forces of attraction

The reason behind decreasing boiling point:
Among nonpolar hydrocarbon molecules, the forces of attraction\which come into play are weak van der Waals forces. These which come into play are weak van der Waals forces. These Waals forces depend on the area of contact between Waals forces depends on the area of contact between the molecules. Branching reduces the area of contact because

A branched compound has a more compact, nearly spherical shape, and spheres touch only at a point. For this reason, branching reduces the van der Waals forces and so it reduces the boiling point. Since branching, for example, increases gradually on going from n-pentane to neopentane, the area of contact gradually decreases and consequently, the boiling point gradually decreases as van der Waals forces go on decreasing
Van der Waals forces of attraction: in non-polar molecules, the center of positive charge density coincides with the center of negative charge density. However, due to the random 300-movement of electrons around the nucleus, a momentary 280 distortion of their distribution may occur.
This results in both momentary loss of electrical symmetry and the formation of a momentary dipole in the molecule. This instantaneous dipole induces a dipole in a second molecule. These dipoles then attract each other which hold the molecules together. These attractive forces are known as van der Waals forces of attraction
2. Alkanes Chemical Properties
Alkanes are generally inert substances. They do not easily react with acids, alkalis, oxidizing agents, and reducing agents. However, under suitable conditions, they form compounds by substitution reactions.
Reasons for chemical internees of alkanes:
The reasons for the chemical reactivity of any compound are polar bonds,
- The presence of one or more lone pairs of electrons,
- The presence of an atom with an expandable octet and
Presence of an atom with an incomplete octet. Alkanes possess none of the above characteristics.
For example:
The C —H and C —C bonds present in alkane molecules are non-polar.
So the polar reagents do not find any suitable site to attack the alkane molecules. There is no lone pair of electrons in alkane molecules. In alkane molecules, the octet of carbon is filled with electrons and hydrogen has also attained the stable electronic configuration of an inert helium atom. Again, due to the absence of any orbital, carbon cannot expand its octet. All these factors collectively contribute to the general inertness of alkanes
3. General reactions of alkanes
1. Oxidation reactions of alkanes:
Combustion:
Alkanes burn in the presence of excess oxygen or air to produce carbon dioxide and water along with the liberation of huge amounts of heat. For this reason, alkanes are used as fuels. Chief constituents of LPG used for household cooking are n-butane, propane and isobutane, and a small amount of ethyl mercaptan.
CH4 + 2O2 → CO2 + 2H2O + 213 kcal.mol-1
2C2H6 + 7O2 → 4CO2 + 6H2O + 368 kcal.mol-1
The general equation for combustion of an alkane may be given as follows:
CxHy + (x+y/4) →x CO + y/2 H2O + heat
When burnt in a limited supply of air or oxygen, alkanes produce different quantities of carbon (carbon black) and carbon monoxide, besides carbon dioxide and water.
Controlled oxidation:
Controlled oxidation of alkanes by oxygen at high temperature and pressure in the presence of metal or metallic oxide catalyst produces alcohols, ‘ aldehydes and carboxylic acids
Example:
1. The controlled oxidation of methane yields methyl alcohol and formaldehyde.

2. Methane is oxidised by ozone to form formaldehyde
CH4 + 2O3→HCHO + H2O + 2O2
3. Alkanes containing tertiary hydrogen are oxidized to tertiary alcohol potassium permanganate

2. Substitution reactions:
The characteristic reaction of saturated hydrocarbons are substitution reactions. In this reaction, the hydrogen atom attached to the carbon atom is displaced by any monovalent atom or group.
1. Halogenation:
In the reaction of halogens with alkanes presence of light, heat (250 – 400°C) or catalyst, the hydrogen atoms of the alkanes are easily replaced by halogen atoms to give haloalkanes and hydrogen halide. This is called a halogenation reaction.
Hydrocarbons Class 11 Chemistry Solutions
Example:
1. When methane is allowed to react with chlorine in the presence of diffused sunlight at ordinary temperature, all the hydrogen atoms of methane are replaced by chlorine atoms successively to yield different substitution products

Mechanism:
The displacement of hydrogen atoms of alkane by chlorine atoms in the presence of diffused sunlight proceeds through the free radical mechanism.
It is a chain reaction which occurs through the following steps:
First step (chain initiation):
In the presence of light or heat, ClCBSE Class 11 Chemistry Notes For Chapter 13 Hydrocarbons First Step Chain Initiation molecules get excited and its covalent bond undergoes homolytic cleavage to form free radicals (Cl ).

Second step (chain propagation):
Two reactions occur in this step.
1. Chlorine-free radical abstracts a hydrogen atom from a methane molecule to form methyl radical

2. The formed methyl radical abstracts a Cl-atom from Cl2 molecule to form CH3Cl and another chlorine-free radical C

The two reactions (1) and (2) are repeated again and again and as a result, the chain gets propagated. Consequently, the amount of CH3Cl in the reaction mixture gradually increases.
[The resulting CH3CI reacts similarly with Cl to form CH2Cl2 which in turn leads to the formation of CHCl3 which subsequently facilitates the formation of CCl4 by similar mechanisms].
Third step (chain termination):
The two free radicals (same or different) combine to terminate the chain

From the above-mentioned mechanism, it is observed that, methyl free radical forms as an intermediate in the halogenation of methane. Two methyl free radicals (C•H3) formed in tills way combine to terminate the chain thereby forming an ethane molecule

Therefore, it can be said that a small amount of ethane may be formed during chlorination of methane
2. Bromine atoms also substitute hydrogen atoms of alkanes, but bromination reaction takes place at a much slower rate than chlorination reaction. This reaction also follows the free radical mechanism.

3. The iodination reaction is reversible because hydroiodic acid produced in this reaction, being a strong reducing agent, reduces the alkyl iodide back to alkane. In this case, an oxidizing agent capable of oxidizing HI such as HIO3, HNO3, HgO etc., is used and as a result, the reaction becomes irreversible
CH4+ I2 ⇌ CH3I +HI
5HI + HIO3 → 3I2 + 3H2O
4. Fluorination of alkanes with pure fluorine has very little practical use. It cannot be controlled under ordinary conditions as fluorination of alkanes is very vigorous. It causes extensive breaking of C —C and C —H bonds and a mixture of products is formed. However it is done by diluting fluorine with an inert gas such as nitrogen or argon. Suitable inorganic fluorides like AsF3, SbF3, AgF, HgF2 etc., are heated with suitable chloroalkanes to get alkyl fluorides.

It is called the Swarts reaction.
The reactivity of halogen towards the alkanes follows the order: of fluorine > chlorine > bromine > iodine.
If any alkane contains two or more non-equivalent hydrogen atoms then all the isomeric monohalogenated derivatives are formed. However as the reactivity of different types of hydrogens differ, the monohalo derivatives are obtained in different quantities, The reaction follows free radical mechanism, and since the stability of free radicals
Follows the order:
3° > 2° > 1°, the reactivity of different hydrogens towards halogenation reaction follows the order: 3°H > 2°H > 1°H.
For example:
2-chloropropane is obtained as the major product in chlorination of propane and 2-bromobutane is obtained as the major product in bromination ofbutane.

Nitration:
The reaction in which the hydrogen atom of an organic compound is replaced by a nitro (-NO2) group is called nitration. When a mixture of an alkane and fuming HNO3 vapours are heated at higher temperatures (400 – 475°C) under pressure, it undergoes nitration to yield a nitroalkane.

All types of hydrogen atoms of an alkane molecule may be replaced by —NO2 group during a nitration reaction. Also, there is a possibility of C —C bond cleavage leading to the formation of a mixture of lower nitroalkanes.

Sulphonation:
The substitution reaction in which the hydrogen atom of an organic compound is replaced by a sulphonic acid group (-SO3 H) is called sulphonation. When an alkane is heated with fuming sulphuric acid (H2SO4 + SO3 ) or oleum (H2S2O7 ) at a higher temperature, an H-atom of alkane is substituted by -SO3 H group to form alkane sulphonic acid

3. Pyrolysis or Cracking:
The thermal decomposition of organic compounds is called pyrolysis and in case of alkanes, it is called cracking. When an alkane containing a large number of carbon atoms is heated at high temperatures (500 – 700°C) in the absence of air, it undergoes cracking to yield a mixture of lower alkane, lower alkene, and hydrogen
Example:

Isomerization:
The conversion of one isomer of a compound into another isomer is called isomerization. When an n -alkane is heated at high temperature (300°C) in the presence of a catalyst (anhydrous AlCl3/HCl or AlBr3/HBr ), it gets converted into abranched chain isomer.
Example:
Isobutane is obtained from n-butane by isomerization. Isobutane is the chain isomer (a type of structural isomer of n-butane

Isomerization is a very important reaction for the preparation of high-quality gasoline (higher octane n
4. Dehydrogenation and dramatization:
When an alkane is passed over a suitable catalyst (oxides of Cr, Mo, Al, etc.) heated at 500-750°C , one molecule of hydrogen is eliminated from a molecule of the alkane to liberate an alkene

When alkanes containing six or more carbon atoms are passed over Pt, Pd or Ni catalysts heated at higher temperatures, benzene or alkylbenzenes are obtained. This reaction is known as aromatization. Aliphatic hydrocarbons may be converted into aromatic hydrocarbons by using this reaction.
Example:
When n -n-hexane is heated at 400-600°C in the presence of Pt catalyst, benzene is obtained. Similarly, n -heptane gives toluene.

4. Use of hydrocarbons as fuel
- Natural gas: The gas found above petroleum deposits at various depths below the earth’s crust is referred to as natural gas. The main constituent of natural gas is methane (90%). This gas is supplied by pipelines for use as a fuel.
- CNG (Compressed Natural Gas): Natural gas kept in steel cylinders under high pressure is called CNG. It is used as an alternative fuel in different vehicles in many metropolitan cities.
- LNG (Liquefied Natural Gas): Liquefied natural gas kept in steel cylinders under high pressure is called LNG. It is also used as a fuel
- LPG (Liquefied Petroleum Gas): Liquefied petroleum gas kept in steel cylinders under high pressure is called LPG.It is mainly a mixture of n-butane and isobutane with a small amount of propane.It is mainly used as a pollution-free fuel in small-scale industries and for household purposes.
Methane
Methane is the simplest paraffin or alkane. Its molecular formula is CH4. The general formula of the paraffin is (CnH2n + 2). When n = 1, it gives the formula of the first member of the alkane series, methane (CH4).
1. Preparation of methane
1. Laboratory preparation:
Methane Principle:
Methane is prepared in the laboratory by heating a mixture of anhydrous sodium acetate (1 part) and sodalimde (3 parts).

Methane Purification:
- Acetylene is eliminated by passing the evolved gas first through the ammoniacal cuprous chloride solution.
- Then ethylene and moisture are removed from the gas by passing it through fuming sulphuric acid.
- The methane gas obtained contains a small quantity of sulphuric acid vapors and hydrogen.
- Vapors of H2SO4 are then eliminated by passing the gas over solid potassium hydroxide and the gas thus obtained is collected by downward displacement of mercury. In this methane, a small quantity of hydrogen is present as an impurity.
- In order to remove hydrogen, this gas is passed through palladium heated to about 100°C. H2 gas is adsorbed by palladium. Pure methane obtained is collected over mercury.
2. Preparation of methane at room temperature:
By hydrolysis of aluminum carbide: At ordinary temperature, methane is prepared by treating aluminum carbide with water. If dilute HC1 is used instead of water, there is less possibility of the formation of aluminum hydroxide layer on aluminum carbide
Al4C3 + 12H2O → 3CH4 + 4Al(OH)3
Al4C3 + 12HCl → 3CH4 + 4AlCl3
Besides this, methane can also be prepared by the hydrolysis of beryllium carbide (Be2C).
Be2C + 4H2O→ CH4+ 2Be(OH)2
By the reduction of methyl iodide:
Almost pure methane can be prepared by the reduction of methyl iodide with ethyl alcohol and Zn-Cu couple or aluminum amalgam. Methane thus obtained contains traces of hydrogen as an impurity

By the hydrolysis of methylmagnesium iodide (a Grignardreagent):
CH3Mgl +H2O →CH4 + Mg(OH)I
By the hydrolysis of zinc dimethyl:
Zn(CH3)2 + 2H2O → 2CH4 + Zn(OH)2
3. Synthetic methods:
Methane is obtained when a mixture of H2 and CO or CO2 is passed through overpowered nickel at 250-400°C.

Methane is obtained in small amounts when the electric spark is produced in H2 gas with the help of carbon electrodes. electric spark Heat

2. Properties of methane
Physical properties:
- At normal temperatures, CH4 is a colorless, odorless, tasteless & non-poisonous gas.
- It is lighter than air and sparingly soluble in water but is highly soluble in organic solvents (alcohol, acetone, and ether).
- On cooling, it turns into its liquid & solid state. Its boiling & melting points are -161.4°C & -183°C respectively.
Chemical properties:
- Methane, being a saturated hydrocarbon, is highly stable.
- At ordinary temperature, it is inert to acids, bases, oxidizing and reducing agents. However, methane participates in different substitution reactions
Will O The Wisp:
Methane is produced in marshy lands due to bacterial decomposition of organic matter and hence, it is called marsh gas. Again, due to the putrefaction of animal bodies, phosphine, (PH3) and diphosphorus tetrahydride (P2H4) are also produced in marshylands. So methane gets contaminated with PH3 and P2H4. P2H4 readily bums in the air.
So when the whole mixture comes in contact with air, P2H4 sets the gases on fire and the heat produced causes methene to bum with a blue flame. As a result, an intermittent source of light is produced, known as will-o’- the-wisp. Thus, light can be seen in stagnant swampy areas, especially graveyards. Will-o’-the-wisp is not a supernatural phenomenon
3. General reactions of methane
Combustion:
Methane does not support combustion but in the presence of air or oxygen, it bums with a non-luminous bluish flame with the formation of carbon dioxide & water.
CH4 + 2O2 →CO2 + 2H2O + 213 kcal-mol-1
A mixture of methane and air or oxygen explodes when comes into contact with fire and this is a possible reason of explosions in coal mines.
Substitution reactions:
1. Reaction with chlorine:
Methane does not react with chlorine in the dark, but in the presence of direct sunlight, methane reacts with chlorine explosively to form carbon (in the form of soot) and hydrogen chloride

In presence of diffused sunlight, methane undergoes substitution reaction with chlorine. In this case, the hydrogen atoms of methane are successively replaced by chlorine atoms to form methyl chloride, methylene chloride, chloroform and carbon tetrachloride respectively

The substitution reaction of methane with chlorine proceeds via free radical mechanism In this reaction, a mixture of different chloro compounds is always obtained. The constituents of the mixture can be separated. The reaction can be restricted to the first step by using excess of methane and consequently, methyl chloride may be obtained as the predominant product.
Hydrocarbons Class 11 Chemistry Solutions
2. Reaction with bromine:
Like chlorine, bromine also reacts with methane. However, the reaction proceeds slowly because bromine is less reactive than chlorine.
3. Reaction with iodine:
The reaction of iodine with methane is extremely slow and the reaction is reversible. So, the reaction is carried out in the presence of an oxidizing agent like HIO3 or HNO3
CH4 + I2 ⇌ CH2 HI, 5HI + HIO3 +3I2 ⇌ 3H2O
4. Nitration: Methane reacts with nitric acid vapour at about

Reaction with fluorine:
Fluorine reacts violently with methane with explosion to form carbon and hydrogen fluoride
CH4 + 2F2→ C + 4HF
Recently, it has been possible to prepare different fluoro compounds by reacting fluorine with methane in the presence of inert gas (dilution of the active reagent).
Reaction with ozone:
Methane undergoes oxidation by ozone to yield formaldehyde (HCHO).
CH4 + 2O3 → HCHO + 2O2 + H2O
Controlled oxidation:
Methane on controlled (partial) oxidation by oxygen at much higher temperature (1500°C) produces acetylene.

Oxidation:
- When a mixture of methane and oxygen solution of by volume) is passed through hot copper tube at 200°C under a pressure of 100 atmosphere, methanol is obtained.
- Methane is oxidised by air at 450°C, under pressure in the presence of molybdenum oxide as catalyst to yield formaldehyde

Reaction with steam:
When a mixture of methane and steam is passed over nickel (catalyst) kept on alumina heated at 800 – 900°C, a mixture of CO and H2 is obtained

Thermal decomposition:
At 1000°C, methane decomposes to produce fine carbon powder.It isknown as carbonblack

4. Uses of methane
- Carbon black is used for preparing printers’ ink, black paints and in rubber industry for making motor tyres
- Useful chemicals like methyl chloride, acetylene, formaldehyde, methanol etc. are produced from methane
- 3. The gas mixture (CO + H2) obtained by the reaction of methane with steam is used for the commercial preparation of hydrogen.
- When steam is passed through a catalyst heated at 500°C, CO is converted into CO2 and H2 is obtained:
- CO + H2O→CO2 + H2. Also, when the gas mixture containing CO (1 part) and H2 (2 parts) is passed through a mixture consisting of CuO, ZnO, and Cr2O3 as catalyst under 200 atmosphere pressure, methanol is obtained: CO + 2H2→ CH3OH.
- Methane is used as fuel. Its calorific value:1000Btuper eft
5. Identification of methane
Methane reacts with ozone to yield formaldehyde. So the gas being tested is subjected to react with ozonised oxygen. Ifit is methane, it emits the characteristic pungent smell of formaldehyde. Water is added to the reaction mixture to prepare a dilute solution of formaldehyde and with this solution, Schryver’s colour test is performed.
Schryver’s colour test:
2mL of an aqueous solution of phenylhydrazine hydrochloride is added to lmL 5% aqueous solution of potassium ferricyanide and to this mixed solution, a small quantity of the above test solution is added followed by the addition of 5mL of concentrated HCl. If formaldehyde is present, the solution turns pinkred. It indicates that the gaseous sample is methane
Methane is a saturated hydrocarbon—Proof:
Methane is chemically inert. At ordinary conditions, it does not react with acids, bases and oxidising or reducing agents. When methane gas is passed through red bromine water or through alkaline KMnO4 solution, colours of the reagents do not change, i.e., methane does not react with these reagents.
So methane is not an unsaturated compound. It undergoes a substitution reaction with chlorine to produce four chloro compounds (CH3Cl, CH2Cl2, CHCI3 and CCl4) and HCl. It means that methane is a saturated compound.
5. Structure of methane molecule
The central C-atom of methane is sp³ -hybridised and it has a regular tetrahedral structure. Four C—H bonds of formaldehyde, methanol etc. are produced from methane. bond energy and the same bond length.
If any one of the H-atoms of methane is replaced by a monovalent atom or group (Z), only one type of derivative CH2Z is obtained. This proves that four H-atoms of methane are equivalent.
Methane has a regular tetrahedral structure—Proof:
If two hydrogen atoms of methane are substituted by two similar atoms or groups (Z), only one disubstituted methane (CH2Z2) is obtained
For example:
Only one kind of methylene chloride (CH2Cl2) is known to exist which has no isomer. From the above observation and in the perspective of tetracovalency of carbon, it can be concluded that structurally methane molecule is a regular tetrahedron—it is not square planar, rectangular planar, pyramidal with square or rectangular base.
This is becauseit will not have any isomer only when it becomes tetrahedral but if it assumes any structure other than tetrahedral, then it will have more than one isomer.

Ethane (C2H6)
Ethane is found to exist along with methane in natural gas. Ethane is also obtained in small amount from coal gas.
1. Preparation of ethane
1. By heating sodium propanoate with soda lime:

Ethane thus obtained is impure and contains some amount of methane and hydrogen as impurities.
2. By the reduction of ethyl iodide:
Ethyl iodide, on reduction with Zn-Cu couple or aluminium-amalgam and ethyl alcohol, yields ethane

3. By Wurtz reaction: When metallic sodium reacts with methyl iodide dissolved in dry ether, ethane is obtained

4. By Kolbe’s electrolytic method :
When a concentrated aqueous solution of sodium or potassium acetate is electrolysed by using platinum electrodes, ethane, and CO2 are evolved at the anode. At the cathode, hydrogen gas is evolved and sodium or potassium hydroxide is produced

5. By hydrolysis of ethyl magnesium iodide:
When ethylmagnesium iodide (CH3CH2MgI) is treated with water, pure ethane is obtained.
CH3CH2Mgl + H2O→ CH3 —CH3 + Mg(OH)I
6. By hydrogenation of ethylene or acetylene:

2. Properties of ethane
Physical properties:
- At ordinary temperature, ethane is a colourless and odourless gas. Its boiling and melting points are -89°C and -172°C respectively.
- It is slightly soluble in water but highly soluble in organic solvents such as ether, alcohol etc.
Chemical properties:
- Ethane is a saturated hydrocarbon and hence,itis quite stable.
- Like methane,itis chemicallyinert to acids, bases, oxidising and reducing agents.
- The main reactions that ethane undergoes are substitution reactions similar to methane.
3. Reactions of ethane
1. Combustion:
Ethane burns in air or oxygen with a nonluminous flame, producing CO2 , H2O and considerable amount ofheat.
2C2H6 + 7O2 → 4CO2 + 6H2O 368 kcal-mol-1
2. Halogenation:
In diffused sunlight, ethane undergoes a stepwise substitution reaction with chlorine or bromine to yield different compounds. Ethane contains two methyl groups. So ethane produces two disubstituted, two trisubstituted, and two tetrasubstituted chloro or bromo derivatives in this substitution reaction,

Due to the substitution of H-atoms of C2H6 by P & Cl- atoms, chlorofluoroethanes are formed. Some chloroform-ethanes are known as freons,
For example: Cl2FC—CCIF2 (Freon 113), CH3, (Freon-114) F3C—CClF2(Freon-11 5) etc.
3. Nitration:
Ethane reacts with nitric acid vapor sat 400°C to form nitroethane.

4. Pyrolysis:
When ethane is heated at 700°C in the absence of air, it decomposes to yield mainly ethylene

Uses of ethane:
Ethane is mainly used as fuel.
It is used to prepare ethyl chloride and C2H4 etc., C2H4 is an important raw material for the preparation of various organic compounds
Alkenes Olefins
Unsaturated hydrocarbons in which at least two adjacent carbon atoms are linked by a double bond are called alkenes. Alkenes are also called olefins (Greek: Olefiant = Olt forming) because the lower members (eq, ethene, propene) react with halogens cl or Br to form silly substances. They are represented by general formula CnH2n, where n = 2,3… etc
Nomenclature of alkenes
IUPAC nomenclature of aJJames has been only the IUPAC names of some higher alkenes mentioned here

Structure of the carbon-carbon double bond
The carbon-carbon double bond consists of one cr -bond and one σ-bond. The tr-bond is formed by head-on overlapping or axial overlapping of two sp² -hybridized orbitals of two C-atoms while the σ-bond is formed by lateral or sideways overlapping of the two unhybridized p-orbitals (let us assume, pz -orbital) of the two C-atoms. The n -n-electron cloud remains distributed above and below the plane in which the two C-atoms and the atoms attached to them exist.
If now one of the C-atoms of the double bond is rotated along the axis of the cr -bond keeping the other C-atom fixed the (>. orbitals will no longer be parallel and there will be no overlap between them. As a result, the π -bond will break, However, the breaking of an n -bond requires 60 kcal mol-1 of energy which Is not provided by the collision of the molecules at room temperature.
Consequently, free rotation of the doubly bonded carbon atoms Is not possible at room temperature. So the relative positions of the four groups (a, b, and a, b) attached to the two doubly bonded C-atoms remain fixed. The value of each of the three bond angles originated around the doubly bonded C-atoms (sp² -hybridized) is 120°

Isomerism of alkenes
Alkenes exhibit both structural isomerism and geometrical or ds-trans isomerism.
Structural isomerism:
Alkenes containing three or more carbon atoms can exhibit position, chain, and ring-chain isomerism.
1. Position isomerism:
This isomerism arises due to the difference in the position of the double bond in a particular carbon chain. For example, but-l-ene and but-2-ene are two position isomers
CH3CH2CH=CH2 (But-l-ene)
CH3CH =CHCH3(But-2-ene)
Hydrocarbons Class 11 Chemistry Solutions
2. Chain isomerism:
This type of isomerism arises due to differences in the carbon skeleton. For example, but-l-ene and 2-methyl prop-l-ene are two chain isomers

Ring-chain isomerism:
This type of isomerism arises due to ring closure. For example, propene and cyclopropane are two ring-chain isomers

2. Geometrical or cis-trans isomerism:
Due to restricted rotation about die carbon-carbon double bond, alkenes exhibit geometric or ds-trans Isomerism.
For example The two geometric isomers of 2-butene are as follows

As each of the doubly bonded carbon atoms Is attached to two different groups, two types of spatial arrangements of groups are possible. The geometric isomers which have similar groups on the same side of the double bond are called c/s-isomers while the geometric isomers which have similar groups on the opposite sides of the double bond are called trans-isomers. Both the isomers have same structure but they have different configurations (arrangements of groups in space)
4. Relative stabilities of alkenes
Hydrogenation of alkenes leads to the formation of relatively more stable alkanes and the amount of heat evolved when 1 mol of an alkene is hydrogenated is cailed heat of hydrogenation. Higher the heat of hydrogenation of an alkene, less is its stablity. Therefore, the stabilities of alkenes can be predicted from the values of their heats of hydrogenation

Order of stabilities of alkenes based on their heat of hydrogenation values: 1 >2 >3 > 4 > 5 > 6.
Explanation of relative stabilities of alkenes:
Relative stabilities of alkenes can be explained based on hyperconjugation. The greater the number of a -H atoms i.e., the greater the number of hyperconjugative structures, higher the stability of the alkene.
A number of a-H atoms present in the alkenes I, II, III, IV, V, and VI are 12, 9, 6, 6, 3 and 0 respectively. Therefore, the order of stability is: I >II >III = IV > V > VI. However, due to presence of two methyl (-CH3) groups on the same side ofthe double bond in ds-2-butene (IV), it is somewhat less stable than the trans-isomer (III) due to steric hindrance. Hence, the correct order of stabilityis: I >II >III > IV > V > VI.

Generalalkenes Methods Of Preparation Of Alkenes
By elimination reactions
Reactions that involve the removal of two atoms or groups from two adjacent carbon atoms of an organic compound resulting in the formation of a double (or triple) bond in between those two carbon atoms are called elimination reactions.
By dehydration of alcohols:
- When alcohols are heated with molecule of water gets eliminated to form alkenes.
- In these reactions, —OH group is lost from the a -carbon atom while H atom is lost from the p -carbon atom.
- Therefore, for a dehydration reaction, the alcohol must contain a ft hydrogen atom. 0 Concentrated phosphoric acid can be used instead of concentrated sulphuric acid’

Mechanism of dehydration:
It is an El (Elimination unimolecular) reaction which proceeds via three steps.
These are
- Protonation of alcohol
- Removal of H2O and formation of carbocation
- Loss of proton by the carbocation.
- The second step (slowest step) is the rate-determining step of the reaction.

Some important points related to the dehydration of alcohols:
- For dehydration of primary and secondary alcohols, concentrated H2SO4 and for dehydration of tertiary alcohols, dilute H2SO4 are effective.
- For different alcohols, ease of dehydration follows the one order: 3° alcohol > 2° alcohol > 1° alcohol.
- Alcohol vapors, when passed over phosphorous pentoxide (P2O5) or heated alumina(Al2O3) produce alkene with the elimination of one water molecule

- Rearrangement during dehydration of alcohols: During dehydration of alcohols, sometimes unexpected or rearranged alkenes are formed. These rearrangements happen due to 1, 2-hydride or 1, 2- methyl shift in order to form a more stable carbocation intermediate.
Examples:

By dehydrohalogenation of alkyl halides:
When alkyl halides are heated with alcoholic caustic potash (KOH dissolved in ethanol) solution, alkenes are produced.
In this reaction, the halogen atom is lost from the a carbon atom while H-atom is lost from the β -carbon atom and therefore, it is also β -elimination reaction.

Mechanism:

Example:

Important points related to dehydrohalogenation of alkyl halides:
1. Due to the greater solubility of KOH than that of NaOH in ethanol, ethanolic potassium hydroxide is a more effective reagent. In this reaction, alcoholic solution of sodium or potassium alkoxide can also be used. 3-1
2. In that case, alkoxide ion (RO–) acts as a base. For example, potassium ethoxide (C 2H 5O–K+) dissolved in ethanol, potassium ferf-butoxide (Me 3CO–K+) dissolved in tertbutyl alcohol.
3. Alkyl halides undergo hydrolysis in the presence of KOH (or NaOH) dissolved in the more polar solvent water to give mainly alcohols through substitution reactions.
4. On the other hand, they undergo dehydrohalogenation reaction, i.e., elimination reaction in the presence of KOH (or NaOH) dissolved in the less polar solvent ethanol to produce alkenes as the major product

5. When an alkyl halide is heated in the presence of ethanolic KOH solution or potassium alkoxide dissolved in alcohol, ether is obtained as a side product along with alkene. If a primary alkyl halide is used, the possibility of the formation of ether becomes much higher because in that case reaction is more likely to proceed through SN2 mechanism.

6. The order of reactivity of different types of alkyl halides towards de hydrohalogenation reaction is: alkyl iodide > alkyl bromide > alkyl chloride. In case of alkyl groups, order of activity is: tertiary > secondary > primary.
By heating 4° ammonium hydroxide:
Alkene is obtained by heating 4° ammonium hydroxide.
For example: When tetraethylammonium hydroxide is heated, C2H4 is formed.

Saytzeff and Hofmann rules:
During the preparation of alkenes via elimination reaction (E2 mechanism), more than one alkene can be produced if two or more carbon atoms adjacent to the carbon atom containing the leavinggroup
For example: —X, —+NR3, –+SR2) have available H-atom.
1. According to the Saytzeff rule, if the leaving group be halide (except fluoride) or sulphonate (neutral substrate), the E2 reaction leads to the formation of a highly substituted alkene as the major product. This is called Saytzeff product. For example, when 2- bromobutane is heated with ethanolic KOH solution, 2-butene (80%) is obtained as the major product.

2. According to Hofmann rule, If the leaving group be a charged one
For example: +NR3 or – +SR2),
Then the E2 R — C = C —R- frans-alkene (major) reaction leads to the formation of a less substituted alkene as the major product. This is called the Hofmann product. For example, when cetyltrimethylammonium hydroxide is heated, 1- butene (95%) is obtained as the major product

NCERT Solutions Class 11 Chemistry Chapter 13 Hydrocarbons
By dehalogenation of vicinal dihalides:
Dihalogen derivatives of alkanes in which the two halogen atoms are attached to adjacent carbon atoms are called vicinal dihalides or 1,2- dihaloalkanes. When a methanolic solution of a vicinal dihalide is heated with Zn-dust, one molecule of halogen is eliminated from the dihalide molecule to produce an alkene.

By the reduction of alkynes
When alkynes are reduced with sodium in liquid ammonia, (rans-alkenes are obtained as the major product.
Reduction of alkynes by hydrogen in the presence of Lindlar’s catalyst [Pd-CaCO3 partially poisoned with lead acetate, Pb(OAc)2 ] gives cfs-alkene predominantly


By Kolbe’s electrolysis
When an aqueous solution of sodium or potassium salts of saturated dicarboxylic add are electrolyzed, alkenes are obtained.

By pyrolysis or cracking of alkanes
When alkanes are passed through a tube heated at 500-600°C in the absence of air, they undergo thermal decom¬ position to produce lower alkenes, alkanes and hydrogen.

By pyrolysis of some other compounds
Alkenes are obtained by the pyrolysis of esters, xanthates and N-oxides of tertiary amines

Cope elimination reaction:
Tertiary amine oxides on thermal decomposition produce alkenes and dialkylhydroxylamines. This reaction is known as Cope elimination reaction
General Properties Of Alkenes
1. Physical properties of Alkenes
- The first three members of alkene family, i.e., ethene, propene and butene are colourless gases, the next fourteen members (C5-C18) are liquids while the higher members are solids at room temperature. All are colourless.
- Except for ethene which has a pleasant odour, all other alkenes are odorless.
- All alkenes are lighter than water and insoluble in water. However, they are soluble in non-polar organic solvents like benzene, petroleum ether, carbon tetrachloride, alcohol, chloroform etc.
- Due to the presence of double bond (C=C), some alkenes exhibit geometrical isomerism.
Boiling points:
The boiling points of alkenes increase regularly with increase in molecular mass and for the addition of each CH2 group to the chain, the boiling points increase by 20- 30.
In cls-alkene, the alkyl groups lie on the same side of the double bond but in trans-alkene, the alkyl groups lie on the opposite sides of the double bond. For this reason, the molecules of cis-isomer are polar but the molecules of trans-isomer are non-polar or less polar. Thus, in the liquid state, the intermolecular attractive forces are relatively stronger in the case of the cis-isomer and hence it has a higher boiling point than the trans-isomer.

Melting points:
In trans alkene, the molecules are more symmetrical, and therefore, they can pack more closely in the crystal lattice than the molecules of cis-alkene. Due to this, the intermolecular forces operating in frans-alkene are stronger and iso, a larger amount of heat is required to break the crystal lattice of trans-alkene than the corresponding lattice ofc/s-alkene. Thus, the melting point of trans-alkene is higher than that of c/s-alkene
2. Chemical properties of Alkenes
The double bond present in alkenes consists of a strong cr-bond and a weaker 7r-bond. The 7T -electrons are loosely held and are easily polarisable. So the n-bond takes part in chemical reactions easily.Itis for this reason, alkenes are more reactive than alkanes. The typical reactions of alkenes are addition reactions in which the rr -bond breaks and two new (r-bonds are formed. Two monovalent atoms or groups become attached to the doubly bonded carbon atoms to form a saturated compound.
General Reactions Of Alkenes
Chemical reactions of alkenes are generally divided into five classes:
- Combustion reaction
- Addition reaction,
- Oxidation reaction
- Polymerization reaction and
- Substitution reaction.
- Combustion reactions
Alkenes are combustible substances. They bum in air or in O2 with a luminous flame to yield CO2 and H2O with the evolution of heat. The percentage of carbon content in alkenes is higher than that in alkanes and so during combustion alkenes produce black smoke due to liberation of free carbon.
2CnH2n + 3nO2→ 2nCO2+ 2nH2O + heat
Example: CH2=CH2 + 3O2 →2CO2 + 2H2O + heat
1. Addition reactions Definition
The reactions in which two reactant molecules are added together to yield a single molecule of product are called addition reactions
Characteristics:
- The compounds obtained in addition reactions are called addition products.
- Alkenes give additional products by reacting with halogens (Cl2, Br2 or I2), hydrogen halides (HX, X = Cl, Br, I), ozone etc.
- The molecule which gets attached to the unsaturated molecule in addition reaction is known as addendum.
Addition of hydrogen:
Presence of finely divided platinum, palladium or Raney nickel at ordinary temperature or finely divided nickel at 200-300°C hydrogen adds to the double bond of the alkene to give an alkane

Addition of halogen:
1. At ordinary temperature, halogens (Cl2 or Br2) participate in addition reactions with alkenes to produce vicinal dihalides. Generally, the alkenes are added to solutions of halogen dissolved in carbon tetrachloride (CCl4).

2. In case of iodine, this reaction is reversible and takes place very slowly. © At ordinary temperature, fluorine does not from additional compounds with alkenes. However, at extremely low temperatures (-78°C) & under controlled conditions, the addition reaction may be carried out by using xenon fluoride as the reagent. In addition reactions, the order of reactivity of halogens is: CI2 > Br2 > I2.
Mechanism of the reaction:
The reaction of halogen with alkene is an electrophilic addition reaction. Bromine molecule is non-polar. However, under the influence ofn -electrons of C=C bond, the displacement of cr -electrons of the bromine molecule takes place.
As a result, the Br-atom which is close to the double bond acquires partial positive charge while the other Br-atom gains partial negative charge.
The reaction of an alkene with this polarised bromine molecule takes place in two steps as follows:
First step:
The σ-electrons of carbon-carbon double bond attacks the bromine atom having partial positive charge. This results in heterolytic fission of Br—Br, σ-bond and the positive bromine atom (electrophile) becomes attached to both the doubly bonded carbon atoms concertedly to form a cyclic bromonium ion. A bromide ion is obtained along with it. It is the slow or rate-determining step of the reaction.

Second step:
The bromoniumion undergoes nucleophilic attack (SN2) by the Br– ion and the cyclic ion opens up Markownikoff’s rule: In the addition reactions of producing 1,2-dibromoethane (addition compound). This step is fast

As the addition reaction is initiated by electrophilic end of the reagent, it is termed an electrophilic addition reaction.
If the electrophile contains no unshared pair of electrons or if one of the double bonded carbon contains an aromatic ring (as in the case of C6H5CH=CH2), then a carbocation intermediate is formedin first step.
Addition of halogen hydracids:
Halogen hydracids HX (X = Cl, Br, I) undergo an addition reaction with alkenes to form alkyl halides. The reactivity of halogen hydracids towards addition reactions of alkenes follows the order: HI > HBr > HCl > HF

Example:
(Ethylene)CH2=CH2 + HBr → CH3CH2Br(Ethyl Bromide)
When a halogen hydracid reacts with an unsymmetrical alkene, there is a possibility of formation of two different alkyl halides.
Example:
Propene is an unsymmetrical alkene. Propene, when reacts with HBr, may give rise to both 1-bromopropane and 2-bromopropane. However, one of the two products is formed predominantly

In addition reactions of this type, i.e., in case ofaddition of an unsynunetrical addendum (as H—X) to an unsynunetrical alkene, out of the two addition compounds, the compound obtained as themajorproduct is governed by a rule known as Markownikoff’s rule.
Markownikoffs rule:
In the addition reactions of producing 1,2-dibromoethane (addition compound). unsymmetrical addendum (adding molecules) with unsymmetrical alkenes, the negative part of the addendum becomes attached mainly to that doubly bonded carbon atom which carries a lesser number of hydrogen atoms
Example:
In the addition reaction of HBr with propene, 2- bromopropane is obtained as the major product because C-2 of propene contains lesser number of hydrogen atoms than C-l and the negative part of her, i.e., bromine (Br) is added to C-2

Similarly, the addition of HI with 2-methylpropene takes place according to Markownikoff’s rule and 2-iodo- 2-methylpropane is obtained as the major product

Mechanism of the reaction:
In the first step of the following electrophilic addition reaction,
CH2CH=CHCH2+ HBr→ CH3CH2CHBrCH3
the electrophilic end (H) of \(\stackrel{\delta+}{\mathrm{H}}-\stackrel{\delta-}{\mathrm{Br}}\) is attracted by the r -electron cloud of the double bond, i.e., the σ-bond attacks the H atom of HBr, thus forming a new C—H bond while breaking the H—Br bond. As the remaining carbon atom of the original double bond is left with six electrons, a carbocation is formed. This is the rate determining step of the reaction. In the second step, the nucleophile Br– ion is added to C+ of the carbocation to form 2-bromobutane as the only additional compound.
Since the addition of the electrophile occurs in the determining step (the first step), the reaction is called an electrophilic addition reaction

Explanation:
When propane (CH3CH=CH2) reacts with HBr, the proton obtained from HBr can add to the number1 carbon atom (C- 1) to form an isopropyl cation (a secondary carbocation) or it can add to the number 2 carbon atom (C- 2) to form n -propyl cation (a primary carbocation).
The secondary carbocation is formed more easily and rapidly because it is more stable than the primary carbocation. Therefore, 2-bromopropane is expected to be formed as the major product in this reaction. In fact, 2-bromopropane is the predominant product obtained in this case

Exception:
The addition reaction of CH2=CH —CF3 with halogen hydracids
For example: HCl takes place contrary to Markownikoff rule.
1. In this case, the carbocation formed by the addition of H+ to C-l, in spite of being a secondary (2°) carbocation, becomes less stable than the primary (1°) carbocation formed by the addition of H+ to C-2 because of the presence of strong electron-attracting CF3 group. So the reaction occurs mainly through the formation of primary carbocation thereby producing ClCH2CH2CF3 as the only addition compound.

2. During addition of halogen acids to the alkene sometimes rearrangement occurs by 1, 2-hydride shift or 1, 2-methyl shift to form a more stable carbocation intermediate


Peroxide effect:
Addition of hydrogen bromide (HBr) to an unsymmetrical alkene in the presence of organic peroxides such as benzoyl peroxide (C6H5CO)2O2, differ-butyl peroxide (Me3COOCMe3) etc., or oxygen, takes place contrary to Markownikoff’s rule (antiMarkownikoff addition), i.e., Br” is added to that carbon atom of the double bond which contains greater number of H-atoms.
This abnormal addition in the presence of a peroxide is known is the peroxide effect or Kharash effect.
Example:
Addition reaction of propene with HBrin presence of an organic peroxide leads to the formation of 1- bromopropane as the major product.

Mechanism of the reaction:
The reaction of HBr with unsymmetrical alkene, e.g., propene (CH3CH=CH2), in the presence of an organic peroxide, follows free radical mechanism. The reaction takes place in four steps—
First step: Homolytic fission of benzoyl peroxide gives rise to phenyl radical

The reactive bromine radical (Br)thus produced uses itselfin the third step to continue the chain reaction.
1. The exception to Markownikoff’s rule in the presence of peroxide is observed only in the case of HBr.
Class 11 Hydrocarbons Important Topics
2. HF, HCI and HI do not exhibit peroxide effect:
Only if the two reactions of the third and fourth steps ofthe reaction mechanism described above are exothermic, then this additional reaction becomes possible. However, if any step becomes endothermic, the reaction will not occur.
It has been observed experimentally that onlyin the case of HBr both the steps are exothermic but in the case of HF, HCI or HI, one or the other of these steps is endothermic. For these reasons, HF, HCI, and HI do not respond to peroxide effect.
3. Alternative explanation:
The H—Cl bond is stronger than H—Br bond so the former is not cleaved by the free-radical. On the other hand, H—I bond is weaker than the H—Cl bond but iodine free radicals combine with each other rapidly to form iodine molecules instead of being added to carbon. Thus, HCI and HI do not exhibit a peroxide effect.
Addition of sulphuric acid:
Alkenes are absorbed by cold and concentrated sulphuric acid to form alkyl hydrogen sulfate (an inorganic ester)
(Alkene) RCH=CHR + H→OSO3H(Sulphuric acid) → RCH2CHR—OSO33H (Alkyl hydrogen sulphate)
Example:
H2SO4 is an unsymmetrical addendum. So the additional reaction of unsymmetrical alkene,
For example: Propene (CH3CH=CH2) with H2SO4 takes place according to
Markownikoff’s rule. Consequently, isopropyl hydrogen sulfate is formed as the predominant product

Alkyl hydrogen sulphates, when heated with water, undergo hydrolysis to give alcohols.

Addition of water (hydration):
Water adds to alkenes in the presence of acid catalysts to produce alcohols.

Example:
As H2O is an unsymmetrical addendum, an unsymmetrical alkene like 2-methylpropene reacts with water to form fert-butyl alcohol following Markownikoff’s rule.

Addition of hypogeous acid:
The addition of hypohalous acid (HOδ -δ+Cl or HOδ -δ+Br ) to an alkene occurs when the alkene is treated with chlorine (Cl2) or bromine (Br2) in the presence of water. As a result, a vicinal halohydrin (a compound that contains both an —OH group and a halogen atom bonded to adjacent carbons) is produced. A small amount of vicinal dihalide is also obtained.

Hydroboration (Reaction with diborane):
When alkene reacts with diborane (B2H6), trialkylborane, an addition compound of BH3, is produced.

When trialkyl borane is subjected to oxidative hydrolysis by treating with H2O2/NaOH, alcohol is produced. As a result of hydroboration and oxidative hydrolysis, antiMarkownikoff hydration of an unsymmetrical alkene

Oxymercuration-demarcation:
The reaction between an alkene and an aqueous solution of mercuric acetate leads to the formation of p -hydroxyalkylmercuric acetate (an addition compound). This reaction is known as oxymercuration. In this reaction, tetrahydrofuran is used as a solvent

When the compound produced is reduced with alkaline sodium borohydride, the —HgOCOCH3 group is replaced by hydrogen to yield an alcohol. This is called demercuration.

Oxymercuration-demarcation is an alternative process for the addition ot water molecule to an alkene:
- In this method, the addition of water to unsymmetrical alkenes takes place by Markownikoff’s rule.
- As there is no possibility of a rearrangement reaction in this process of hydration, it is more effective than the conventional process of acid-catalysed hydration.
Ozonolysis:
The complete process of preparation of ozone by reacting with an alkane and then allowing ozone to decomposition of the resulting ozonide to give carbonyl compounds (aldehyde and/or ketone) Is called ozonolysis.
1. First step:
When ozone gas or a mixture of ozone and oxygen is passed through an alkene dissolved in a solvent with which ozone does not react
For example:
Chloroform, carbon tetrachloride or glacial acetic acid), ozone adds on to the alkene to form an addition compound, called alkene ozonide

2. Second step:
If alkene ozonide is heated with water or acetic acid in presence ofzinc dust, it decomposes (hydrolysis) to give aldehyde or ketone or a mixture of both depending on the structure of alkene. This decomposition of ozonide is referred to as reductive decomposition

Reason for using Zn-dust:
If the ozonide is decomposed by water alone (without zinc dust), the aldehyde formed is oxidised by H2O2 (another product of hydrolysis) to carboxylic acid. As zinc dust destroys (reduces) HO , the possibility of this oxidation reaction can be avoided and hence, aldehyde is obtained
RCHO + H2O2 → RCOOH + H2O
H2O2 + Zn → ZnO + H2O
At present, ozonide is decomposed by dimethyl sulphide (Me2S). As a result of this reaction, dimethyl sulphoxide (Me2S= O) and carbonyl compound arc were produced. This reaction also provides no scope for the oxidation of aldehyde
Dissociation of C=C bond by ozonolysis:
In ozonolysis, the C=C bond of an alkene dissociates and as a result, two molecul of carbonyl compound are produced from one molecule of alkene.

Prediction about the nature compounds to be obtained on ozonolysis: if the structure of an alkene is known, the carbonyl compounds to be obtained on its ozonolysis can be easily determined.
After writing the structure of the alkene, the molecule is divided into two parts by causing fission of the double bond and by adding an oxygen atom to each carbon atom of the double bond. Thus, the nature of the carbonyl compounds to be obtained on ozonolysis can be ascertained

Different products of ozonolysis depending an the nature of alkene:


Determination of the structure of an alkene from the products obtained on its ozonolysis:
The structures of the two carbonyl compounds formed by ozonolysis are written side by side, their carbonyl groups facing each other. The two oxygen atoms are then removed and finally, the two carbonyl carbon atoms are joined by a double bond.
Example:
If ozonolysis of an alkene forms acetaldehyde and acetone as products, it may by concluded that the alkene is 2-methyI-2-butene.

Some important points related to ozonolysis:
1. If formaldehyde is obtained on ozonolysis, then the double bond of the alkene lies at the terminal position of the carbon chain i.e., the alkene must contain a terminal methylene group (=CH2).
2. When ozonolysis gives rise to two molecules of the same carbonyl compound, the original alkene must be a symmetrical one but if two different carbonyl compounds are formed, then the alkene must be an unsymmetrical one.
3. If an alkene, on ozonolysis, leads to the formation of only one dicarbonyl compound, then the original alkene must be a cyclic alkene.
For example, cyclobutene on ozonolysis gives butanediol:

4. Any compound having two double bonds (an alkadiene) on ozonolysis produces one molecule of a dicarbonyl compound and two molecules of carbonyl compound.
For example:
1, 3 -pentadiene, when subjected to ozonolysis, gives one molecule of acetaldehyde, one molecule of glyoxal and one molecule of formaldehyde.

5. When two geometrical isomers are subjected to ozonolysis, they yield the same compounds. For example, both the cis-and trans-isomers of 2-pentene, when subjected to ozonolysis, give acetaldehyde and propanal.

6. If CO2 is evolved on ozonolysis, then the original alkene must be an allene, i.e., an alkadiene in which the double bonds are adjacent to each other.
For example:

7. In the process of ozonolysis, carbon-carbon double bond undergoes fission to yield carbonyl compounds. So ozonolysis is an example of cleavage reaction of alkene
8. Ozonides on being reduced by LiAlH4or NaBH4 gives alcohols instead of carbonyl compounds.
For example:

9. When ozonides are decomposed by water in the absence of Zn, c-atom of the double bond, bonded to two h-atoms is ethylene converted into CO2 whereas die doubly bonded c, bonded to one H-atom and one alkyl group, is converted to carboxylic acid. however, if any doubly bonded c atom bears two alkyl groups, it is converted to a ketone. this decomposition is referred to as oxidative decomposition

Ozonolysis of alkynes:
Alkynes on ozonolysis yields 1,2-dicarbonyl compounds.
For example:
Glyoxal is obtained on ozonolysis of acetylene. 2-oxopropanal is’ obtained on ozonolysis of propyne and butanedione is obtained on ozonolysis of but-2-yne

Class 11 Hydrocarbons Important Topics
2. Oxidation reaction
1. Oxidation by alkaline KMnO4:
1. Cold and alkaline potassium permanganate solution oxidizes an alkene to yield a 1,2-diol or vicinal diol by attaching one hydroxyl group to each of the doubly bonded carbon atoms. This reaction is called hydroxylation of alkenes. In this case, cis-hydroxylation occurs because the addition of two OH groups takes place from the same side of the double bond.

Baeyers test:
Dilute alkaline solution (1-2%) of potassium permanganate is known as Baeyer’s reagent. The colour of the reagent is purple which disappears when it is allowed to react with any compound belonging to the class of alkenes or alkynes. So this reaction is used to test the presence of unsaturation, i.e., carbon-carbon double or triple bond in an organic compound. This is known as Baeyer’s test.
2. A hot and concentrated solution of alkaline KMnO4 oxidises alkenes to give acid or ketone or a mixture of both. In this case, cleavage of the double bond occurs to yield the products.
- If =CH2 group is present in the alkene, then it is oxidised to CO2 and H2O. If the alkene contains =CHR group, then it is oxidised to RCOOH
- If the alkene.contains =CR2 group, then the group gives rise to R2C=O by oxidation

By osmium tetroxide (0sO4):
Osmium tetroxide (OsO4) adds on to the double bond of an alkene to form osmic ester (a cyclic compound). When this cosmic ester is hydrolysed by the aqueous ethanolic solution of sodium bisulphite, 1,2- diol or vicinal diol is obtained.
Therefore, it is also a hydroxylation reaction. In this case, cis-hydroxylation preferentially because the two hydroxyl (—OH) an occurs groups add on to the doubly bonded carbon atoms from the same side of the double bond

2. Epoxidation (Formation of epoxide):
1. Alkene oxides or epoxides are formed when alkenes react with per acids
For example:
Perbenzoic acid, chloroperbenzoic acid etc.) This reaction is called Prileschaiev’s reaction

The resulting epoxide oh hydrolysis with dilute acid or alkali yields 1,2-diol. Epoxidation followed by hydrolysis causes the addition of two OH groups from the opposite sides of the double bond, i.e., in this case, trans-hydroxylation occurs.

Formation of carbonyl compounds (Wackcr process): when a mixture of alkene vapours and oxygen, at high pressure and 50°C, is passed through a solution of palladium chloride containing CuCl2, the corresponding carbonyl compound is formed. Pd(II) ion is reduced to metallic Pd

Example:

3. Polymerisation
Polymerisation Definition:
The reaction in which two or more molecules of a simple compound unite together under the influence of suitable pressure, temperature and catalyst to produce a single large molecule, i.e., a macromolecule of high molecular mass (whose molecular mass is a multiple of the molecular mass of the simple compound) is called polymerization (Greek word poly = many, meros = unit, member, or part).
The product obtained by the reaction is called polymer and the simple small molecules from which the polymer is formed are called monomers. Alkenes possess a tendency to polymerize.
Polymerization of ethylene:
Depending on the reaction conditions and catalyst used,
Two types of polyethylene or polythene may be obtained.
1. Low-Density Polythene (LDPE):

It is used for making waterproof covers, toys, sheets used in construction work etc
2. High-Density Polythene (HDPE):

The tensile strength of this type of polythene is much higher. It is used in making pipes, bottles, water tanks, and as insulation for electric wires and cables.
Polymerisation of propylene:
Polymerisation of propene or propylene leads to the formation of polypropylene

Polypropene is much harder than polyethylene and its tensile strength is also higher. It is used for making ropes, buckets, automotive moulding, seat covers, containers for storing oils and gasoline, carpet fibers and packing.
Polymerization of substituted ethenes:

Teflon is inert towards acids, alkalis and other chemicals. It is used in making chemically resistant pipes and surgical tubes. Due to chemical inertness and high thermal stability, teflon is used for making non-stick utensils
5. Substitution reactions
Although addition reactions are the characteristic reactions of alkenes, yet alkenes also respond to substitution reactions under special condition, e.g., O when a mixture of ethylene and chlorine gas is heated at 350-400°C, vinyl chloride is formed through substitution reaction

6. Isomerisation
When alkenes are heated at 200 – 300°C in the presence of aluminium chloride as catalyst or at 500 – 700°C in absence of a catalyst, they yield isomeric compounds through change in the position ofthe double bond or alkyl groups. Example: When 1-butene is subjected to isomerisation, it gives 2-butene and 2-methyipropene

Ethylene Or Ethene (C2H4)
Ethylene (CH2=CH2) is the first member of the olefin or 1 alkene series. The general formula of alkenes is CnH2n. The formula of ethylene can be obtained by putting n = 2.
1. Preparation of ethylene
Laboratory preparation:
Principle:
Ethylene is prepared in the laboratory by heating a mixture of ethyl alcohol and excess of concentrated sulphuric acid (about three times the volume of ethanol) at 165-170°C . Concentrated sulphuric acid being a strong dehydrating agent eliminates a molecule of water from ethanol to liberate ethylene. In this ethyl alcohol undergoes intramolecular dehydration.

Steps of the reaction:
The reaction takes place in two steps. In the first step ethyl hydrogen sulphate (an inorganic ester) and water are produced. In the second step, ethyl hydrogen sulphate decomposes at 165-170°C to yield ethylene and sulphuric acid.

CBSE Class 11 Chemistry Chapter 13 Hydrocarbons Overview
Collection:
- Concentrated sulphuric acid being a strong oxidising agent oxidises ethyl alcohol to CO2 , itself being reduced to SO2 So, CO2 and SO2 thus produced remain as impurities in ethylene.
- These acidic impurities are removed by passing the gas through NaOH or KOH solution.
- The gas is collected by the downward displacement of water in a gas jar.
Drying:
Ethylene thus collected contains a little water vapour which is removed by passing through a tower filled with fused CaCl2 and the pure gas is collected over mercury
- In the preparation of ethylene, concentrated sulphuric acid (H2SO4) acts as an acid catalyst and water absorbent.
- In this process, if the quantity of cone. H2SO4 is not high or the quantity of alcohol taken is high and the temperature is less than 165 – 170°C, one molecule of water is removed from two molecules of alcohol, yielding diethyl ether (intermolecular dehydration).

- The use of syrupy phosphoric acid at 230°C instead of concentrated sulphuric acid improves the purity of ethylene (free from CO2 and SO2 ) formed.
- Ethylene cannot be dried by concentrated sulphuric acid because it absorbs ethylene to form ethyl hydrogen sulphate
CH2=CH2 + H2SO4 →CH3— CH2 —OSO3H
Other methods of preparation of ethylene:
1. Ethylene Is prepared by heating ethyl alcohol In the presence ofstrong dehydrating agent like phosphorus pentoxide.
3C2H5OH + P2O5 → 3 [CH2=CH2] + 2H3PO4 ]
2. When ethyl alcohol vapours are passed over heated alumina at 350°C, ethylene Is produced (Industrial preparation of ethylene).

3. Ethylene gas is obtained when ethyl chloride, bromide or iodide is heated in the presence of a concentrated alcoholic solution of caustic potash

[X= Cl,Br,I]
4. When alcoholic solution of ethylene dichloride or dibromide is heated with zinc dust, ethylene is produced.

5. Pure ethylene may be prepared from impure ethylene by using Oxidation reactions using this reaction. The impure ethylene is first converted into ethylene dibromide by allowing it to react with bromine. It is then washed with Na2 CO3 solution, dried by anhydrous CaCl2 and finally distilled to obtain pure ethylene dibromide. Pure ethylene dibromide thus obtained gives pure ethylene when heated with zinc dust

6. By partial hydrogenation of acetylene: When a mixture of acetylene and hydrogen is passed over palladium on silica gel support at 200°C, ethylene is produced.
By Kolbe’s electrolytic Process:

By thermal decomposition of petroleum: To meet industrial demand
For example:
Manufacture of polythene, production of ethyl alcohol on industrial scale etc.), ethylene is required in large quantities. Industrial requirements of ethylene are mainly fulfilled by cracking of petroleum
2. Properties of ethylene
Physical properties:
- Ethylene is a mild, sweet-smelling colourless gas.
- Its boiling point Is -105°C and its melting point Is -IG9.5°C.
- It Is almost as heavy as air. Its vapour density is 14.
- It is liquefied at 0°C and 44atm pressure. h is sparingly soluble in water but is highly soluble in alcohol and ether.
- It possesses anaesthetic properties.
Chemical properties:
In ethylene, the two C-atoms are linked by a double bond, one of which is a strong cr -bond and the other is a weak σ-bond. Hence, ethylene is easily oxidised and participates in an addition reaction. Due to the presence of π-bond, ethylene is more reactive than ethane. During the addition reaction,π -bond in ethylene is broken and two new σ -bonds are formed by which two monovalent atoms or radicals get attached to the C- atoms
General Reactions Of Ethylene
1. Oxidation reactions
Combustion:
Ethylene is a combustible gas but it is not a supporter of combustion. It bums with a sooty luminous flame with liberation of large amount of heat
C2H4 + 3O2→2CO2 + 2H2O + 337 kcal-mol-1
Due to the presence of unsaturation, the percentage of carbon in ethylene is more than that in ethane, having the same number of carbon atoms. So, as a result of its partial oxidation in air, some carbon particles are produced and the presence of hot carbon particles results in the emission of luminous flame. In excess of oxygen, ethylene undergoes complete combustion.
Reaction with potassium permanganate:
1. When ethylene gas is passed through cold and dilute (1-2%) alkaline KMnO4 solution, the purple (reddish-violet) colour of permanganate is discharged. Ethylene gets oxidised to form colourless ethylene glycol. This proves that ethylene is an unsaturated compound

2. Ethylene is oxidised to CO2 entered alkaline solution of KMnO4 and H2° by hot an

Formation of ethylene oxide:
When a mixture of air and ethylene is passed under pressure over silver catalyst at 250-400°C, ethylene undergoes partial oxidation by O2 to yield ethylene oxide.

Formation of acetaldehyde:
At 50°c, when a mixture of ethylene and O2 under high pressure is passed through a solution of palladium chloride (PdCl2) containing cupric chloride (CuCl2), acetaldehyde is produced.
CH2 = CH2 + PdCl2 +H2O →CH3CHOI = Pd +2HCl
2. Addition reactions
1. Addition of hydrogen:
At ordinary temperature and pressure in the presence of Pt or Pd or Raney nickel catalyst or at 200-300°C in the presence of Ni catalyst, hydrogen combines with ethylene to form the saturated hydrocarbon, ethane.

2. Addition of halogen:
1. Chlorine combines directly with ethylene in the presence of sunlight to yield additional compound 1,2-dichloroethane. It is an oily liquid which is commonly known as Dutch oil.
CH2 = CH2 + Cl →ClCH2CH2Cl
- When a mixture of ethylene and twice its volume of chlorine is ignited, ethylene bums with a red flame with the formation of carbon (soot) and hydrogen chloride. This reaction proves the presence of carbon in ethylene.
CH2 = CH2 + 2Cl2 → 2C + 4HCl
- At 350 – 450°C ethylene undergoes substitution reaction with chlorine to give vinyl chloride.
H2=CH2 + Cl2 → CH2 =CHCl (Vinyl chloride) + HCl
3. When ethylene gas is passed through a red-brown solution of bromine in CCl4 or CHCl3, the solution becomes colourless due to the formation of colourless 1,2-dibromoethane. This reaction is used as a test for detecting ethylene and ethylenic unsaturation

3. The reaction of ethylene with iodine is very slow and in fact, the reaction is reversible.

4. Fluorine is highly reactive, so it does not participate in addition reaction. It decomposes ethylene to give hydrogen fluoride and carbon
3. Addition of halogen hydracids:
The addition reaction of halogen hydracids with ethylene produces ethyl halides.
CH2=CH2 + HX → CH3CH2X [X = I, Br, Cl, F]
Order of reactivity of halogen hydracids: HI > HBr > HC1 > HF
4. Formation of halohydrin (addition of HOX):
When ethylene reacts with chlorine water or bromine water, ethylene chlorohydrin or 2-chloroethanol and ethylene bromohydrin or 2-bromoethanol are respectively formed the reaction with chlorine water, some amount of 1,2-dichloroethane (ClCH2CH2Cl) and with bromine water, (BrCH2CH2Br) are produced.

In this reaction, the red colour of bromine water some amount of 1,2 dibromoethane is discharged. So it can be used as a test for the detection of ethylene or ethylenic unsaturation in an organic compound.
2. Addition of sulphuric acid:
When ethylene gas is passed through concentrated sulphuric acid (98%), it gets absorbed by sulphuric acid to form ethyl hydrogen sulphate.
Ethyl hydrogen sulphate, when heated with a dilute sulphuric acid solution, undergoes hydrolysis to give ethyl alcohol and H2SO4 alkaline solution, Baeyer’s reagent (a drop or two) is added. If the reddish-violet or purple colour of permanganate is discharged and brown-coloured MnO2 is precipitated, then it indicates that the compound contains ethylenic unsaturation

1. It is to be remembered that in the test for unsaturation in an unknown organic compound, the disappearance of ; the reddish-violet or purple colour of cold dilute KMnO4 solution does not necessarily confirm the presence of unsaturation in the compound. If the organic compound contains any group
For example:
—CHO ) which is easily oxidised by KMnO4, the purple colour of KMnO4 may be discharged in spite of the absence of unsaturation in the compound.
2. Again, while dissolving the test sample in an organic i solvent in place of water, it should be remembered that the solvent must not be oxidised by KMnO4.
For example:
In this experiment, acetone can be used as a solvent but not ethanol because the latter is readily oxidised by KMnO4
3. Test with bromine solution
Dilute solution of bromine (2%) in carbon tetrachloride or chloroform is added dropwise to the solution of the organic sample in the same solvent. If the reddish-brown colour of bromine solution is discharged and no HBr gas is evolved (moist blue litmus paper held over the mouth of the test tube does not turn red), then it indicates the presence of ethylenic
unsaturationin the organic compound under consideration

- The above reaction is an addition reaction. HBr is not liberated in this reaction. The colour of bromine may also be discharged by substitution reaction, but in that case, the evolution of HBr takes place.
For example:

- Again, when an organic compound contains a group (e.g., —CHO group) which can be oxidised by bromine, then the colour of bromine gets decolourised with the liberation of HBr.
For example, glucose discharges the colour of bromine water

- In fact, for the detection of ethylenic unsaturation, both Baeyer’s test and Br2/CCl4 test are performed. If both the tests give positive results, then it can be concluded that the given organic compound contains ethylenic by unsaturation.
Alkynes Or Acetylenes
The unsaturated hydrocarbon containing carbon-carbon triple bond (C= C) are called alkynes. Each family member has four hydrogen atoms less than the corresponding alkane. The general formula of alkynes is CnH2n-2 where, n = 2, 3, 4, ••• etc.
The first member of the series is acetylene and so the other members of this series are also collectively known as acetylene
1. Nomenclature of alkynes
In trivial or common system of nomenclature, the name of the simplest alkyne is acetylene. The names of the higher alkynes are given as substituted acetylenes.
The IUPAC names of alkynes are derived from the names of the corresponding alkanes by replacing the suffix -ane by -yne. IUPAC nomenclature of alkynes has been discussed thoroughly in
Only the IUPAC names of some higher alkynes are mentioned here

2. Structure of the carbon-carbon triple bond
1. The C = C bond present in the simplest alkyne acetylene consists of one sigma (cr) bond and two pi (a-) bonds. Each carbon atom of acetylene has sp-hybrid orbitals. One sp -hybrid orbital of each C undergoes head-on (axial) overlap with sp -hybrid orbital of another carbon to form a C —C σ-bond. The second sp-hybrid orbital of each carbon overlaps axially with the ls-orbital of each of the two hydrogen atoms to form two C —H sigma bonds.
2. Each carbon atom has two unhybridised p -orbitals (let us assume, py and pz) which are perpendicular to each other as well as to the internuclear axis. The two parallel py orbitals, one on each carbon, overlap sideways to form a n -n-bond. Similarly, the sideways overlapping of two parallel pz orbitals, one on each carbon, leads to the formation of a second σ-bond. These two; r -electron clouds are perpendicular to each other.
3. The value of H —C= C bond angle in acetylene is 180° and therefore, the shape of the molecule is linear

4. The C—H bond length is 1.06A and the C = C bond length is 1.20Å. The C = C bond length is shorter than the C=C bond (1.34Å) and C—C bond(1.54Å)
3. Isomerism in alkynes
Alkynes exhibit 4 types of structural isomerism:
1. Position isomerism:
Alkynes (except ethyne & propyne) exhibit position isomerism due to difference in position of the triple bond in the carbon chain. For example,
CH3CH2C = CH (But -1- yne) and CH3C= CCH2 ((But -2- yne)
CH3CH2CH2C (Pent-1-yne)= CH and CH3CH2C = CCH3 (Pent-2-yne)
2. Chain isomerism:
Alkynes having five or more carbon atoms show chain isomerism due to differences the type of carbon skeleton. For example,
CH3CH2CH2C (Pent-1-yne)= CH and (CH3)2CH – C≡ CH
3. Functional group isomerism:
Dienes, i.e., compounds containing two double bonds, and alkynes are functional isomers of each other.
For example:
CH3CH2C = CH (But -1- yne) and CH= CH-CH = CH2 (Buta – 1,3 – diene)
4. Ring-chain isomerism: Alkynes are ring-chain isomers of cycloalkenes.
For example:

4. Classification of alkynes
Alkynes are classified into two types
- Terminal alkynes and
- Non-terminal alkynes.
In terminal alkynes, the C = C bond is present at one end of the carbon chain and in non-terminal alkynes, the C= C bond is present in any position other than the terminal positions
Terminal alkynes:
CH3CH2 C ≡ CH (But -1- yne)
CH3CH2CH2C ≡ CH(Pent -1- yne)
Non-terminal alkynes:
CH3C ≡ C CH3 (But -2- yne)
CH3C≡ C CH2 CH3 (Pent -2- yne)
CBSE Class 11 Chemistry Chapter 13 Hydrocarbons Overview
General Methods Of Preparation Of Alkynes
Like alkenes, alkynes can also be prepared by elimination
1. By dehydrohalogenation of vicinal dihalides:
1. When vicinal dihalides are refluxed with ethanolic KOH solution, alkynes are obtained due to the removal of two molecules of hydrogen halide.

2. Alkynes are formed at lower temperatures when sodamide (NaNH2) dissolved in liquid NH3 is used instead of alcoholic KOH

3. When vicinal dihalides are heated with ethanolic KOH solution, vinyl halides are obtained due to the removal of one hydrogen halide molecule. The resulting vinyl halides on treatment with NaNH2/liq. NH3 at lower temperatures lose another molecule of hydrogen halide to yield alkynes

Mechanism:

When NaNH2/liq. NH3 Is used as the reagent, the alkyne is obtained as a salt because acetylenic hydrogen is weakly acidic. This salt, on acidification, liberates the alkyne.
For example:
In the above reaction, propyno, as soon as it is produced, reacts with NaNH2 to form the corresponding salt. When dilute HCl is added to this salt, propyne is liberated.

Preparation of alkynes from alkenes:
Acetylene, for example, may be prepared from ethylene as follows:

2. By the dehydrohalogenation of gem-dihalides:
Dihalo compounds having two halogen atoms attached to the same carbon atom are called gem-dihalides.
1. When gem-dihalides are heated with ethanolic KOH solution, haloalkenes or vinyl halides are obtained. Alkynes are produced by treating the vinyl halides thus obtained with NaNH2 /liq. NH3


2. When gem-dihalides are refluxed with ethanolic KOH, alkynes are obtained

3. By dehalogenation of tetrahaloalkanes:
When tetrahaloalkanes (each of two adjacent carbon atoms is linked to two halogen atoms) dissolved in ethanol are heated with zinc dust or vapours of tetrahaloalkanes are passed over zinc dust, then alkynes are formed by the removal of four halogen atoms (as two molecules of ZnX2).

In fact, Tetra haloalkanes are generally prepared from alkynes and hence, this method loses its importance for the preparation of alkynes in general.
Preparation of higher alkynes from alkynes containing acetylenic hydrogen atom:
When vapors of acetylene or terminal alkynes (RC = CH) are passed over heated metallic sodium or made to react with soda mide dissolved in iquid ammonia, their sodium salts (sodium alkynes) are produced. When these salts are allowed to react with primary alkyl halides
For example:
CH3Br, CH3 CHBr etc) higher alkynes are formed. In the case of acetylene, both the H-atoms can be replaced by alkyl groups

Examples:

Higher alkynes can also be prepared from lithium salts of terminal alkynes. These lithium salts are prepared by the action of lithium amide (LiNH2) or n -n-butyllithium (n-BuLi) on terminal alkynes
General Properties And Chemical M Reactions Of Alkynes
1. General properties
The first three alkynes (ethyne, propyne, and butyne) are colorless gases at ordinary temperature and pressure, the next eight members (C5-C12) are liquids and higher alkynes are solids.
- Alkynes are sparingly soluble in water but highly soluble in organic solvents like alcohol, benzene, acetone etc.
- The melting and boiling points of alkynes are higher than those of the corresponding alkenes and alkanes.
Reason for higher melting and boiling points of alkynes:
Due to the presence of triple bonds, lower alkynes have linear structures. Hence, their molecules remain more closely packed in the crystal lattice as compared to those of the corresponding alkenes and alkanes.
Consequently, alkynes have higher melting and boiling points. Comparison of reactivities of alkenes and alkynes; Due to the smaller distance between the two triply bonded carbon atoms and better p -orbital overlap, the electrons in the triple bond are held more tightly. Therefore, they are poorly available to an electrophile as compared to that of a double bond.
Again, when electrophilic addition involves bridged-ion intermediates (a sin die case of Br2 addidon), those arising from triple bonds are more strained [i.e., less stable) than those arising from double bonds.
Hence, although there is a higher concentration of electrons between the carbon atoms of a triple bond than in a double bond, alkynes are in general less reactive than alkenes towards electrophilic addition reactions
2. Chemical reactions
- The reactions of alkynes can be divided mainly into five classes, such as—
- Combustion reaction,
- Addition reaction,
- Oxidation reaction,
- Reactions due to acidic behaviour of acetylenic hydrogen and
- Polymerisation reactions.
Acetylene Or Ethyne (C2H2)
Acetylene (HC = CH) is the first member of the alkyne series. The general formula of alkynes is CnH2n-2. When n = 2, the general formula reduces to C2H2 which is the formula of acetylene. In 1865, Edmund Davy discovered this gas. It is not available in nature. Coal gas contains a very littie amount (0.06%) of acetylene.
1. Preparation of acetylene
Laboratory preparation:
Acetylene Principle:
At room temperature, acetylene is prepared by the action of water on calcium carbide
CaC2 + 2H2O→Ca(OH)2 + C2H2↑
Acetylene Impurities:
Acetylene gas thus produced is not pure but contaminated with small amounts of phosphine (PH3 arsine (AsH3), hydrogen sulphide etc, Due to the presence of these gases, acetylene gas emits a foul smell.
Pure acetylene gas is sweet-smelling.

Acetylene Purification:
The impure gas is first passed through an acidified copper sulphate solution and then through a suspension of bleaching powder in water. As a result, lf 2S and NIL are absorbed in acidified copper sulphate solution and PH3 and AsH3 are absorbed in the suspension of bleaching powder in water. Acetylene gas thus obtained is moderately pure
Preparation of acetylene gas of high purity:
1. Acetylene gas prepared in the laboratory is passed through ammoniacal cuprous chloride solution when a red precipitate of copper acetylide (CuC2) is obtained while the other gaseous Impurities escape without any reaction
C2H2+ 2CuCl +2 NH4OH Cu2C2 (Red)↓+2NH4Cl+2H2O
2. The precipitate thus obtained is filtered, washed thoroughly with water and then heated with concentrated hydrochloric acid or aqueous solution of potassium cyanide when acetylene gas is liberated.
The issuing acetylene gas, after drying over P2O, is collected over mercury.In this way, highly pure acetylene is obtained.
Cu2C2+2HCl 2CuCl + C2H2↑
Cu2C2+8KCN + 2H2O → 2K3 [Cu(CN)4]+ 2 KOH + C2H2↑
2. Other methods of preparation of acetylene:
1. By dehydrobromination of ethylene dibromide:
Acetylene may be prepared by boiling ethylene dibromide with an alcoholic solution of potassium hydroxide

The reaction occurs in two steps. In the first step, vinyl bromide (CH2=CHBr) is produced. In the second step, the dehydrobromination of vinyl bromide results in the formation of acetylene.
However, for the second step, KOH is not a very effective reagent Sodamide (NaNH2) dissolved in liquid ammonia is a much better and effective reagent. So acetylene can be prepared easily from ethylene dibromide by using NaNH2 dissolved in liquid NH3

Similarly, acetylene may be prepared from ethylidene chloride (1,1-dichloroethane)

2. By debromination of tetrabromoethane:
When vapours of 1,1,2,2-tetrabromoethane are passed over heated zinc dust, the compound undergoes debromination to yield acetylene.

3. By heating a haloform with Ag powder:
When iodoform lUMftl Physical properties (or any haloform) is heated with silver powder, acetylene gas is produced

4. By Kolbe’s electrolytic method:
Electrolysis of concentrated aqueous solution of Na or K-salt of maleic acid or fumaric acid liberates acetylene gas at the anode

5. Industrial preparation of acetylene:
A mixture of coke and quick lime is heated to a very high temperature (2500- 3000°C) in an electric furnace when calcium carbide is produced. Acetylene is prepared by treating calcium carbide with water.

When natural gas, rich in methane, is heated at 1400-1500°C for a fraction of a second (0.1s) at ordinary atmospheric pressure, methane undergoes thermal decomposition (cracking) to yield acetylene.

Acetylene can be prepared by passing a stream of pure hydrogen gas through an electric arc struck between two carbon electrodes.

General Properties And Chemical Reactions Of Acetylene
1. Acetylene Physical properties
- Pure acetylene is a colourless sweet-smelling gas having boiling point -84°C .
- It is slightly lighter than air.
- Acetylene is sparingly soluble in water but its solubility in acetone is much higher (at 15°C and 10-12 atmospheric pressure, 1 volume of acetone dissolves 300 volumes of acetylene).
- Acetylene can be liquefied at 0°C and 26 atm pressure but on liquefaction It is converted into a highly explosive substance.
- For this reason, acetylene is transported from one place to another in cylinders by dissolving in acetone under high pressure
2. Reactions of acetylene
Combustion:
- Acetylene is a combustible gas but it does support combustion. It bums in air with a luminous flame. As the percentage of carbon content in acetylene.
- Is higher than that in the corresponding alkane, it undergoes incomplete combustion which leads to the formation of hot carbon particles for which a luminous flame is produced
- It is for this reason acetylene Is used for illuminating purpose.
- Acetylene forms an explosive mixture with air. Acetylene burns in excess of oxygen with an explosion, forming CO and H2O. In this luminous flame is produced which has a high temperature (3300°C). This flame is known acetylene flame
2C2H2 + 5O2→4CO2 + 2H2O + 312kcal .mol-1
Addition reactions:
1. Addition reactions of acetylene are:
Addition of hydrogen (hydrogenation): At 200 – 300°C in the presence of powdered nickel catalyst or at ordinary temperature and pressure, in the presence of Raney nickel or powdered platinum or palladium catalyst, the reaction of acetylene with hydrogen gas occurs in two steps. In the first step, one molecule of hydrogen adds to acetylene to give ethylene and in the second step, another molecule of hydrogen adds to ethylene to form ethane

2. Partial hydrogenation:
- In the presence of Lindlar’s catalyst, acetylene combines with only one molecule of hydrogen to form ethylene

- Partial hydrogenation of acetylene can also be affected in the presence of nickel boride (Ni2 B), commonly known as P-2 catalyst
- Lindlar’s catalyst is finely divided palladium deposited on calcium carbonate Pd-CaCO3, partially poisoned by lead acetate, Pb(CH3COO)2 and quinoline.
- 2-butyne reacts with hydrogen gas in the presence of Lindlar’s catalyst to produce mainly cis-2-butene

- Catalytic hydrogenation is an exception to the generalisation that alkenes are more reactive than alkynes towards addition reaction. The reason is as follows. Alkenes are adsorbed on the surface of the catalyst only when the plane of the n -bond approaches perpendicularly. Due to the cylindrical nature of the n bond of alkynes, any approach along the axis of the cylinder can be successful.
3. Addition of halogen (halogenation):
- Addition of chlorine:
- Direct combination of chlorine with acetylene may occur with explosion, HC = CH + Cl2 → 2C + 2HCl. If the reaction is carried outin the presence of kieselguhr (a silicate compound) and iron powder, the heat liberated is absorbed and no explosion takes place. In this case, the reaction takes place in two steps.

- Westron is a poisonous substance. It is used as a solvent for oils, fats and resins. When it is made to react with steam and lime, trichloroethylene (aerosol) is obtained on the elimination of a molecule of HCl. Westrosol is also used as a solvent

- Addition of bromine:
- The reaction of acetylene with bromine takes place at a comparatively slower rate. When acetylene gas is passed through red-coloured brominewater, 1, 2-dibromoethene or acetylene dibromide is produced and as a result, the colour of bromine-water is discharged. The reaction confirms the presence of unsaturation in acetylene

- When acetylene is made to react with gaseous bromine or liquid bromine, it combines with two molecules of bromine to form 1,1,2,2-tetrabromoethane or acetylene tetrabromide

- Addition of Iodine: Acetylene reacts extremely slowly with iodine. It combines with only one molecule of iodine dissolved in ethanol to form 1,2-diiodoethene.

4. Addition of halogen hydracids (HX):
Halogen hydracids react with acetylene in two steps. The reactivity of halogen hydracids in this reaction follows the order: HI > HBr > HCl.
In the first step, the addition of one molecule of HX gives vinyl halide (1-haloethene). The second step involves the addition of one more molecule of HX to vinyl halide to yield ethylidene halide (1,1-dihaloethane). This second step of the reaction occurs according to Markownikoff’s rule
Reaction with HBr:

Reaction with Hl:

Reaction with HCl:
At 65°C, in the presence of mercuric chloride (HgCl2) catalyst, acetylene forms vinyl chloride with hydrochloric acid by addition reaction.
Hydrocarbons Chapter 13 NCERT Notes

5. Addition of hypochlorous acid (HOCl):
When acetylene is passed through a cold HOCl solution, in first, combines acetylene with one molecule of HOCl to produce 2-chloroethenoI. In the second step, another molecule of HOCl is added to the resulting enol to yield an unstable addition compound that readily eliminates a molecule of water to give dichloroacetaldehyde

6. Addition of water or hydration:
When acetylene gas is passed through a dilute H2SO4 solution (20%) containing 1% HgSO4 at 60 – 80°C, it combines with one molecule of water to form the unstable addition compound vinyl alcohol which rapidly tautomerises to yield stable acetaldehyde.

In case of higher alkynes, this reaction follows Markownikoff’s rule, and ketone is obtained as the major product,
For example: Acetone is obtained from propyne

8. Addition of hydrocyanic acid (HCN):
In the presence of a barium cyanide catalyst, one molecule of hydrogen cyanide combines with acetylene to form the additional compound vinyl cyanide or acrylonitrile.

9. Addition of acetic acid (CH3COOH):
When vapours of acetic acid and acetylene are passed over zinc acetate placed on charcoal at 170°C, vinyl acetate is obtained. Polyvinyl acetate, an important plastic, is prepared by the polymerisation of vinyl acetate.

- When acetylene gas is passed through hot acetic acid in the presence of Hg2+ catalyst, in the first step one molecule and in the second step, another molecule of acetic acid is added to it to form ethylidene diacetate

- Acetaldehyde and acetic anhydride are obtained when ethylidene diacetate (liquid) is heated at 300 – 400°C.

10. Addition of arsenic trichloride (AsCl3):
Acetylene combines with arsenic trichloride AsCl3 in the presence of AlCl3 or HgCl2 to give the poisonous gas 2-chlorovinyl dichloramine or lewisite

Addition of CH3OH:
CH3OH adds on to acetylene in the presence of potassium methoxide (CH3OK) to form methyl vinyl ether. This is a nucleophilic addition reaction.

Oxidation reactions
1. Oxidation by potassium permanganate:
When acetylene is treated with dilute alkaline potassium permanganate solution, it is oxidised first into glyoxal and then into oxalic acid (which exists as potassium salt) and the reddish-violet or purple colour of permanganate is discharged.


Ozonolysis:
When a stream of ozonized oxygen is passed through acetylene dissolved in an inert solvent
For example:
Chloroform, carbon tetrachloride etc.), acetylene ozonide is formed. The ozonide on decomposition (hydrolysis) is converted into glyoxal and hydrogen peroxide. H2O2 oxidises glyoxal partially into formic acid. Consequently, a mixture of both glyoxal and formic acid is obtained.

If the decomposition of the ozonide is carried out in the presence of zinc dust, H2O2 is no longer available (reduced by zinc) to convert glyoxal into formic acid and hence, glyoxal is obtained as the only product.

The acidic nature of acetylene
The H-atom attached to a triply bonded carbon atom (= C —H) is called an acetylenic hydrogen atom. Since the acetylenic carbon atom is sphybridised, its s -character is much higher (50%) and consequently electronegativity of acetylenic carbon is relatively higher.
For this reason, the Csp-H bonding electrons are strongly attracted by the carbon nucleus and the hydrogen atom can be removed as aproton (H+), i.e., an acetylenic hydrogen atom can exhibit mild acidic character.
The acidic character of acetylenic hydrogen may also be explained by the fact that the conjugate base (HC = C-) is considerably stable and this is because the unshared electron pair lying in an sp-hybrid orbital remains tightly held by the carbon nucleus. The acetylenic hydrogen atom can, therefore, be replaced by some metal atom to form metal acetylides.
Since the s-character decreases from sp→ sp² → sp³ – hybridised carbon atoms, the acidic character of hydrocarbons follows the order:
HC = CH (Ka = 10-25) > , CH2=CH2 (Ka = 10-35) >CH3– CH3 (Ka = 10-40)
1. Reaction with sodium:
When acetylene gas is passed over molten sodium at 180°C, both mono and disodium acetylide are produced and H2 gas is liberated. However,if excess acetylene is used, monosodium acetylide is obtained as the major product.

2. Reaction with sodamide:
Acetylene reacts with sodamide in liquid ammonia to form monosodium acetylide. However, when excess sodamide is used, disodium acetylide is obtained

Sodium acetylide undergoes rapid hydrolysis in the presence of water to give acetylene and NaOH

3. Reaction with lithium amide:
Lithium amide dissolved in liquid NH3 reacts with acetylene to form lithium acetylide.

4. Reaction with butyllithium:
When n -n-butyllithium reacts with acetylene, lithium acetylide is produced quantitatively.

Reactions of NaNH2, LiNH2 and BuLi with acetylene are acid-base reactions in which acetylene acts as an acid and other compounds act as bases. These reactions occur because acetylene is more acidic than NH3.
5. Reactions with heavy metal cations (Cu+, Ag+):
Acetylene and the terminal alkynes
For example:
CH3C = CH, CH3CH2C= CH etc.) react with heavy metal cations like Cu+ and Ag+ to form insoluble salts. These salts, when absolutely dry, are explosive.
When acetylene gas is passed through ammoniacal cuprous chloride solution, a red precipitate of insoluble cuprous acetylide (Gu2C2) is obtained. With the help of this reaction, acetylene present even in trace amounts can be detected.

Again, when cuprous acetylide is boiled with aqueous solution of potassium cyanide, pure acetylene is regenerated.

Moreover, if cuprous acetylide is boiled with concentrated hydrochloric acid, acetylene is again obtained but not in pure form
CuC≡CCu + 2HCl →HC ≡ CH ↑ + 2CuCl
When acetylene gas is passed through ammoniacal stiver nitrate solution, an insoluble white precipitate of Ag2C2 is obtained.

Silver acetylide is also produced when acetylene is allowed to react with an alcoholic silver nitrate solution.

Again, when silver acetylide is boiled with aqueous solution of potassium cyanide or nitric acid, pure acetylene is regenerated

Propyne also exhibits acidic characteristics similar to that of acetylene.
For example:

Acetylene can be regenerated from the metal derivatives by taking advantage of the weak acidic property of acetylene.
The corresponding reactions are as follows:

1. With the help of the above reactions (formation of cuprous and silver acetylides):
- Acetylene and terminal alkynes:
- For example: CH3C = CH ) can be detected,
- Terminal and non-terminal:
- For example CH3C≡ CH3 ) alkynes can be distinguished from each other and
3. Acetylene and any terminal alkyne can be separated and purified from a mixture containing alkane, alkene and non-terminal alkyne.
2. Purification o( impure 1-pentyne):
Aqueous solution of silver nitrate is added to a sample of impure 1-pentyne dissolved in 95% ethyl alcohol. As a result of the reaction, a white precipitate of silver 1 -pentoxide is obtained

The white precipitate is filtered, washed with ethanol, and refluxed with an aqueous solution of sodium cyanide when 1-pentyne is regenerated. Pure 1-pentyne is separated from the mixture by distillation

6. Formation of Grignard reagent:
Acetylene reacts with ethyl magnesium bromide in ether to yield the corresponding Grignard reagent. This is an acid-base reaction.

Polymerisation reactions
Acetylene molecules may polymerise to form cyclic or linear polymers.
1. Formation of cyclic trimer:
When acetylene gas is passed through Fe or Cu-tube heated at 600°C, 3 molecules of acetylene combine to form a benzene molecule. Benzene is the cyclic polymer of acetylene. From this reaction, aromatic compounds can be prepared from aliphatic compounds

Formation of cyclic trimers of propyne and 2-butyne:
When acetylene homologues are passed through red hot Fe or Cu tube, they polymerise to produce cyclic polymers.
For example:
1,3,5-trimethyl benzene and hexamethyl benzene can be prepared from propyne and 2-butyne respectively

Hydrocarbons Chapter 13 NCERT Notes
2. Formation of cyclic tetramer:
When acetylene gas is passed over nickel cyanide catalyst at high pressure, the cyclic tetramer, cyclooctatetraene is obtained.

3. Formation of open-chain polymer:
When acetylene gas is passed through a mixed solution of cuprous chloride and ammonium chloride, the simple linear polymers, vinylacetylene and divinylacetylene, are produced.

In the additional reaction of vinylacetylene and concentrated hydrochloric acid in the presence of cuprous and ammonium chlorides, chloroprene (2-chlorobuta- 1,3-diene) is obtained. Polymerisation of chloroprene leads to the formation of the artificial rubber, neoprene.

Identification And Uses Of Acetylene
1. Proof of unsaturation in acetylene
- Acetylene discharges a reddish-brown color of bromine in carbon tetrachloride without the liberation of hydrogen CH3 bromide (HBr)

- Acetylene decolorises reddish-violet or purple-colored cold dilute alkaline solution of KMnO4
- Acetylene reacts with ozone to form the additional compound acetylene ozonide
2. Identification of acetylene
- A red precipitate of cuprous acetylide is obtained when acetylene gas is passed through ammoniacal cuprous chloride solution.
- A white precipitate of silver acetylide is obtained when acetylene is passed through ammoniacal silver nitrate solution.
- Acetylene can be identified by the above-mentioned tests along with the tests for unsaturation.
3. Uses of acetylene
- Acetylene is used for producing oxy-acetylene flame (temperature nearly 3300°C), and used for welding and cutting steel and other metals.
- It is used for producing bright illuminating flame in
carbide lamp or Hawker’s lamp. - It is used in the manufacture of acetaldehyde, acetic acid, ethyl alcohol, acetone etc
- It Is also used in the manufacture of industrial noninflammable solvents like acetylene tetrachloride (western, C2H2Cl4) and trichloroethylene (aerosol, Cl2C=CHCl ), used for dissolution of fats, oils, resins etc.
- It is an important raw material for the large-scale production of vinyl plastics, synthetic rubber such as buna-N and synthetic fibre such as orlon.
Separation of methane, ethylene and acetylene from their mixture:
1. The mixture of methane (CH4), ethylene (C2H4) and acetylene (C2H2) gases is at first passed through ammoniacal cuprous chloride solution. Acetylene is absorbed in ammoniacal cuprous chloride (Cu2Cl2) solution with the formation of red precipitate of cuprous acetylide. Methane and ethylene pass out without undergoing any reaction.

2. The red precipitate is filtered, washed with alcohol and then boiled with concentrated HCl or KCN solution when acetylene gas is evolved. It is dried by P2O5 and collected.
CCu ≡CCu + 2HCl →HC = CH↑ + Cu2Cl2
3. The gas mixture (CH4, C2H4) which escapes is then passed through fuming sulphuric acid. CH4 comes out without any reaction. It is collected after removing acid vapours by passing through KOH.
4. Ethylene reacts with fuming sulphuric acid to form ethyl hydrogen sulphate. Ethyl hydrogen sulphate thus produced is heated at 170°C when ethylene gas is liberated. It is collected after removing acid vapours bypassing through KOH
H2C=CH2 + H2SO4 →CH3CH2OSO3H

Comparison among methane, ethylene and acetylene:

Alkadienes
Unsaturated hydrocarbons containing two carbon-carbon double bonds (C=C) are called alkadienes. An alkadiene molecule contains four H-atoms less than the corresponding alkane. Therefore, general molecular formula for an alkadiene isCnH2n2 (n = 3, 4–). These are isomeric with alkynes
1. Classification of alkadienes
Depending on the relative positions of the two double bonds,
Alkadienes are classified into three types:
1. Isolated dienes:
Dienes in which two double bonds are separated from each other by more than one single bond are called isolated dienes

2. Conjugated dienes:
Dienes in which the two double bonds are separated by one single bond are called conjugated dienes i.e., there are alternate single and double bonds in the compound.

3. Cumulated dienes:
Dienes in which two double bonds are adjacent to each other, i.e., two double bonds are attached to the same carbon atom are called cumulated dienes. These are also called allenes

The cumulated dienes or allenes have the general structural formula molecules and are always optically active.
molecules and are always optically active.
2. Relative stabilities of dienes
The heat of hydrogenation of a conjugated diene is less than that of an isolated diene having the same molecular formula. Therefore, a conjugated diene is relatively thermodynamically more stable than the corresponding isolated diene

Reasons for greater stability of conjugated diene:
There are two reasons for additional stability of conjugated dienes as compared to isolated dienes. These are as follows:
1. Carbon-carbon single bond between two double bonds in a conjugated diene is derived from the overlap of two sp² – orbitals of carbon, i.e., it is sp²-sp² single bond. This is a shorter and stronger bond than one formed by sp³-sp² overlap. As a conjugated diene has one stronger single bond than an isolated diene, the conjugated diene is more stable

2. Second factor that causes a conjugated diene to be more CH2—CH=CH-CH2stable than an isolated diene is resonance, which means diene has delocalised electrons, n -electrons in each of the double bonds in an isolated diene are localised between 2 carbon atoms.
But in a conjugated diene, the 2p-orbitals on the 4 carbons are in parallel alignment which is necessary for overlap; the 4 electrons are delocalised over 4 carbon atoms. Due to such electron delocalisation, a conjugated diene is relatively more stable than an isolated diene

The resonance hybrid shows that in 1,3-butadiene, the single bond flanked by two double bonds is not a pure single bond but has a partial double bond character

3. Electrophilic addition reaction of1,3-butadiene
1. Reaction with bromine (1:1 molar ratio):
In this case, two types of addition compounds are formed. These are 3,4-dibromo but-l-ene (1,2-addition product) and l,4-dibromo but-2-ene (1, 4-addition product)

Mechanism of the reaction:

Carbocation obtained in the first step is a hybrid of two resonance structures. The positive charge of the carbocation exists partially on two carbon atoms. In the second step, (Br®) (the nucleophile) attacks either of the positive carbon atoms to yield two different dibromo compounds
2. Reaction with HBr (1:1 molar ratio):
In this reaction also, two addition compounds, 1-bromobut-2-cne and 3-bromo but-1-ene, are formed.

Mechanism to the reaction:

3. Diels-Alder reaction:
When 1,3-butadiene is heated with acetaldehyde, an addition reaction leading to the formation of a six-membered ring occurs due to the formation of two different C—C bonds.
This type of reaction is called the Diels-Alder reaction. It is an example of 1,4-addition. The conjugated diene is referred to as a diene, the compound containing the double is called a dienophile and the product is called an adduct

Preparation of Methane, Ethylene and Acetylene and their Reactions
Preparation of Methane Reactions :

Preparation of Ethylene Reactions:

Preparation of Acetylene Reactions:

Transformation
Synthesis of organic compounds is one of the main objectives of organic chemistry and for this, one compound is to be transformed into another. Soit is necessary to devise a suitable transformed into another. Soit is necessary to devise a suitable These conversions are carried out with the help of different reactions. During writing each reaction, the necessary conditions are mentioned.
In case, if any preparation involves multistep reactions, then the yield of the desired product is increased by minimising the number of steps. However, if the desired product is contaminated with other side products, the separation of which is difficult or the end product further participates in a reaction and gets partially converted into other substance(s) or the yielding any step is very low, then the route of the preparation with more number of steps is selected. The relation between different homologous series and their inter¬conversions are shown by the following flow charts.
Hydrocarbons Class 11 Chemistry Solutions
Conversion of methane and ethylene into different compounds

Conversion of acetylene into different compounds:

Various transformations obtained from the chart:




The distinction between two compounds chemical tests:
Acetylene and ethylene:

1-butyne and 1-butene:

2-butene and 1-butene:

Butane and 1-butene:

1-butyne and 2-butyne:

Problems related to alkanes, alkenes and alkynes and their solutions
1. A gaseous hydrocarbon decolourises bromine i in CCI4. One molecule of acetone and one molecule of acetaldehyde are obtained as a result of its ozonolysis. Determine the structural formula of the compound and write its IUPAC name. Give all reactions involved
Answer:
- The given hydrocarbon decolourises the bromine solution. Hence,it is an unsaturated hydrocarbon.
- The hydrocarbon, on ozonolysis, produces 1 molecule of acetone and1 molecule of acetaldehyde. So, the structure of the unsaturated hydrocarbon may be obtained by writing the formula of the two carbonyl compounds side by side facing formula of the two carbonyl compounds side by side facing formula of the two carbonyl compounds side by side facing formula of the two carbonyl compounds side by side facing hydrocarbon is 2-methyl but-2-ene

The reactions are as follows:

2. One molecule of an olefinic compound, on ozonolysis, produces one molecule of acetone, one molecule of glyoxal and one molecule of formaldehyde. Identify the compound and write its IUPAC name
Answer:
The structure of the olefinic hydrocarbon may be obtained by arranging the carbonyl compounds obtained on ozonolysis properly (each of the two oxygen atoms of acetone and formaldehyde placed in front of the 2 oxygen atoms of glyoxal) followed by eliminating the four oxygen atoms and joining the carbonyl carbons by double bonds. Thus, the IUPAC name ofthe starting olefinic compound is 4-methylpenta-1,3-diene

3. An organic compound having the molecular formula C5H10O3, on hydrolysis in the presence of Zn, gives acetone and acetaldehyde. Write the structure of the organic compound
Answer:
The molecular formula of the compound is C5H10O3 = CnH2nO3 (n = 5)> ie-the compound is an addition compound of an alkene and O3 The compound reductive hydrolysis, produces acetone and acetaldehyde Hence, the compound must be an alkene ozonide.
The structure of the alkene is obtained by writing the carbonyl compounds side by side facing their carbonyl groups for each other followed by removing the two oxygen atoms and joining the two carbonyl carbons by a double bond. So the compound is 2- methyl but-2-ene
Hence, the starting compound is 2 methylbut – 2 – ene ozoined and its structure is:

4. A gaseous hydrocarbon (A) is converted into another (B) by consuming 2 mol H2 in the presence of Ni catalyst. B can be prepared by the reaction between methyl iodide and metallic sodium in a dry ether medium. Identify the two hydrocarbons (A andB) and mention the reactions involved.
Answer:
In the hydrogenation (H2/Ni) reaction, one mole of the hydrocarbon (A) accepts two moles of hydrogen. So, the n hydrogenation (H2/Ni) reaction, one mole of the hydrocarbon (A) accepts two moles of hydrogen. So, the contains two double bonds or one triple bond.
In either medium, the reaction between methyl iodide and metallic sodium yields the hydrocarbon (JB) . This is the Wurtz reaction and in this reaction, ethane (CH3CH3) is formed,

Therefore, the hydrocarbon (B) is ethane. Since the hydrocarbon (A) gives ethane by absorbing 2 moles of H2, it must contain a triple bond (two double bonds cannot be formed between two carbon atoms) and obviously, it is acetylene (H—C = C—H)

5. An alkene (A) having formula C4H8, on ozonolysis, gives propanal & methanal. A reacts with HBr to produce a compound of molecular formula, C4H9Br. This compound, when heated with alcoholic KOH, produces another alkene (B) which is isomeric with A. Identify the alkenes (A) and (B).
Answer:
The products of ozonolysis of the alkene (A) are propane (CH3CH2CHO) and methanal (HCHO). Hence, the alkene(A) is 1-butene (CH3CH2CH=CH2)

1-butene, being an unsymmetrical alkene reacts with HBr according to Markownikoff’s rule to give 2-bromobutane. Its molecular formula is C4H9Br.
CH3CH2CH=CH2+ HBr → CH3CH2CHBrCH3 (2- Bromobutane)
When 2-bromobutane is heated with alcoholic KOH solution, it undergoes dehydrobromination and according to Reaction: Saytzeff’s rule, 2-butene (an isomer of 1-butene) is obtained as the major product. Therefore, the alkene (B) is 2-butene (CH3CH=CHCH3).

6. A compound (A) having molecular formula C4H9Cl, on heating with alcoholic KOH solution, gives two isomeric alkenes (B) & (C). The mixture of (B) & (C), on ozonolysis, produces three compounds:
- HCHO
- CH3CHO
- CH3CH2CHO.
Ascertain the structures of(A), (B) and (C).
Answer:
The compound (A), when heated with alcoholic KOH solution, undergoes dehydrochlorination to give two isomeric alkenes (B) and (C). So, the molecular formula of these two alkenes is C4H8

A mixture of (B) and (C), on ozonolysis, produces three carbonyl compounds (HCHO, CH3CHO & CH3CH2CHO). Now, the total number of carbon atoms of the compound of compounds obtained by ozonolysis must be equal to the number of carbon atoms present in the compound undergoing ozonolysis. So, letus suppose that as a result of ozonolysis of the alkene B, 1 molecule of CH3CH2CHO and 1 molecule of HCHO (totalnumber of carbon atoms of the two compounds = 4) are obtained and the ozonolysis of( C) produces 2 molecules of CH3CHO (total number of carbon atoms of the two molecules = 4).
So, the structure of the alkene B is CH3CH2CH=CH2 (1-butene) & the structure of alkene C is CH3CH=CHCH3 (2-butene)
CH3CH2CH= O + O = CH2 ⇒ CH3CH2CH= CH2
CH3CH= O +O = CHCH3 ⇒ CH3CHCH = CHCH3
Since the compound (A), on dehydrochlorination produces 1 – butene and 2 – butene , the structure of (A) is:
CH3CH2CH(Cl)CH3( 2- chlorobutane)
Reaction:

7. A hydrocarbon containing two carbon atoms decolourises bromine water and undergoes hydration by H2SO4 in the presence of H2SO4 to produce a compound which produces chloroform when heated with solution of bleaching powder. Write the name of the hydrocarbon and mention the reactions in support of your arguments.
Answer:
The hydrocarbon decolourises bromine water. Hence, it is an unsaturated hydrocarbon. Since the hydrocarbon undergoes hydration by H2SO4 in the presence of H2SO4 it is an alkyne. The compound obtained on hydration of the hydrocarbon reacts with bleaching powder solution to produce chloroform.
This reaction is a haloform reaction and the compounds containing the CH3CO —group participate in the haloform reaction. Now, acetylene is the only hydrocarbon containing two carbon atoms which reacts with H2SO4/HgSO4 to form acetaldehyde (CH3CHO) having a CH3CO —group.
Acetal dehyde, on reacting with bleaching powder, produces chloroform
Therefore, the hydrocarbons acetylene (HC = CH).

8. A sweet-smelling organic liquid (A) consisting of C, H and O boils at 78°C. (A) on heating with a cone. H2SO4 liberates a gaseous substance (B). The empirical formula of (B) is CH2. (JB) decolourises bromine water and alkaline KMnO4 solution. Again, each mole of(B) absorbs one mole of H2 at high temperature in the presence of Ni catalyst. Identify A and B.
Answer:
Since the gaseous substance (B) decolourises bromine KMnO4 water and alkaline KMnO4 solution and consumes one mole of H2 permolein catalytic hydrogenation, it must be alkene.
Since the empirical formula of (B) is CH2, it may be the gaseous alkene ethylene, (CH2), or C2H4. Ethylene may be obtained when the sweet-smelling organic liquid ethyl alcohol (CH3CH2OH) hating a boiling point 78°C is dehydrated by heating with concentrated H2SO4. Therefore, the organic liquid (A) is ethyl alcohol

Hydrocarbons Class 11 Chemistry Solutions
9. Ahydrocarbon having the molecular formula C6H10, on catalytic hydrogenation, absorbs one mole of H2. The product obtained on ozonolysis of the compound is OHCCH2CH2CH2CH2CHO. Determine the structure of the compound
Answer:
As the compound absorbs one mole of hydrogen in the presence of a catalyst, the compound is an alkene. On ozonolysis, it gives a dicarbonyl compound. So, the alkene is cyclic.
The structure of the alkene is obtained by writing the 2 O-atoms of hexanedial (OHCCH2CH2CH2CH2CHO) face to face followed by removing the two oxygen atoms and joining the two carbonyl carbons by a double bond. Hence, the hydrocarbon (C6H10) is cyclohexene

10. A hydrocarbon (A) (molecular formula C5H10), on catalytic hydrogenation, produces 2- methylbutane. The compound (A) combines with HBr according to Markownikoff’s rule to form the compound (B) which reacts with silver hydroxide to produce an alcohol (C) having the molecular formula C5H12O. On oxidation, the alcohol (C) yields a ketone (D). Identify A,B,C, and D and give the reactions involved
Answer:
The given changes are as follows:

The molecular formula, C5H10 agrees with the general formula of alkenes (CnH2n) and on catalytic hydrogenation, (A) produces the saturated hydrocarbon, 2- methylbutane. Therefore, the compound (A) is an alkene. Now, the alcohol (C), on oxidation, produces the ketone (D). So, the alcohol must be a secondary (2°) alcohol. Again, this alcoholis obtained the reaction of (B) with AgOH.
So, (B) is a secondary bromide.

Now, (B) is produced by the reaction of (A) with HBr according to Markownikoff’s rule. So, the structure of(A) is

Reactions:

11. A hydrocarbon (X) discharges the colour of cold (reddish violet) HgS04 alkaline KMnO4 solution and reacts with warm dilute sulphuric acid containing HgSO4 to form another compound (Y). (Y) gives a positive iodoform test but does not react with Tollens’ reagent. (Y), when distilled with 80% H2SO4, gives sym-trimethylbenzene. Identify the compounds (X) and (Y) and write the reactions involved with proper reasons.
Answer:
The compound (X) discharges the colour of cold dilute 80% H2SO4 alkaline KMnO4 solution. So it may be an unsaturated hydrocarbon. Since (X) reacts with dilute H2SO4 to yield a carbonyl compound ( Y) which gives a positive iodoform test but does not react with Tollens’ reagent, the carbonyl compound must be a ketone containing a CH3CO— group.
And the unsaturated hydrocarbon (X) is a terminal alkyne (R- C = CH) which, on hydration, produces a methyl ketone (RCOCHg). Now, the alkyne, when distilled with 80% H2SO4, gives jym-trimethylbenzene. Hence, the terminal alkyne (X) is propyne. Naturally, the ketone (Y) is acetone, CH3COCH3
Reactions:

CBSE Class 11 Chemistry Notes For Chapter 13 Hydrocarbons Aromatic Hydrocarbons
The Greek word aroma means fragrance and so the term ‘aromatic compounds’ was originally applied to various fragrant organic compounds. Chemical analysis of most of the natural fragrant organic compounds has shown that they are made of one or more benzene rings containing six carbon atoms and the carbon content in them is higher than the corresponding aliphatic compounds. Moreover, these aromatic compounds can be converted into benzene and benzene derivatives by chemical reactions. Similarly, different aromatic compounds can be synthesised from benzene. Thus, benzene is called the parent hydrocarbon of aromatic compounds and all aromatic compounds are considered as benzene derivatives. Hence, it may be simply said that the aromatic compounds are benzene and benzene derivatives.
Aromatic compounds containing benzene ring are also called benzenoids. Later on, some polycyclic compounds like naphthalene, anthracene, etc., and some heterocyclic compounds like pyrrole, furan, pyridine etc., are also considered as aromatic compounds. Again, some 3, 4, 5 or 7-membered cyclic polyenes (cations, anions or neutral compounds) are also included in the aromatic group. These aromatic compounds are called non-benzenoids. Therefore, the presence of benzene ring in an aromatic compound is not essential. All aromatic compounds do not necessarily possess sweet smell. Many aromatic compounds are odourless while some have bad odour. There is also a large number of non-aromatic compounds having characteristic sweet smell. Hence, the concept that all sweet-smelling organic compounds are aromatic compounds and all aromatic compounds must possess sweet smell is baseless.
Arenes
Aromatic hydrocarbons containing one or more benzene rings are called arenes. If arenes contain more than one benzene ring, then the rings may remain fused or isolated.
Some examples of arenes containing only one benzene ring are as follows:

Aromatic hydrocarbons containing fused rings
Two benzene rings are said to be fused when they remain attached through a common bond. For example, in naphthalene molecule, the two benzene rings A and B are attached through the common bond C9 — C10. So, this is a fused bicyclic arene. Similarly, anthracene and phenanthrene are two examples of used tricyclic arenes. In these compounds, the benzene rings A and B are attached with each other through a common bond and the benzene rings B and C are attached with each other through a common bond

Aromatic hydrocarbons containing fused rings are also called polynuclear aromatic hydrocarbons. Their general formula is CnHn2n-6m, where n = the number of carbon atoms and m = the number of rings. For the bicyclic arene naphthalene, n = 10 and m = 2. For tricyclic arenes such as anthracene and phenanthrene, n = 14 and m = 3
Arenes containing isolated rings:
These are also polynuclear hydrocarbons

Structure Of Benzene Molecule
1. The molecular formula of benzene is C6H6 , but the molecular formula of the corresponding open-chain saturated hydrocarbon (alkane) is C6H14. It means that, benzene has eight hydrogen atoms less than the corresponding saturated hydrocarbon. Therefore, benzene is expected to be a highly unsaturated compound which will easilyform addition compounds.
2. It has been observed in practice that benzene undergoes addition reactions only under drastic conditions

These reactions suggest that benzene is an unsaturated compound containing 3 double bonds. However, from the conditions of these reactions, it is clear that benzene does not contain a high degree of unsaturation
3. In spite the presence of three double bonds, benzene does not discharge the reddish-violet colour of cold alkaline KMnO4 solution, does not decolourise bromine in carbon tetrachloride solution and does not react with halogen acids.
Therefore, the type of unsaturation present in benzene is quite different from that of aliphatic unsaturated compounds.
4. Like saturated compounds, benzene undergoes substitution reactions
For example: Chlorination, nitration, and sulphonation) easily. In these reactions, one or more H atoms of benzene are substituted by different atoms or groups.
For example:
So, it can be said that in spite of the presence of three double bonds, benzene behaves mostly like a saturated compound.
1. Kekule structure of benzene
The first acceptable ring structure for benzene was proposed by Friedrich August Kekule (1865). He proposed that the six carbon atoms of benzene molecule are joined to each other to form a ring resembling a regular hexagon, with each carbon atom carrying one H-atom. In order to satisfy the fourth valency of each carbon atom, he proposed the presence of three double bonds at the alternate positions in the ring Presence of three double bonds and the equivalency of six supported by this structure of benzene.

Drawbacks of Kekule Structure:
There are two drawbacks of the Kekule structure of benzene and these are as follows:
1. In spite of the presence ofthree double bonds, benzene is a very stable molecule and behaves like a saturated compound.
2. Two disubstituted compounds (I & II) should result from benzene when H-atoms attached to two adjacent carbon atoms in a benzene molecule are substituted bytwo similar or different groups. The reason is that in one of the isomer, the bond between the substituted C-atoms is a single bond while in the other isomer, it is a double bond. However, in fact, only one 1,2-disubstituted compound is obtained.

To account for the non-existence of two types of ortho-disubstituted benzene derivatives, Kekule slightly modified his proposed structure for benzene. He proposed a dynamic equilibrium between two structures (III & IV)

Positions of carbon-carbon double and single bonds in benzene are not static but oscillate back and forth between adjacent positions. That means each C— C pair has a single bond for half of the time and a double bond for the other half. In other words, each molecule spends half of its time in (III) and the other half in (IV)
This new proposition of Kekule was known as the Oscillation theory. According to this theory, the two ort/io-disubstituted benzenes (1 and II) are identical, i.e., benzene will form only one type of ortho -disubstituted compound.
2. Valence bond or resonance theory regarding the structural formula of benzene
The relative stability of tire double bonds and the unusual behaviour of benzene as a whole have been explained with the help of valence bond theory.

According to this theory—
- Neither of the two Kekule structures (1 & 2) represents the actual structure of benzene. None of these structures of benzene can individually give the exact identity of the benzene molecule. They do not have any real existence.
- The real structure of benzene is a resonance hybrid of these two structures. Since the two structures, I and II are equivalent and equally stable, they contribute equally to the resonance hybrid, Le., the contribution of each of these resonance or canonical structures is 50%.
- Consequently, the resonance hybrid becomes highly stable. Due to resonance stability, the chemical reactivity of benzene due to unsaturation decreases and stability increases. The resonance energy of benzene is 36 kcal-mol-1 (calculated value)
Previously benzene was considered as a resonance hybrid of two Kekule structures (1 & 2) and three Dewar structures (3, 4 & 5). However, benzene having a Dewar structure has been prepared later on in the laboratory. So structures 3, 4 and 5 are excluded from the resonance hybrid of benzene. It is to be noted that structures involved in resonance has no real existence.

Explanation of the abnormal behaviour of benzene, by resonance:
1. The three double bonds of benzene are not active in forming addition compounds like the olefmic double bonds because their participation in addition reaction causes loss of resonance stability of benzene. However, in substitution reaction there is no net effect on the hybrid structure of benzene. So, benzene prefers to participate in substitution reaction than in addition reactions.
2. Due to the hybrid structure, all the carbon-carbon bonds In benzene arc equivalent. So, there is no difference between two apparently different disubstituted ortho- isomers— 1,2 and 1,6. Two H-atoms of benzene, on being replaced by the same or different atoms or groups, can form three isomers (ortho, meta & para).
3. Since benzene exists as a resonance hybrid, there is no real existence of carbon-carbon double bonds (C=C) or carbon-carbon single bonds (C —C) in the molecule. All the carbon-carbon bonds are equivalent. It has been observed experimentally that in a benzene molecule all the carbon-carbon bonds are equal in length and its value (1.39A) is intermediate between carbon-carbon single bond length (1.54 Å) and carbon-carbon double bond length (1.34 Å).
4. Orbital structure of benzene
1. Each carbon atom in benzene molecule is sp2 – hybridised, i.e., to form -bond, each carbon atom uses three sp2 -hybrid orbitals.
2. Out of these three hybrid orbitals, one orbital forms a C—H cr-bond by axial overlapping with ls-orbital of hydrogen atom and the other two overlap axially with two sp2 -hybrid orbitals of two adjacent carbon atoms to form two C —C tr -bonds. So, in the benzene molecule, there are six carbon-carbon (sp2 – sp2) and six carbon-hydrogen (sp² -s) cr -bonds.
3. Since all the carbon atoms in benzene forming the ring system are sp2 -hybridised, the benzene molecule has a planar regular hexagonal structure.
Calculation of resonance energy:
The resonance energy of benzene can be calculated from heat of hydrogenation data. When one mole of an unsaturated compound is hydrogenated, the amount of heat liberated is called heat of hydrogenation. The resonance energy of benzene can be calculated by the process as follows

Heat of hydrogenation involving one double bond,
ΔH = -28.6 kcal-mol-1

The calculated heat of hydrogenation involving three double bonds is given by, All = -28.6 ×3 = -85.8 kcal-mol-1

Experimentally observed heat of hydrogenation of benzene is given by, ΔH = -49.8 kcal-mol-1
∴ Resonance energy
Observed heat of hydrogenation – calculated heat of hydrogenation
= -49.8(85.8- 49.8)(-85.8)kcal-mol-1kcal-mol-1
= 36.0 kcal-mol-1
4. One carbon atom resides at each corner of the hexagon.
Each C —C — C and C — C — H bond angle is 120°.
5. Each carbon atom contains one unhybridised 2pz -orbital. Each 2pz -orbital contains one electron and the six 2pz orbitals are perpendicular to the plane of the hexagon, i.e., they are parallel to each other

6. Each pz -orbital can overlap laterally with either of the adjacent pz -orbitals. So, overlap can occur in two different ways as shown below. As the extent of overlap of each pz -orbital on both sides is equal, ;r -electrons remain no longer localised, but undergo delocalisation.
These six n -n-electrons form two continuous rings of n -electron clouds—one lying above and the other below the plane of the hexagonal ring. This ring-like electron cloud formed by six 2pz -orbitals is called aromatic sextet The benzene molecule acquires its stability due to this delocalisation of electrons coupled with the presence of an aromatic sextet

X-ray and different spectral analysis (UV) IR, NMR and Mass spectroscopy) have offered a complete idea about the structure of benzene and now it is known that —
- Benzene is a planar cyclic compound consisting of six carbon atoms
- Each of its carbon-carbon bonds are equal in length and its value is 1.39A
- All the six H-atoms are equivalent and each C— H bond length is 1.09A and
- All H— C — C and C— C—C bond angles are equal and the value is 120°
Representation of benzene molecule
The hexagonal structure of benzene is known as benzene ring. Benzene ring is generally represented by any one of the two Kekule structures. Benzene ring is also represented by drawing a circle inside a regular hexagon. While representing benzene molecule, C & H-atoms are not generally written

Aromatic Character Or Aromaticity
Aromatic character or aromaticity is the collective representation of the characteristic properties of aromatic compounds, which are responsible for the different behaviour of such compounds from their aliphatic or alicyclic analogues.
These are as follows:
1. All aromatic compounds are relatively more stable than the system of the corresponding aliphatic compounds of similar molecular formula. They are resistant to oxidation. Low heat of combustion and hydrogenation gives an idea about their unusual stability.
2. Although aromatic compounds contain several double bonds, yet they do not easily participate in addition reactions like aliphatic unsaturated compounds. However, aromatic compounds undergo substitution reactions easily. Therefore, the nature of unsaturation associated with aromatic compounds is different from that in aliphatic compounds.
3. Aromatic rings containing — OH group exhibit acidic property
For example: Phenol, but aliphatic compounds containing — OH group possess alcoholic properties and alcohols are neutral in nature
4. Aromatic rings containing — NH22 group exhibit weak basic properties
For example: Aniline), but aliphatic compounds containing the — NH2 group are relatively stronger bases
5. In the molecules of aromatic compounds, planar or almost planar rings composed of carbon atoms (in some cases, formed by C, N, O etc., atoms) are present. Such rings are called aromatic rings. Generally, the cyclic compounds which have conjugated systems of double bonds and have very high resonance stability are called aromatic compounds. However, all conjugated monocyclic polyenes are not highly stable.
For example:
Although benzene is extremely stable, cyclobutadiene and cyclooctatetraene are not similarly stable. Therefore, it is found that the special stability of aromatic compounds is not only due to π -electron delocalisation but also due to the presence of a definite number of it -electrons. Huckel’s rule gives a clear idea about this matter
1. Huckel’s rule for aromaticity or (4n+2) rule
According to German scientist Huckel, monocyclic planar conjugated polyene systems (cation, anion or neutral species) containing (4n + 2) delocalised it -electrons [n = 0, 1, 2, 3,…) exhibit aromatic properties. Therefore, monocyclic conjugated polyene systems containing 2(n = 0), 6(n = 1), 10 (n = 2), 14 (n = 3), ••• etc., delocalised 7T -electrons possess aromatic character and they are unusually stable

From Huckel’s rule, it is evident that the following conditions must be fulfilled by a compound to be aromatic:
- The molecule must have planar ringsystem.
- Each atom involved in the formation of ring system must have an unhybridised p-orbital.
- These p -orbitals must be parallel and undergo continuous overlap to cause the delocalisation of it -electrons.
- Each p -orbital may contain 1 electron, 2 electrons or even no electron.
- The delocalised it -electron system must contain 2, 6, 10, 14… etc., electrons.

It must be remembered that due to such it -electron delocalisation, the electronic energy of an aromatic compound decreases and hence, stability increases. Aromatic compounds are more stable than their open-chain analogues.
The general formula of monocyclic conjugated polyenes is CnHn (where n = 4, 6, 8, 10, etc.). The general formulas of cationic & anionic polyenes are CnH+ & CnHn– (where ” ~ 3’5, 7’ etc….)
CBSE Class 11 Chemistry Chapter 13 Hydrocarbons Summary
Classification Of Aromatic Compounds
The compounds that exhibit aromatic properties are two Types.
These are:
- Benzenoid aromatic compounds and
- Non-benzenoid aromatic compounds.
1. Benzenoid aromatic compounds:
Aromatic compounds containing one ormore benzene rings are called benzenoid aromatic compounds or simply benzenoids.

2. Non-benzenoid aromatic compounds
Some compounds, where n = 3] in (Antiaromatic)Less stable c spite of having no benzene ring in their molecules, are able to display aromatic properties. These are called nonbenzenoid aromatic compounds or simply non-benzenoids

1. Antiaromatic compounds
If a compound contains 4nπ -electrons (where n – 1, 2, 3, … etc.) in its planar ring system with a continuous overlap of p-orbitals, it becomes an antiaromatic compound.
Antiaromatic compounds Definition:
Monocyclic planar conjugated polyene systems ions) containing delocalised π -electrons (n = 1, are called antiaromatic compounds.
Therefore, the compounds having 4(n = 1), 8(n = 2), 12(/i = 3)… etc., delocalised π -electrons in their planar ring m system behave as antiaromatic compounds

It should be remembered that due to the delocalisation of π -electrons, electronic energy of antiaromatic compounds increases and consequently stability decreases. Antiaromatic compounds are less stable than their open-chain analogues.

2. Non-aromatic compounds
Non-aromatic compounds Definition:
Cyclic compounds which do notpossess delocalised system of π -electrons, i.e., compounds which are neither aromatic nor antiaromatic, are called non-aromatic compound
The stabilities of non-aromatic compounds are similar to that of their open-chain analogues.

Isomerism Of Benzene Derivatives
When one or more H-atoms of benzene are substituted by any other atom or group, the product obtained is known as benzene derivative. All the six H-atoms of benzene are equivalent. So, if one H-atom of benzene is replaced by a monovalent atom or group, only 1 monosubstituted benzene derivative is obtained.
Monosubstituted benzene has no isomer, i.e., it exists in one form only.
For example: Chlorobenzene (C6H5Cl) or nitrobenzene (C6H5NO2) exists in one form only

1. When two H-atoms in a benzene molecule are substituted by two atoms or groups (same or different), depending on the relative position of the two substituents, three positional isomers are possible.
For example: Three isomers each of dibromobenzene (C6H4Br2) and nitrotoluene (CH3C6H4NO2) are known
Isomeric dibromobenzenes:

Isomeric Nitrotoluenes:

2. In the case of trisubstituted benzene derivatives, the number of isomers depends on the nature of the substituents. O If the three substituents are identical then three isomers are possible.
For example: Trinitrobenzene [C6H3(NO2)3], tribromobenzene (C6H3Br3), etc., are known to exist in three isomeric forms. When two substitutents are identical and one is different, then six isomers are possible.
For example: Dibromophenol (Br2C6H3OH), dichlorobenzoic acid (Cl2C6H3COOH) etc., are found to exist in sixisomeric forms.
When all the three substituents are different, ten isomers are possible.
For example – Bromochlorotoluene (CH3CgH3ClBr) , bromochlorobenzoic acid (BrClC6H3COOH) etc., are found to exist in ten isomeric forms
Isomeric Tribromobenzenes:

Nomenclature Of Benzene Derivatives
1. Monosubstituted benzene derivatives
1. In the IUPAC system, some monosubstituted benzene derivatives are named by their trivial names which may have no resemblance to the name ofthe substituent

2. For many of these derivatives, the name of the substituent is simply added to the word ‘benzene’, as a prefix leaving no gap between the name of the substituent and the word, benzene.

3. In some cases, the names of the substituents are written as suffixes after the word benzene.

4. In some cases, the phenyl group, originated from the benzene molecule, is considered as the substituent to write the IUPAC names.

2. Disubstituted benzene derivatives
In disubstituted benzene, when two substituents are attached to adjacent carbon atoms, the compound is called ortho-isomer, if the substituents are on the alternate carbon atoms, then the compound is called meta-isomer and if the substituents are attached to diagonally opposite carbon atoms, the compound is called para-isomer, ortho, meta and para- are abbreviated as o-, m- and p-respectively.
Nomenclature according to nature & position of substituent:
When the two substituents are identical, then their relative positions are indicated by the prefix ortho, meta or para followed by the word to denote two substituents. Then the name of the substituent along with the word ‘benzene’ are written. In that case, 1,2- disubstituted benzene is called ortho, 1,3-disubstituted benzene is called meta-and 1,4-disubstituted benzene is called para.

Alternatively, relative positions ofthe substituents are also indicated by using numbers. In that case, any one of the carbon atoms having a substituent attached to it is marked as number ‘1’ carbon atom. The other carbon atoms of the benzene ring are numbered consecutively (clockwise or anticlockwise) in such a way as to give lowest number to the carbon atom carrying the second substituent

3. If the substituents are different, the ring containing a substituent is considered as parent, and using its name as the root name, the position of the second substituent is indicated by ortho-, meta- or para- or alternatively by 2, 3 or 4.
The name of the second substituent, in this case, comes before the root name. In the given compound, the benzene ring containing —OH group (out of —OH and — NO2 ) i.e., phenol, is taken as the parent compound. Now, since the substituent — NO2 exists in para-position with respect to —OH, so the compound is named as p-nitrophenol. Alternatively, the carbon atom to which the —OH group is attached is taken as number-1 carbon atom, and then the other carbon atoms of the ring are marked with numbers in succession in a clockwise or anticlockwise manner in such a way that the carbon atom containing the second substituent — NO2 is assigned the lowest possible number. Now, the substituent — NO2 is attached to the number-4 carbon atom. Hence, the name ofthe compound is 4-nitrophenol.

Order of priority of groups for determining root name:
—COOH > —SO3H > —CONH2 > — CN > —CHO > NC=O> -CH2OH > -CH3 > -OH > -NH2 > — NO2> Halogens (F, Cl, Br, I)

If the substituents are two different halogen atoms, then theyare mentioned alphabeticallyfollowing the English spelling of their names. The relative positions of the substituents are indicated bynumbers orwith the prefix ortho-, meta- or para-. Substituent whose name comes first (alphabetically) is considered to be attached to carbon number-1, and the other carbon atoms are so numbered as to give the lowest possible number to the carbon carrying the second substituent
Examples:
In naming the first compound given below, at first bromo and then chloro have been written and this is because alphabetically the initial letter ‘b’ ofbromo comes before the initial letter ‘d of chloro. The carbon to which bromine is attached has been marked with number-1 and the other carbon atoms have been numbered serially in the clockwise direction because, as a result ofsuch method of marking the carbon atom containing Cl gets the lowest possible number.

3. Tri- or polysubstituted benzene derivatives
1. When more than two (same or different) substituents are present in the benzene ring, their relative positions are indicated by numbers. Carbon atom containing principal substituent (according to relative preference order is othercarbon atoms are denoted by serial numbers 2, 3. 4, 5. 6. The name of the benzene ring containing principal substituent is takenas the root name

2. Except die principal substituent other substituents are expressed by position numbers and this is done insuch a way that the first difference between the possible position numbers assumes the lowest value. Their positions in die benzene ring are indicated by writing these numbers before the names ofthe substituents.
3. The substituents are mentioned according to the alphabetical order of the initial letters in the English spelling of their names and finally the word benzene or the root name of benzene derivative is added to the substituent concerned.
For example: In the compound shown above, principal substituent is —CH3. So, correct name of the compound is 6-bromo-3-iodo-2-nitrotoluene.
4. 2-bromo-5-iodo-6-nitrotoluene is not correct nomenclature because in the first nomenclature, the numbers assigned to the carbon atoms containing substituents are 1, 2, 3 and 6 while in the second nomenclature, numbers assigned to the carbon atoms earning substituents are 1,2, 5 and 6. So, in the first nomenclature, the third number is ‘3’ while in the second nomenclature, it is ‘5’. Since ’3′ is less than‘5’ the first nomenclature is correct.

Common and IUPAC names of some typical compounds:

Homologous Series Of Aromatic Compounds
Like aliphatic compounds, depending on the nature of the functional group present, the aromatic compounds are also classified into different homologous series.
1. Aromatic hydrocarbons
Hydrocarbons derived by replacement of one or more H-atoms of the benzene ring by hydrocarbon substituents such as alkyl, alkenyl, alkynyl or aryl groups are called aromatic hydrocarbons or arenes. They can be divided into two categories
1. Arenes containing one benzene ring

2. Arenes containing more than one benzene ring are called polynuclear hydrocarbons

2. Aromatic halogen compounds
Compounds obtained by replacing one or more H-atoms of benzene ring by halogen atoms are called aromatic halogen compounds or aryl halides

3. Aromatic nitro compounds
Compounds obtained by replacing one or more H-atoms of benzene ring by -NO2 group are called aromatic nitro compounds.

4. Aromatic amino compounds
Compounds obtained by replacing one or more H-atoms of benzene ring by — NH2 group or substituted amino group ( — NHR, — NR2) are called aromatic amino compounds

5. Aromatic hydroxy compounds
Aromatic hydroxy compounds are of two types—
1. The compounds in which the hydroxyl (—OH) group is attached directly to the benzene ring are called phenols

2. When the —OH group, instead of being directly linked to the benzene ring, is attached to the side chain, then the compounds are called aromatic alcohols

It is better not to consider aromatic alcohols as aromatic hydroxy compounds because their properties are similar to those of aliphatic alcohols. So, they are usually considered as aryl derivatives ofaliphatic alcohols
6. Aromatic carbonyl compounds
1. Aromatic aldehydes: The compounds in which a —CHO group is directly linked to the benzene ring are known as aromatic aldehydes

2. Aromatic ketones: When an aryl group and an alkyl group aryl groups are joined to a carbonyl group (>C=O), the compounds so obtained are called aromatic ketones

7. Aromatic carboxylic acids:
Compounds containing (one carboxyl (—COOH) group directly attached to the aromatic ring are called aromatic carboxylic acids.

1. Aryl groups: The groups obtained by expulsion of one H -atom from arenes are called aryl (Ar — ) groups,
For example:

2. Aralkyl groups: The groups obtained by expulsion of one or more H-atoms from the side chain of arenes are called aralkyl groups,
For example: The groups that may derived from toluene are:

Nucleus And Side Chain Of Aromatic Compounds
An aromatic compound consists of two parts
- Nucleus and
- Side chain.
1. Nucleus: The benzene ring present in an aromatic compound is called the nucleus.
2. Side chain: If one or more hydrogen atoms of benzene are substituted by alkyl groups, different types of alkyl benzenes are obtained. These alkyl groups are known as side chains.

For example:
The benzene ring in the toluene molecule is its nucleus and the —CH3 part is the side chain. The carbon chains directly attached to the benzene ring are called side chains. Both the nucleus and side chain of aromatic compounds take part in substitution reactions.
Oxidation of the side chain
When an aromatic compound is subjected to oxidation, only the side chain is oxidised but the benzene ring or the nucleus, being sufficiently stable, remains intact.
1. Oxidising agents:
Commonly used oxidising agents dilute HNO3, alkaline KMnO4 solution, K2Cr2O7 acidified with sulphuric acid etc.
For example: Toluene, on oxidation by alkaline KMnO4 and subsequent acidification, yields shining white crystals of benzoic acid.

2. Special feature:
Any side chain (saturated, unsaturated or substituted) containing benzylic hydrogen (i.e., the hydrogen atom attached to the carbon atom directly linked to the benzene ring), on oxidation, becomes converted into a carboxyl ( —COOH) group.

The side chain of test-butylbenzene cannot be oxidised because it does not contain any benzylic hydrogen

Benzene rings containing two side chains give dicarboxylic acid on oxidation

Using mild oxidising agent such as chromyl chloride (CrO2Cl2), toluene gives benzaldehyde. This reaction is called the Etard reaction.

Oxidation of the benzene ring or nucleus:
The benzene ring or nucleus of any alkylbenzene can be oxidised to a carboxyl group by ozonolysis (oxidative workup)

CBSE Class 11 Chemistry Chapter 13 Hydrocarbons Summary
Orientation Of Substituents In The Benzene Ring
1. Ortho/para-directing groups
Ortho/para-directing groups Definition:
Substituent groups present in the benzene ring which direct the incoming group to the ortho- and para-positions of the ring are called ortho-/para- directing group
Groups:
—CH3, —C2H5, — C6H5, —Cl, —Br, —I, —OH,
-OCH3, -NH2, —NHR, —NR2, —NHCOCH3
Examples:
In phenol molecule, an —OH group is already present in the benzene ring. So, phenol on nitration produces mainly a mixture of o- and p -p-nitrophenols.

Similarly, chlorination of toluene in which a — CH3 group is already present in the benzene ring results in the formation of mainly mixture of o – and p -chlorotoluenes.

One characteristic feature of ortho-para- directing groups except alkyl and aryl groups ( —CH3, —C2H5 , —C6H6 ) is that the atom, through which these groups are attached to the benzene ring, contains one or more lone pair of electrons. In fact, due to presence of these unshared pairs of electrons, these groups become ortho-/ para- orienting.
Increase in the activity of the benzene ring :
The ortho/ para directing groups (except halogen atoms), by increasing the electrondensity of the benzene ring, activate the ring more concerning unsubstituted benzene in electrophilic substitution reactions. Thus these groups are called activating groups. In fact, due to an increase in the electron density of the ring by the activating group, the electrophile (E) is more easily attracted by the ring to form a covalent bond (o’ -complex) than the unsubstituted benzene ring. As a result, the electrophilic substitution reaction of the aromatic compound containing activating group occurs at a faster rate than benzene’

2. Meta-directing groups
Definition: Substituent groups present in the benzene ring
Groups:
—NO2, — CN, — CF3, — CHO, — COR,
— COOH, —COOR, —SO3 H etc.
Example: In the nitrobenzene molecule, a nitro (— NO2) group is already present in the benzene ring. So, nitration of nitrobenzene yields mainly m-dinitrobenzene

One characteristic feature of these groups is that the atom which is directly attached to the ring is usually linked to another more electronegative atom by a double or triple bond. In fact, due to the displacement of electrons of the multiple bonds towards the more electronegative atom, these groups become meta-orienting.
Decrease in activity of the benzene ring:
The meta-directing groups, under their electron withdrawing effect, decrease the electron density in the benzene ring and make the ring less reactive towards further electrophilic substitution than unsubstituted benzene.
Thus, these groups are called deactivating groups. As the deactivating group decreases the electron density of the ring, the electrophile (E+) is less easily attracted by the ring than the unsubstituted benzene ring and consequently, the formation of σ -complex, rather the electrophilic substitution reaction occurs at a much slower rate compared to benzene

Ortho-/ Para And Meta Directing Groups:

3. Theory of orientation
The position occupied by the incoming group In monosubstituted benzene is determined by the electronic character, i.e., inductive effect, hyperconjugation effect and resonance effect of the substituent already present In the ring.
Orientation in case of phenol (C6H5 :O:H)
Due to the participation of an unshared pair of electrons of the —:O:H group In resonance (+R effect), the electron density at ortho-and para-positions of the ring increases. For this reason, the —OH group acts as an ortho-/para- directing group in the second electrophilic substitution

Since oxygen is more electronegative than carbon, —OH group in phenol withdraws electrons from the ring by -1 effect. Again, the group donates electrons to the ring by + R effect.
As + R is a more effective than-I effect, —OH group by net electron release activates ring towards further electrophilic substitution, i.e., it is an activating group.
Orientation in the case of toluene (C6H5CH3):
Carbon atom in —CH3 group of toluene contains no lone pair of electrons and therefore, — CH3 group cannot release electrons by + R effect. However, it increases electron density at ortho and para-positions of the ring by hyperconjugation effect. For this reason, the — CH3 group acts as an ortho-/para directing group in the second electrophilic substitution.
+1 effect of —CH3 group has also some contribution in playing the role ofortho-/para- directing group. This can be explained in terms of the relative stabilities of different cr complexes. — CH3 group by its +1 and hyperconjugation electron release activates the ring towards further electrophilic substitution, i.e., it is an activating group
Orientation in case of halobenzene (C6H5 X):
Halogens behave abnormally in electrophilic substitution reactions ofhalobenzenes. In spite of being deactivating groups, they are undoubtedly ortho-/para- directing groups. Halogen atoms withdraw electrons from the ring by -I effect and donate electrons by +R effect 1. However, in case of halobenzene, -I > + R.
Thus the net electron displacement occurs from the ring towards the halogen atom. So, the benzene ring, as a whole, becomes deactivated towards further electrophilic substitution. In fact, halogen atoms withdraw electrons from all positions of the ring by -I effect and send electrons to ortho-Zparapositions by +R effect. Thus, electron densities of ortho-/ para- positions are less reduced, i.e., these positions are comparatively electron-rich and naturally these positions are attacked more easily by electrophiles. So, halogens, in spite of being deactivating groups, are eventually o-/pdirecting. In this case, +R effect of halogen governs the orientation.

Orientation in the case of nitrobenzene (C6H5NO2):
As tlie N=O moiety of — NO2 group in nitrobenzene remains conjugated with the nucleus, the —NO2 group decreases the electron density in the benzene ring by -R effect. Again, it also reduces the electron density of the ring by-1 effect.
In fact, — NO2 group decreases the electron density of all positions of the ring by -I effect but reduces the electron density of only the ortho- and para- positions by -R effect. As a result, the electron density at the meta-position becomes relatively higher and naturally, this position becomes more susceptible towards electrophilic attack. Thus, in the second substitution, the —NO2 group acts as a meto-directing group

In this case, displacement of electrons caused by -R and -I effects occurs from the ring towards the group. Hence, the —NO2 group is a deactivating group.

Almost in each of the substitution reactions of monosubstituted benzene, a mixture of ortho-, meta- and para- isomer is obtained. For ortho-/para- orienting groups, the ortho- and para-isomers are the chief products, the metaisomer being produced in negligible amounts. However, in I the case °i meta- directing groups, the meta-isomer is the major product and the yields of ortho-and para-isomers are negligible. Again, for ortho/para-directing groups, the ortho/ para- isomers are frequently obtained in different amounts
Substitution Reactions Of Aromatic Compounds
Like aliphatic compounds, aromatic compounds undergo three types of substitution reactions:
- Electrophilic substitution reactions: In this type of reaction, the benzene ring is attacked by electrophilic reagents or electrophiles in the first step.
- Nucleophilic substitution reactions: In this type of reaction, the benzene ring is attacked by nucleophilic reagents or nucleophiles in the first step.
- Free radical substitution reactions: This type of reaction involve the attack of free radicals on the benzene ring.
Cause of participation of aromatic compounds in substitution reactions:
- Aromatic compounds gain extraordinary stabilisation due to the resonance and delocalisation of n –electrons. In the formation of an additional product, the aromaticity of the parent aromatic compound is no longer retained due to loss of conjugation and consequently, the extra stability of the compound is lost.
- For this reason, the tendency of aromatic compounds to undergo addition reactions is much lower. On the other hand, in the substitution product, the’ aromatic stability or aromaticity of the starting organic compound remains intact. For this reason, benzenoid aromatic compounds prefer to undergo substitution rather than addition reaction.
- Aromatic compounds prefer electrophilic substitution rather than nucleophilic substitution: The substitution reactions of aromatic compounds are mainly ionic.
- Due to the presence of π -electron cloud above and below the plane of the system, the aromatic rings serve as a source of electron-rich centre and so welcome any attacking electrophile. On the other hand, the benzene ring being electron-rich repels any approaching nucleophile. For this reason, the tendency of aromatic compounds to undergo electrophilic substitution is much higher than nucleophilic substitution.
Mechanism type of reactions of electrophilic take place substitution in two steps reaction:
First step:
The electrophilic reagent or the electrophile (E+) being attracted by the n -electron cloud of the benzene ring forms a sigma (σ) bond with any one of the ring carbon atoms. As a result, a carbocation is formed and in forming this carbocation, the benzene ring loses its aromatic character. The carbocation, however, is stabilised by resonance. It is a resonance hybrid of three resonance structures 1, 2 and 3.
It is generally represented by a single non-Lewis structure 4. As it Is produced by the formation of a sigma bond, it is called σ -complex. It is also known as cyclohexadienyl cation, benzeniumi on or Wheland intermediate. As this step involves loss of aromatic character of the benzene ring, it is slow and hence it is the rate-determining step of the reaction

Second step:
Although the carbocation produced attains some degree of stability through resonance, yet it is less stable than benzene. For this reason, the σ -complex, by rapid expulsion of a proton from the carbon bonded to the electrophile, reverts to the more stable substituted product. Any base (B”) present in the reaction medium helps in the formation of substituted benzene by accepting a proton. In this step, benzene regains its aromatic character or aromaticity

This type of reaction pathway is called bimolecular electrophilic substitution (aromatic) or SE2(Ar) pathway
Electrophiles involved in various electrophilic substitution reactions:

Benzene (C6H6)
Michael Faraday discovered benzene in 1825. In 1845,
Hof&nann was able to recover benzene from coal tar. 80-110°
1. Fractional distillation of coal tar: Isolation of benzene
When coal is subjected to destructive distillation, coal gas, coal tar (black liquid having high viscosity), ammoniacal liquor and coke are obtained. It is a mixture of about 200 compounds, the majority of which are aromatic compounds. Coal tar consists of compounds which are acidic, alkaline and neutral. Isolation of benzene from coal tar: when coal tar is subjected to fractional distillation, the fraction collected upto 170°Cis called light oil.
Its colour is yellow. Light oil contains mainly neutral hydrocarbons like benzene, toluene, xylene etc. Besides these, it contains small amounts of acidic phenol, basic aniline, pyrrole, pyridine, neutral sulphur-containing compound, thiophene and water.
Preparation of 90% benzol from light oil:
- At first the light oil is fractionally distilled and the fractions which are distilled out at temperatures above 70°C are collected.
- The distillate is shaken with cold cone. H2SO4 when the basicsubstances like aniline and pyridine get converted into their sulphate salts and dissolve in acid. A portion of thiophene also dissolves in the acid after being converted into thiophene sulphonic acid. The mixture forms two separate layers. The acid layer is separated from the organic layer.
- The organic layer is then shaken with 10% NaOH solution when acidic phenol dissolves in an alkali solution forming phenatesalt. Excess H2SO4 being neutralised also dissolves in an alkali solution. Two separate layers are formed. The aqueous layer containing caustic soda is then separated from the organic layer.
- The upper organic layer is repeatedly washed with water to remove .excess alkali. The washed fight oil thus obtained is subjected to fractional distillation and the fractions obtained at different range of temperatures are collected
Fractions obtained at different temperatures by fractional distillation of light oil:

Preparation of pure benzene from 90% benzol:
- When 90% benzol is subjected to fractional distillation, the fraction that is collected at 80-82°C is impure benzene. This benzene is contaminated with a small amount of toluene and thiophene.
- When it is cooled in a freezing mixture, benzene is converted into a solid mass at 5.5°C. It is separated from liquid toluene by filtration. When this solid benzene is kept at room temperature, liquid benzene is obtained. Benzene, thus obtained, is not absolutely pure. It contains traces of thiophene (0.05%).
- Test for the presence of thiophene in benzene:o addition of a yellow solution of isatin (dissolved in H2S04 ) to a sample of benzene causes the development of a blue colour, then it indicates that the sample is contaminated with thiophene. This test is very sensitive even to very small amounts of thiophene.
- Alternatively, on shaking a sample of benzene with a cone. H2SO4 two layers are formed. If the lower layer assumes a yellow colour, then it indicates that the sample is
Removal of thiophene from benzene:
- The boiling point of benzene is 80.4°C. So, thiophene (b.p. 84°C) cannot be removed completely from benzene by fractional distillation. i] When benzene containing thiophene is shaken with cold and cone. H2SO4 thiophene sulphonic acid (a yellow liquid) is formed. It dissolves in sulphuric acid. contaminated with thiophene
- Benzene does not react with cold and cone. H2SO4 This process of washing with cone. H2SO4 is repeated several times till the layer of H2SO4 becomes no longer yellow. It suggests the complete removal of thiophene from the sample of benzene.
- Benzene thus obtained is washed with water thoroughly to make it free from acid. It is then dried with fused CaCl2 & redistilled when pure benzene is obtained.
1. Preparation of benzene
Industrial preparation:
The chief source of benzene is coal tar. Besides this, benzene is also obtained from mineral petroleum. Manufacture of benzene from coal tar has been discussed earlier
Laboratory preparation:
1. From acetylene:
When acetylene gas is passed through red hot copper tube (600°C) , three molecules of it combine to form benzene (polymerisation reaction)

2. By decarboxylation of sodium benzoate:
Anhydrous sodium benzoate, on being heated in the presence of soda lime, yields benzene

3. Benzene From phenol:
Phenol, when distilled in the presence of zinc dust or the vapours of phenol, when passed over zinc dust, produces benzene.

4. From diazonium salts:
When a solution of benzene diazonium salt, C6H5N2Cl is heated with hypophosphorous acid or dry alcohol, benzene is produced.

5. From benzenesulphonic acid :
When benzenesulphonic acid is heated to 150-200°C under pressure in the presence of dilute HCl or H2SO4 , benzene is obtained (This reaction is used for the removal of
—SO3H group from benzene ring is called desulphonation)

2. Physical properties of benzene
- Benzene is a colourless liquid with a characteristic smell. Its boiling point is 80.4°C. When cooled by freezing mixture, it forms a crystalline solid.’Hie melting point of crystalline benzene is 5.5°C
- Benzene is lighter than water (specific gravity : 0.B7). It is insoluble in water but dissolves In alcohol, ether and acetone.
- Benzene is a good solvent for oils, fats, rubber, resin, iodine, sulphur, phosphorus etc.
- Benzene is a highly inflammable substance.lt burns with a sooty luminous flame. Due to the high percentage of carbon, elementary carbon is produced during the burning of benzene. Due to this black smoke is formed. The presence of hot
carbon particles in a flame makes the flame luminous. - Benzene is a highly toxic substance. At present, it has been identified as a carcinogenic compound.
All aromatic compounds bum with sooty flame but aliphatic compounds do not bum with such sooty flame. r So, aliphatic and aromatic compounds can be distinguished with the help of an ignition test.
Two special precautions:
Benzene is highly
It is highly injurious to inhale benzene vapours. So, it should never be allowed to vaporise in open air and for this purpose, fume chamber must be used.
3. Chemical properties and reactions of benzene
- Despite the presence of three double bonds, the chemical properties of benzene are quite different from those of olefins.
- Although in some cases benzene takes part in addition reactions
- Its chief and characteristic reactions are substitution reactions because in substitution products, aromaticity of the ring system is preserved.
Substitution Reactions Of Benzene
1. Halogenation of benzene
1. Chlorination:
The reaction in which an H-atom of benzene ring is displaced by a Cl-atom is known as chlorination reaction. When benzene is allowed to react with chlorine at ordinary temperature in the presence of Lewis acid catalysts such as FeCl3 , AlCl3 , I2 etc., (act as halogen carrier), chlorobenzene is obtained.
Iron (catalyst) is most commonly used, being converted to the Lewis acid FeCl3 by chlorine. In the absence of halogen carrier, such a substitution reaction does not take place

Reaction mechanism:
It is an electrophilic substitution reaction. The electrophile involved in this reaction is a positive chlorine ion (Cl+) or the chlorine-iron (3) chloride complex which leads to the formation of that ion. This Cl+ ion displaces H+ from the ring.
Formation of electrophile:
Fe reacts with Cl2 to form Lewis acid, FeCl3. The Lewis acid then forms a complex with excess CI2. The complex finally dissociates to give Cl+ and FeCl+ ions:
2Fe + 3Cl2 → 2FeCl3 (Lewis acid)

Substitution:

The number of H-atoms of the ring that will be displaced by chlorine depends on the quantity of chlorine used. For For example, 1 mole of Cl2 (in addition to that consumed by Fe to form FeCl3) leads to the formation of monochloro benzene (C6H5Cl) . When the reaction is continued for a long period using 2 moles of Cl2 , a mixture of maple ortho- and para- dichlorobenzenes (C6H4Cl2) is obtained. In this case, chlorobenzene is formed first. Chlorine atom of chlorobenzene is ortho-/para-directing. So, the second chlorine atom enters the ortho- or para-position with respect to the first Cl-atom

2. Bromination:
Like Cl2,Br2 also reacts with benzene in the presence of iron filings, AlBr3 or iodine (halogen carrier) to form bromobenzene.

The reaction mechanism of the bromination reaction is similar to the chlorination reaction
3. Iodination:
Iodination of benzene cannot be accomplished like chlorination or bromination because iodine is the least active of the halogens. Iodination may, however, be carried out smoothly in the presence of an oxidising agent like nitric acid, mercuric oxide etc. When a mixture of benzene, iodine and cone. HNO3 is refluxed, and one H-atom of benzene ring is substituted by one I-atom to give iodobenzene in good yield

Removal of a halogen atom from the benzene ring:
A halogen atom present in the benzene ring may be removed through the i formation of Grignard reagent i.e., halobenzene can be converted into benzene

2. Nitration of benzene
Nitration of benzene Definition:
The reaction in which a hydrogen atom of benzene is displaced by nitro (-NO) group is called a nitration reaction. 05 tot. (C6H4Cl2)
Reagent:
Generally a mixture of cones. HNO3 and H2SO4 is used as nitrating agent. This mixture of two acids is called mixed acid.
1. Preparation of nitrobenzene:
When benzene is heated at 50 – 60°C in the presence of a mixture of one. HNO3 and cone. H2SO4 one H-atom ofbenzene ring is replaced by a nitro (— +NO2) group to produce nitrobenzene.

Reaction mechanism:
It is an electrophilic substitution reaction. The electrophile involved in this reaction is nitronium ion ( NO2 ) & it is the actual nitrating agent in this reaction.
Formation of the electrophile:
HNO3 accepts a proton from H2SO4 to form its conjugate acid (O2N—OH2). It then dissociates to form a nitronium ion

If only hot and cone. HNO3 is used, and the reaction takes place at a relatively slower rate. Again aromatic compounds which are susceptible to oxidation, the explosion may take place.
Substitution:

2. Preparation of dinitrobenzene :
Whenandbenzenecone. H2SO4 is heated at boiling water bath), and m-dinitrobenzene is produced. At first, nitrobenzene is formed. Since the ( —NO4) group is meta-directing, the second nitro group mainly enters the meta-position

Nitro group, being an electron-attracting group, decreases the electron density of ring. So, incoming ion cannot be easily attracted by the ring and consequently, the nitration reaction becomes somewhat difficult. For this reason, in the case of second nitration, higher temperature and fuming nitric acid are used
3. Preparation of trinitrobenzene:
When benzene is refluxed with fuming HNO3 and fuming H2SO4 for 5-6 days, 1,3,5-trinitrobenzene (TNB) is produced. It is an explosive. The third nitro group takes the meta-position with respect to the two nitro groups already present in ring

- The two nitro groups present in m-dinitrobenzene cause a decrease in the electron density of the ring to such an extent that the introduction of the third nitro group becomes extremely difficult.
- For this reason, the third nitration requires much higher temperature and a mixture of fuming nitric acid and fuming sulphuric acid becomes necessary.
- Nitration of benzene can also be carried out by using stable nitronium salts like nitronium tetrafluoroborate (NO2BF4), nitronium trifluoromethane sulphonate (NO2 CF3SO3 etc.

3. Sulphonation of benzene
Sulphonation of benzene Definition:
The reaction in which a hydrogen atom of the benzene ring Is substituted by sulphonic acid (-SO3H) group is known as the sulphonation reaction. Reaction and conditions: At ordinary temperature, cone. H2SO4 does not react with benzene. However, when a mixture of benzene and cone. H2SO4 is heated at 80°C about hours, benzene sulphonic acid Is produced

Sulphonation with fuming H2SO4 (7% SO3 dissolved in cone. H2SO4) can be carried out even at ordinary temperature. Sulphonation may also be affected by chlorosulphonic acid (C1S03H).
Sulphonation of benzene Reaction mechanism :
This electrophilic substitution reaction is reversible. In this reaction, sulphur trioxide (SO3) acts as the electrophile. In some cases, protonated sulphur trioxide (HSO3+) acts as an electrophile.
Formation of electrophile:
Two molecules of sulphuric acid interact in the following way to form sulphur trioxide
Substitution:
It occurs through three steps.
First step: Formation of carbocation (σ-complex).

Second step: Expulsion of proton

The second step occurs at a slower rate than the first step. Thus, the second step is the rate-determining step

Preparation of di- & tri-sulphonic acid:
Benzene, on sulphonation with fuming H2SO4 at 200-245°C gives benzene-m-sulphonic acid which on further heating at 280-300°C produces1,3,5-benzenetrisulphonic acid

At first benzene sulphonic acid is formed. The sulphonic acid group(—SO3H) is meta-directing. So, the second
—The SO3H group occupies the meta-position and the third
—SO3H group takes the meta-position concerning the other two sulphonic acid groups. The sulphonic acid group is a deactivating group. So, the introduction of second and third
—SO3H group becomes more and more difficult.
Thus, to introduce the second and third
—SO3H groups, the reaction temperature is to be gradually increased.
Removal of —SO3 H group from benzene sulphonic acid:
- When benzene sulphonic acid is heated to 150°C with dilute HCl or H2SO4 or is brought in contact with superheated steam, the
- —SO3H group is replaced by hydrogen to form benzene. The reaction is called desulphonation reaction. In this reaction, proton (H+) acts as the electrophile.

- If superheated D2O is used instead ofsuperheated steam, the —SO3H group is replaced by D to produce C6H5D. O Importance: ‘Sulphonation-de-sulphonation’ may serve as a useful tool for synthesis of various organic compounds.
- This may well be illustrated in the synthesis of o-nitroaniline from aniline. To avoid oxidation, aniline is first converted into acetanilide. The para-position of acetanilide is then blocked by the —SO3H group.
Due to steric reason, the —SO3H group enters mainly the para-position. In the next step, the — NO2 group enters the position ortho to -NHCOCH3
Finally, —SO3H group is removed from the ring. The sulphonic acid group here acts as a blocking group.

If para-position of acetanilide is not blocked by the —SO3 H group, — NO2 enters mainly para- position instead of the sterically more crowded ortho-position to give p-nitroaniline.
4. Friedel-Crafts reaction:
The reaction in which a H-atom of the benzene ring is Q) + CH3CH2CI Anhyd. AlCl3 substituted by alkyl (R— ) or acyl (RCO— ) group in the presence of a catalyst is called Friedel-Crafts reactions
Catalysts used: The best catalyst used for this reaction is anhydrous aluminium chloride (AlCl2). Lewis acids such as boron trifluoride (BF3) , ferric chloride (FeCl3) , zinc chloride (ZnCl2) etc., and protonic acids like HF, H2SO4, H2PO4 etc., may also be used as catalyst
Solvents used:
Suitable solvent for this reaction is nitrobenzene. Nitrobenzene with highly deactivated ring (due to -R and -I effects of -NO2 group) does not take part in this reaction as the relatively weak electrophile R+ cannot attack the ring i.e., substitution or Fridel-Crafts reaction does not take place. Being polar, nitrobenzene dissolves anhydrous AICI3. Again, benzene and alkylating or acylating reagents also dissolve in it. As all the reagents remain dissolved in liquid phase, the reaction takes place smoothly.
Moreover, as the boiling point of nitrobenzene is high (211°C), the reaction may be conducted atappreciably high temperature. Sometimes, carbon disulphide (CS2) is also used as a solvent for the reaction.
Friedel-Crafts alkylation:
When benzene is allowed to react with alkyl halide (RX) in the presence of anhydrous aluminium chloride as a catalyst, a hydrogen atom of benzene ring is replaced by alkyl group to produce alkylbenzene. This reaction is called the Friedel-Crafts alkylation. The different homologues of benzene can be prepared with the help of this reaction.

Examples:
- Methylbenzene (toluene) may be prepared by allowing benzene to react with methyl iodide in the presence of anhydrous AlCl3.
- Methyl chloride may also be used, but as methyl iodide is a liquid at ordinary temperature, it is generally preferred

Similarly, ethylbenzene is obtained when benzene reacts with ethyl chloride in the presence of anhydrous aluminium chloride

Friedel-Crafts Reaction mechanism:
The effective electrophile in this electrophilic substitution reaction is a carbocation (R+). Although 2° or 3 alkyl halide reacts with the Lewis acid, AICI3 to form R, the reaction of
alkyl halide or methyl halide with AICI3 does not produce R+ ion. This is because the 1° carbocation and CH2 are relatively very unstable. In that case, the initial complex formed in the reaction between the alkyl halide and AlCl3 acts as the electrophile.
Formation of the electrophile:

Substitution: It occurs in two steps
First step: Formation of carbonation(-complex)

Second step: Explusion of Proton

In this reaction, besides alkyl halides, aliphatic alcohols, may also be used as alkylating agents. Apart from AlCl3, BF3, HP, cone. H2SO4 etc., are used as catalysts.

Limitations of the Friedel-Crafts alkylation reaction:
1. In this reaction, there is always a possibility of polyalkylation of the benzene ring because, once an alkyl group enters the benzene ring, the electron density of the ring increases due to electron-repelling effect of the alkyl group. Consequently, the ring becomes activated towards further electrophilic substitution. Now, all the molecules of benzene and alkyl halide do participate in the reaction simultaneously.
So, the alkyl halide molecules present in the reaction medium reacts with the resulting alkylbenzene molecules at a rate faster than benzene. As a result, more than one alkyl group enters the benzene ring, i.e., polyalkylation occurs. Thus, for the preparation of monoalkyl benzenes, the Friedel-Crafts reaction is not suitable.

Polyalkylation in Friedel-Crafts alkylation reaction may be considerably reduced by using excess of substrate (benzene).
2. If alkyl halide used in the Friedel-Crafts alkylation reaction is a primary alkyl halide containing three or more carbon atoms, then instead of the corresponding alkyl benzene, the alkyl benzene containing a secondary or tertiary alkyl group is obtained as the major or the sole product by rearrangement ofthe alkyl group

Reaction mechanism:
Lewis acid-Lewis base complex obtained in the reaction of AlCl3 with the 1° alkyl chloride undergoes dissociation and rearrangement simultaneously to form a stable 2° or 3° carbocation. These carbocations participate in substitution reactions and as a result, alkyl benzenes isomeric with the expected alkyl benzene are obtained as the major or the sole product.

3. The following compounds do not participate in FriedelCrafts alkylation and acylation reactions:

Groups such as nitro, carboxyl, acyl and trimethyl-ammonium (— +NMe3) etc., withdraw electrons from the benzene ring and deactivate the ring to such an extent that it cannot be attacked by tyre relatively weak electrophile (R+). So, the substitution reaction does not take place.
Aniline does not react because the —NH2 group gets converted into a powerful electron-withdrawing group by coordinating with the Lewis acid.

Any vinyl halide, vinyl chloride (CH2=CH—Cl) ] or halobenzene [e.g., chlorobenzene (C6H5Cl) ] cannot be used as an alkylating agent. As, due to resonance, the C—X bond acquires some double bond character, AlCl3 cannot snatch the halogen atom to form the corresponding carbocations.
Friedel Crafts Acylation:
When benzene is treated with acyl chloride (RCOCl) in the presence of anhydrous aluminium chloride as catalyst, the H-atom of benzene ring is substituted by the acyl (RCO— ) group to form acylbenzene (aromatic ketone).
This reaction is called Friedel-Crafts acylation. Besides acyl chlorides, acid anhydrides are also used as acylating agents.

Hydrocarbons Class 11 NCERT Notes
Friedel Crafts Acylation Reaction mechanism:
The electrophile involved in this electrophilic substitution reaction is an acylium ion (R —C=O) substitution reaction is an acylium ion (R —C=O)
Formation of the electrophile:

Two special synthetic advantages of acylation reaction:
1. Although polyalkylation is a common feature of benzene, its polyacylation never occurs. An electron-attracting acyl (RCO— ) Group decreases the electron density of the ring.
As a result, despite the presence of excess RCOCl in the reaction medium, the second acylation does not take place, i.e., not more than one RCO— Group can enter the benzene ring. Therefore, pure aromatic ketone can easily be synthesised with the help of this reaction.
2. During the acylation reaction, the carbon chain of the acyl halide does not rearrange. So, there is no possibility of formation ofa ketone isomeric with the desired ketone. Due to such an advantage, the acylation reaction is used to prepare alkyl benzenes containing 3 or more carbon atoms.
For example:
The alkylation reaction for preparing propylbenzene leads to the formation of iso propyl benzene as the major product. However, if benzene is first allowed to form a ketone and the ketone is then reduced (Clemmensen reduction), the desired propylbenzene is obtained as the only product

Removal of alkyl or acyl group from benzene ring:
Toluene or acetophenone on oxidation by alkaline KMn04 solution followed by acidification produces benzoic acid which, when heated with soda lime, gives benzene

If the alkyl group is tertiary (Me3C— ), then it cannot be removed in this way because the tertiary alkyl group cannot be oxidised. However, the tertiary alkyl group can easily be removed from the ring
By the following reaction:

Chloromethylation:
When benzene is heated with HCHO and HCI in the presence of anhydrous zinc chloride, H-atom of the benzene ring is replaced by the chloromethyl (—CH2Cl) group to form benzyl chloride. This reaction is called chloromethylation

CH2Cl group may be removedfrom benzene ringbythe same process applied for removal ofalkyl (—R) group.
Gattermann-Koch aldehyde synthesis:
When a mixture of carbon monoxide and hydrogen chloride is passed through benzene dissolved in nitrobenzene or etherin the presence of anhydrous AlCl3 and small amount Cu2Cl2 as catalyst, benzaldehyde is obtained. This reaction is known as Gattermann-Koch aldehyde synthesis

—CHO group may be removed from benzene ring by the same procedure applied for the removal of an alkyl group.
Gattermann aldehyde synthesis:
Benzene reacts with hydrogen cyanide and hydrogen chloride in the presence of anhydrous AlCl3 to produce an imine which on hydrolysis gives benzaldehyde. This reaction is known as Gattermannaldehydesynthesis

Addition Reactions Of Benzene
1. Reduction—addition of hydrogen
1. Reduction into cyclohexane:
When a mixture of benzene vapour and hydrogen gas is passed through powdered nickel catalyst heated at 200°C, benzene combines with three molecules of hydrogen to form hexahydrobenzene or cyclohexane.

In this reaction, cyclohexadiene  and cyclohexane
and cyclohexane  are not obtained as intermediates because these compounds are more reactive than benzene.
are not obtained as intermediates because these compounds are more reactive than benzene.
2. Birch reduction:
Benzene is reduced by Na, K or Li in liquid ammonia in presence ofmethanol or ethanol to give 1,4-cyclohexadiene. This reaction is called Birchreduction

2. Addition of halogen
When chlorine gas is passed through boiling benzene or through benzene in the presence of
UV-rays, benzene combines with three molecules of chlorine to form benzene hexachloride or 1,2,3,4,5,6- hexachlorocyclohexane

Like chlorine, bromine also reacts with benzene to form benzene hexabromide.

lodine doesn’t undergo such additional reaction with benzene. benzen
BHC is used as an insecticide. It is a mixture of eight geometrical isomers and out of them the y-isomer (overall 18%), known as gammexane or lindane, possesses insecticidal property. So, BHC too possesses insecticidal property. However, because of its many adverse effects, its use is gradually declining.
3. Addition of ozone
When ozonised’ oxygen is passed through benzene at ordinary temperature, benzene combines with three molecules of ozone to form an unstable addition compound known as benzene triozonide. When the triozonide is hydrolysed in the presence of zinc, three molecules ofglyoxal and H2O2 are obtained. H2O2 thus produced is reduced by zinc to give water

The formation of 3 mol glyoxal from 1 mol benzene suggests that benzene is a six-membered carbocyclic compound containing 3 double bonds in alternate positions
Oxidation Of Benzene
When a mixture of benzene vapour and air is passed over a vanadium pentoxide (V2O5) catalyst heated at much higher temperature (450°C), benzene is oxidised to maleic anhydride

Effect of high temperature on benzene
When benzene vapours are passed through a hot (600-800) iron tube packed with pumice stones, biphenyl or diphenyl is obtained.

Uses of benzene
The preparation of ethyl benzene is an important use of benzene because, ethyl benzene is used in the preparation of styrene (C6H5CH=CH2), the starting material for the manufacture of polystyrene and artificial rubber.
It is used to prepare many important compounds,
For example: Nitrobenzene, aniline, phenol, etc., and detergents (alkyl benzenesulphonates).
It is used as a solvent for fats, oils, resins, rubber, sulphur, iodine, phosphorus etc..
Carcinogenicity And Toxicity
Benzene and polynuclear hydrocarbons containing fused rings or are highly toxic. Many of these are carcinogenic substances. These are formed by Incomplete combustion of organic materials such as coal, petroleum, tobacco etc. and extensively mix with the surrounding air, These compounds enter our body during inhalation and destroy our DNA by performing various biochemical reactions. This ultimately leads to cancer.

Hydrocarbons Class 11 NCERT Notes
Action of carcinogenic substances in the human body:
- From experimental results, it has come to our knowledge that these polynuclear hydrocarbons (PNH) after entering the human body are first converted into epoxides and then into dihydroxy epoxides.
- The resulting dihydroxy epoxides react with the purine bases such as guanine present in DNA and RNA of the human cells.
- Due to the attachment of the hydrocarbon part, the purine bases become large in size and can no longer accommodate themselves in the double helix of DNA.
- This damage in the double helix of DNA causes mutation and ultimately leads to cancer. Carcinogenic effect of polynuclear hydrocarbons (PNH)

Preparation of benzene:

Special reactions of benzene:

Flow chart for substitution reactions of benzene:

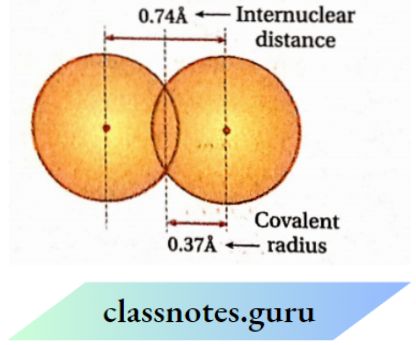























































































































































































































































































 molecules and are always optically active.
molecules and are always optically active.





























































































































































 are not obtained as intermediates because these compounds are more reactive than benzene.
are not obtained as intermediates because these compounds are more reactive than benzene.












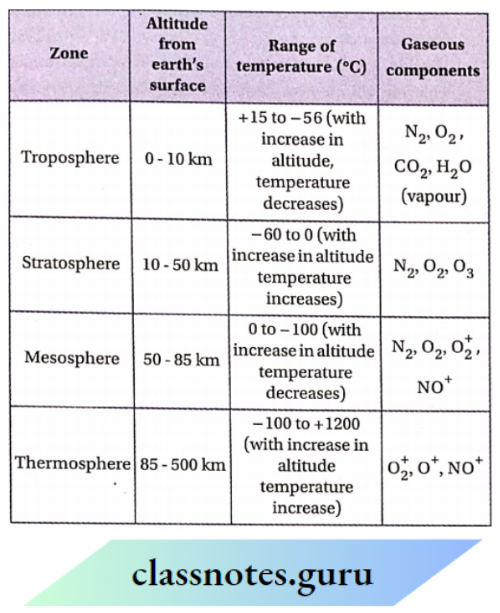


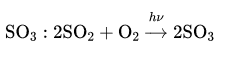









































 (M = Be, Mg or Ca)
(M = Be, Mg or Ca) (M = Ba, Sr)
(M = Ba, Sr)




















































































































































































































 What happens ether?
What happens ether?




































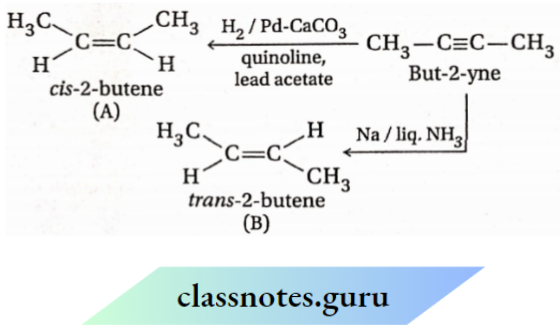



















 formed.
formed.













































 A and B are the two geometrical isomers. Identify them.
A and B are the two geometrical isomers. Identify them.

















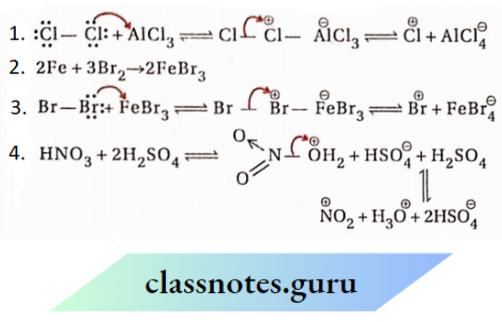
















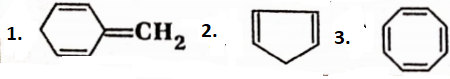


 Cyclooctatetraene has a non-planar structure and there are 8π -electrons in it. So, cyclooctatetraene is a non-aromatic compound.
Cyclooctatetraene has a non-planar structure and there are 8π -electrons in it. So, cyclooctatetraene is a non-aromatic compound.






