Concept Of PH And PH Scale
In the case dilute solution of an acid or a base, the concentration of H30+ or OH’ ions Is generally expressed in terms of the negative power of 10.
For instance, the concentration of H3O+ negative power of 10. For instance, the concentration of H3O+ concentration of OH– ions In 0.0002(M) NaOH solution is 2 x 10-4 mol. L-1 . However, it is very inconvenient to express the concentration of H3O+ or OH– ions in terms of such negative power.
To overcome such difficulty encountered in the case of dilute solutions, Sorensen introduced the system of expressing the concentration of H3O+ ions by pH (pH stands for Potein of hydrogen ion; the German word ‘Potenz’ means ‘power’).
PH Scale Definition:
The ph of a solution is defined as the negative i logarithm to the 10 of Its H30′ Ion concentration In mol-1
Therefore, pH=-log 10[H3O+]
Example:
If the concentration of If. O– Ions In a solution is 10-3(M), then all of the solution = -log 10 (10-3) =3
Read and Learn More CBSE Class 11 Chemistry Notes
Important points to remember about all of the solutions:
1. pH – log10[H3O+] . According to this equation, If (the concentration of Ions in a solution Increases, then the of the solution decreases, and vice-versa. Thus, the higher the value for a solution, [H3O+] lower the pH of the solution. Conversely, the lower the value for a solution, the higher the pH of the solution.
Example:
If [H3O+] = 10-3 (M) In an aqueous solution, then all of the solution = 3. Now, If the solution Is diluted such that [H3O+ ] = 10-5 = (M), then the pH of that solution increases and becomes 5.
2. If the pH of an aqueous solution is increased or decreased by one unit, then the concentration of H8O+ Ions In the solution undergoes a ten-fold decrease or increase in its value.
CBSE Class 11 Chemistry Notes Concept of pH and pH Scale
Example:
Let the pH of an aqueous solution be 3. So, [H3O+] in the solution = 10-pH= 10-3(M). Now, by diluting the solution, If the pH of (lie solution Is made 4, then, the concentration of H3O+ ions i.e., H3O+ will be =10-pH= 10-1 (M).
Hence, when the pH of (lie solution is increased by one unit, the concentration of H3O4 Ions In the solution decreases by a factor of ten. Similarly, the decrease In pH by one unit corresponds to a ten-fold Increase In [H3O+.] The acidity of a solution Increases with a decrease In pH and decreases with an Increase In pH.
The POH of a solution Is defined as the negative logarithm to the base 10 of Its OH- Ion concentration In mol. L-1.
Therefore POH = – log10 [OH–]
Important points about the pH of a solution:
- With a decrease or Increase In (lie concentration of OH- ions In the solution, the pOH of the solution Increases or decreases respectively
- The basicity of a solution increases with a decrease in pOH and decreases with an increase in pOH.
Relation holen pH, pOH, and pKw
At a fixed temperature, for pure water or an aqueous solution,
⇒ \(\left|\mathrm{H}_3 \mathrm{O}^{+}\right| \times\left[\mathrm{OH}^{-} \mid=K_w\right.\)
Taking negative logarithms on both sides, we get
-log10[H3O+]-log10[OH–]= – log10 Kw
Or, p+HpOH =PKw
Therefore, at a fixed temperature, for pure water or an aqueous solution, pH+POH= pk
At 25C, pKw = J 4. Hence, at 25°C, for pure water or any aqueous solution pH+POH =14
Values of pH and pOH for pure water:
Pure water is neutral. Hence, in pure water [H3O+] = [OH–]
⇒ \(\text { or, }-\log _{10}\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=-\log _{10}\left[\mathrm{OH}^{-}\right] \text {or, } p \mathrm{HOH}\)
From the relation, pH + pOH = pKw, we have 2pH= 2pOH = p Kw
Or \(p H=p O H=\frac{1}{2} p K_w\)
At 25 °C, pKw= 14 . Hence, in the case of pure water at 25 °C,
⇒ \(p H=p O H=\frac{1}{2} \times 14=7\)
At 100°C, pKw = 1 2.26. Hence, in the case of pure water at 100 °C,
⇒ \(p H=p O H=\frac{1}{2} \times 12.26=6.13\)
pH of pure water at 100 °C is lower than that at 25 °C. Hence, at 100 °C, the molar concentration of H2O+ ions is higher than that at 25°C. However, this does not mean that pure water is acidic at 100 C. Because the concentration of H2O+ and OH– ions are always the same in pure water, it Is always neutral irrespective of temperature
pH of a basic solution
To calculate the pH of a basic solution, the equation pH + pOH = pK w is used. If the temperature is 25°C, then pKw = 14. Therefore, at 25°C, if the pOH of an aqueous solution lies at 3, then,
pH = 14 -pOH =14-3 = 11.
PH -scale
PH -scale Definition: The scale in terms of which the acidity or basicity of any aqueous solution is expressed by its pH value is called the pH scale.
Range of pH-scale at 25°C:
Generally in dilute solution, the concentration of H3O+ or OH– ions is not more than 1mol.L-1. If in the solution, [H3O+] = 1 mol.L-1, then pH = —log 10- = 0.
If in the solution, [OH–] = 1 mol.L-1, then [H3O+] = 10-14 mol.L-1 [since At 25°C, Kw = 10-14]. Hence, for a dilute solution.
pH = -log 10-14
=1 4
Therefore, at 25 °C, the pH of a dilute aqueous solution ranges from 0 to 14.

Range of pW-scale vs. temperature:
The value of pKw of water determines the range of pH -scale. Since the value of pKw varies with the temperature change, the range of pH -scale also changes with the temperature change.
The range of the pH -scale can generally be expressed in the following way:

The value of pKw decreases with an increase in temperature. As a result, the range of pH -scale also decreases. For instance, at 25°C, pKw = 14. Hence, at this temperature, the pH scale extends from 0 to 14. On the other hand, at 100°C, pKw = 12.26. Thus, at this temperature pH scale extends from 0 to 12.26. For a neutral aqueous solution at 25°C, pH = 7, and at 100°C, pH =6.13.
a pH of neutral, acidic, and basic solutions at 25’C:
pH of neutral aqueous solution:
In the case of a neutral aqueous solution at 25°C,
[H3O+]=[OH–] =, O-14
= 10-7mol.L-1. Hence, for a neutral solution at 25°C, pH =-Iog10 [H3O+] =log1010-7 = 7.
pH of aqueous acidic solution:
In the case of an aqueous acidic solution at 25 °C, [H3O+] > 10-7 mol.L-1 or, -log10 [H3O+]<7 or, pH < 7.
pH of aqueous basic solution:
For an aqueous basic solution at 25 °C, [H2O+] < 10-7 mol.L-1 or, -log10[H3O+] >7 or, pH> 7.
At 25 °C, Forneutralaqueoussolution: pH = 7
For acidic aqueous solution: pH< 7

The pH of a solution of a weak acid and that of a weak base
Weak acids or weak bases ionize partially in their aqueous solutions. So, the concentration of H3O+ or OH– ions in an aqueous solution of a weak acid or a weak base cannot be determined directly from their initial concentrations.
To determine the pH (or pOH) of a weak acid (or weak base), the initial concentration of the acid (or base) as well as the degree. of ionization of the add (or base) or ionization constant of the acid (or base) should be known.
pH and pH Scale Class 11 Chemistry Notes
Determination of a solution of a weak monobasic acid:
Let a weak monobasic acid be HA. In an aqueous solution,
Hapartiallyionises to establish the given equilibrium:
HA(aq) + H2O(l) ⇌ H3O+(aq) + A– (aq)
If the initial concentration of HA = c (M), its degree of ionization at equilibrium = a and its ionization constant
⇒ \(=K_a \text {, then }\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\alpha c=\sqrt{\frac{K_a}{c}} \times c=\sqrt{c \times K_a}\)
∴ For HA, pH = -log10[H2O+] = -log10(αc)……………………………….(1)
⇒ \(\text { or, } p H=-\log _{10}\left(c \times K_a\right)^{1 / 2}=-\frac{1}{2} \log _{10} K_a-\frac{1}{2} \log c\)
∴ \(p H=\frac{1}{2} p K_a-\frac{1}{2} \log c\) ……………………………….(2)
Thus, if the initial concentration (c) of the solution and the degree of ionization (α) of the acid are known, the pH of the solution can be determined by applying equation (1) Or, from the knowledge of the initial concentration (c) of the solution and the ionization constant (Ka) of the acid at the experimental temperature, it is possible to determine the of that solution with the help of equation (2)
Since the ionization constant of a weak acid (or base) is very small similar to the concentration of H3O+ (or OH–) ions in very dilute solutions, the ionization constant can also be expressed in terms of ‘p’ pKa = -log10Ka and pKb = -log10Kb.
So, a smaller value of Ka (or Kb) corresponds to a large value of pKa (or pKb) and vice-versa. The stronger an acid, the larger its Ka, and hence the smaller its pKa.
For this reason, between two weak acids, the one with a smaller value of pKa is stronger than the other in water. Similarly, between two weak bases, the one with a small value of pKb is stronger than the other in water.
Determination of pH of a solution of a weak monoacidic base:
Let B be a weak monoacidic base. In an aqueous solution, B reacts with water and forms the following equilibrium.
⇒ \(\mathrm{B}(a q)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{BH}^{+}(a q)+\mathrm{OH}^{-}(a q)\)
In aqueous solution \(\left[\mathrm{OH}^{-}\right]=\sqrt{c \times K_b}\)
∴ \(-\log _{10}\left[\mathrm{OH}^{-}\right]=-\log _{10}\left(c \times K_b\right)^{1 / 2}\)
⇒ \(\text { or, } p O H=-\frac{1}{2} \log _{10} K_b-\frac{1}{2} \log c\)
∴ \(p O H=\frac{1}{2} p K_b-\frac{1}{2} \log c\)
Hence, if we know the initial concentration (c) of the weak monoacidic base in the solution and its ionization constant (Kb) at the experimental temperature, then we can calculate the pOH of the solution by applying equation (1).
Now, pH+ pOH = 14 [at 25°C]
∴ For a solution of weak monoacidic base,
⇒ \(p H=14-p O H=14-\left(\frac{1}{2} p K_b-\frac{1}{2} \log c\right)\)
∴ \(p H=14-\frac{1}{2} p K_b+\frac{1}{2} \log c\)
Therefore, by putting the values of pKb and concentration (c) of the solution in equation (2), the pH of the solution can be determined.
pH (or pOH) of an aqueous solution of acid (or base) having concentration <10-7(m)
It is apparent that for an aqueous solution of 10-7(M)HCl, pH = 7,
And an aqueous solution of 0-8(M) NaOH, pOH = and i.e., pH = 14 -pOH
= 14-8
= 6.
However, these values are not acceptable because the pH of an acidic solution and the pOH of a basic solution are always less than 7. Similarly, the pOH of an acidic solution and the pH of a basic solution are always greater than 7.
1. Generally, in the calculation of the pH or pOH of an aqueous acidic or basic solution, the concentration of H3O+ or OH– ions produced by the ionization of water is considered to be negligible. However, we cannot neglect them when the concentration of the acidic or basic solutions is very small [≤10-7 (M)].
2. The total concentration of HgO+ ions in a very dilute aqueous acid solution = the concentration of H6O+ ions produced by the ionization of acid + the concentration of H6O+ ions produced by ionization of water. If the acid solution is calculated by using this total concentration of H3O+, then the value of pH is always found to be less than 7.
The total concentration of OH– ions in a very dilute aqueous base solution is the concentration of OH– ions produced by the ionization of base + the concentration of OH- ions produced by the ionization of water.
If pOH of the baste solution is calculated by using this total concentration of OH-, then the value of pOH is always found to be less than 7.
NCERT Solutions Class 11 Chemistry pH and pH Scale
Numerical Examples
Determine the pH of the following solutions:
- 0.01(N)HCl
- 0 0.05(M) H2SO4
- 0 0.001(N) H2SO4.
Answer:
1. 0.01(N) HCl = 0.01(M)I-HCl solution HCl is a monobasic acid] In 0.01(M) [since HCl is a monobasic acid]
[since 1 molecule of HCl ionizes to give a single H3O+ ion] In case of an aqueous 0.01(M) HCl solution, pH = -log10[H3O+]
= -log10(0.01) = 2.0
2. In 0.05(M) H2SO4 solution, [H3O+]= 2 × 0.05= 0.1(M)
Since Each H2SO4 molecule ionises to give two H30+ ions] For an aqueous 0.05(M)H2S04 solution,
pH = -log10[H3O+] = -log10(0.1) = 1.0
3. In 0.001(N) H2SO4 solution, [H3O+] = 0.001(M) In case of an aqueous 0.001(N) H2SO4 solution,
pH = -log10[H3O+] = -log10(0.001)
= 3.0
Question 2. Determine the pH of the following solutions:
- 0.1(N)NaOH
- 0 0.005(M)
- Ca(OH)2
Answer:
1. 0.1(N)NaOH = 0.1(M)NaOH [NaOH is amino acid base]
In 0.1(M)NaOH solution, [OH– ] = 0.1(M)
∴ For an aqueous 0.1(M)NaOH solution pOH = -log10[OH–]
= -log10(0.1)
= 1.0
∴ pH = 14- pOH
= 14-1
= 13
2. In case of 0.005(M) Ca(OH)2 solution, [OH-] = 2 × 0.005 = 0.01(M)
Since Each Ca(OH)2 molecule ionizes to give 2 OH– ions]
∴ In case of 0.005(M) Ca(OH)2 solution,
pOH = -log10[OH–]
= -log10(0.01) = 2
∴ pH = 14- pOH
= 14- 2
= 12
Question 3. Calculate the concentrations of HaO+ and OH– ions in the solutions with the following pH values at 25 °C. 0
- pH = 5.0
- pH = 12
Answer:
1. In the case of a solution with pH = 5,
[H3O+] = 10-PH(M) = 10-5(M)
∴ In this solution,
⇒ \(\left[\mathrm{OH}^{-}\right]=\frac{10^{-14}}{\left[\mathrm{H}_3 \mathrm{O}^{+}\right]}=\frac{10^{-14}}{10^{-5}}(\mathrm{M})=10^{-9}(\mathrm{M})\)
2. In case of a solution with pH = 12
[H3O+] = 10-pH (M)
= 10-12 (M)
∴ In the solution
⇒ \(\left[\mathrm{OH}^{-}\right]=\frac{10^{-14}}{\left[\mathrm{H}_3 \mathrm{O}^{+}\right]}\)
= \(\frac{10^{-14}}{10^{-12}}(\mathrm{M})=10^{-2}(\mathrm{M})\)
Question 4. Calculate the amount of Ca(OH)2 required to be dissolved to prepare 250mL aqueous solution of pH = 12.
Answer:
As given in the question, the pH of the solution =12.
∴ pOH = 14- 12 = 2
∴ In the solution, [OH–] = 10-pOH
= 10-2(M)
Common Ion Effect On The Ionisation Of Weak Acids And Weak Bases
In a solution of two electrolytes, the ion which is common to both electrolytes, is called the common ion.
Example:
Acetic acid on its partial ionization forms CH3COO–(aq) and H3O+(aq), and sodium acetate on its complete ionization forms CH3COO–(aq) and Na+(aq). As the CH3COO– (aq) ion is common to both CH3COOH and CH3COONa, it is a common ion in this system.
Common Ion:
Effect When a strong electrolyte is added to a solution of a weak electrolyte having an ion common with a strong electrolyte, the extent of ionization of the weak electrolyte decreases. This phenomenon is called the common ion effect.
Common ion effect on ionization of a weak acid:
Effect of common anion:
Acetic acid is a weak acid. It partially ionizes in its aqueous solution, forming the following equilibrium
CH3COOH(aq) + H2O(l) ⇌ H3O+(aq) + CH3COO–(aq)
If CH3COONa is added to this solution, then CH3COONa, being a strong electrolyte, ionizes almost completely into Na+ and CH3COO– ions (common ion) in the solution. Consequently, the equilibrium involved in the ionization of acetic acid gets disturbed.
So, according to Le Chatelier’s principle, some of the CH3COO– ions combine with an equal number of H3O+ ions to form the unionized CH3COOH and H2O molecules, thereby causing the equilibrium to shift to the left.
As a result, the degree of ionization of CH3COOH and the concentration of H3O+ ions in the solution decreases. This leads to increased pH of the solution.

Effect of common cation:
When a strong acid such as HCl is added to a solution of acetic acid, it almost completely ionizes into H3O+ and Cl– ions. The complete dissociation of HCl increases the concentration of H3O+ (ag) ions (common ion) in the solution. As a result, the equilibrium formed by the ionization of CH6COOH gets disturbed.
According to Le principle, some of the H3O+ ions combine with an equal number of CH6COO- ions to form unionized CH6COOH and H2O molecules. As a result, the equilibrium shifts to the left, causing a decrease in the degree of ionization of CH3COOH.

Common ion effect on ionization of a weak base:
Effect of common cation: Ammonia (NH3) is a weak base. In aqueous solution, NH3 reacts with water to establish the following equilibrium:
NH3(aq) + H2O(l) ⇌ NH+(aq) + OH– (aq)
If a strong electrolyte such as, NH4Cl is added to this solution, it almost completely ionizes to form NH4+(aq) and Cl– (aq). The complete ionization of NH4Cl gives rise to a high concentration of NH3(aq) ions (common ions) in the solution. As a result, the equilibrium involved in the ionization of NH3(aq) gets disturbed.
According to Le Chatelier’s principle, some NH3(aq) ions combine with an equal number of OH– ions to form unionized NH3 and H2O molecules and thereby cause the equilibrium to shift to the left. Consequently, the degree of ionization of NH3 as well as the concentration of OH– ions in the solution decreases. This results in a decrease in the pH of the solution.

Effect of common anion:
When NaOH (a strong base) is added to an aqueous solution of NH3, it almost completely ionizes into Na+ and OH– ions. This increases the concentration of OH– ions (common ions) in the solution.
As a result, the equilibrium involved in the ionization of NH3 gets disturbed. According to Le Chatelier’s principle, some OH– ions combine with an equal number of NH– ions to form unionized NH3 and H2O molecules, thereby causing the equilibrium to shift to the left. This results in a decrease in the degree of ionization of NH3.

Hydrolysis Of Salts
A normal salt (Example; NaCl, KCl, Na2SO4 CH3COONa, NH4Cl ) does not contain any ionizable H-atom or OH- ion. The solutions of normal salts are therefore expected to be neutral as they are formed by the complete neutralization of an acid. and a base. However, the aqueous solutions of many normal salts are found to be acidic or basic.
Example:
Aqueous solutions of NH4Cl, FeCl3, AlCl3, etc., acidic, and those of CH6COONa, NaF, and KCN are basic. This is because the cations or the anions produced by the dissociation of these salts react partially with water to produce an H3O+ or OH– ions solution. This increases the concentration of H3O+ or OH– ions in the solution. As a result, the solutions become acidic or basic. Such a phenomenon is known as hydrolysis.
Hydrolysis Definition
The process in which the cations or the anions or both of a normal salt in its aqueous solution react with water to furnish H3O+ or OH– ions, thus making the solution acidic or alkaline is known as hydrolysis of the salt.
Types of normal salts that undergo hydrolysis
A salt forms due to the reaction of an acid with a base. An acid or a base may be strong or weak. Four different normal salts are possible depending upon the nature of acids and bases involved in their formations.
Among these salts, those produced by reactions of strong acids and strong bases do not undergo hydrolysis. So, if the acid and the base that react to form a salt are weak or one of them is weak, then the salt formed will undergo hydrolysis.
Consequently, aqueous solutions of these salts are either acidic (pH < 7) or basic (pH > 7).On the other hand, an aqueous solution of a salt strong acid, and strong base is always neutral (pH = 7).

Hydrolysis of salts obtained from strong acids and strong bases
A salt of this type does not undergo hydrolysis in its aqueous solution because neither its cation nor its anion reacts with water.
As a result, the concentration of H+ ions or OH– ions in the solution is not affected; that is, the equality of their concentrations is not disturbed. This is why an aqueous solution of a salt derived from a strong acid and a strong base is neutral (pH = 7).
Concept of pH and pH Scale Chapter 11 NCERT Notes
Explanation:
NaCl is a salt of strong acid (HCl) and strong base (NaOH).
1. NaCl dissociates almost completely In aqueous solution to produce Na+ (aq) and Cl (aq) ions
[NaCl(aq) → Na+(aq) + Cl–(aq)].
Water also ionizes slightly to produce an equal number of
H3O+ (aq) and OH–(aq) ions
[2H2O(l) ⇌ H3O+(aq) + OH–(aq)].
2. Na+ (aq) is a very weak Bronsted acid and it is unable to react with H2O to produce a proton:

3. On the other hand, Cl– ion is the conjugate base of strong acid HCl. Hence, it is a very weak Bronsted base. For this reason, Cl– is unable to react with water to produce a proton:
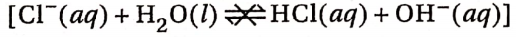
4. Thus, an aqueous solution of NaCl contains H3O+ and OH– ions in equal concentration. As a result, the aqueous solution of NaCl is neutral. For the same reason, other salts obtained from strong acids and strong bases form neutral aqueous solutions.
Hydrolysis of salts obtained from weak acids and strong bases
A salt of this type undergoes hydrolysis in its aqueous solution as its anion reacts with water to form OH– ions. As a result, the concentration of OH– in the solution becomes higher than that of H3O+, thereby making the solution basic (pH > 7). Since anion of such a salt takes part in hydrolysis, this type of hydrolysis is called anionic hydrolysis.
Explanation: KCN is a salt-weak acid (HCN) and strong base (KOH)
1. It dissociates almost completely in its aqueous solution and produces K+(aq) and CN–(aq) ions
[KCN(aq)⇌ K+(ag) + CN–(ag)].
Water also ionizes slightly to produce an equal number of H3O+(aq) and OH–(aq)
2H2O(l) ⇌ H3O+(aq) + OH–(aq)
2. K+(aq) is a very weak Bronsted acid. So, it cannot react with to produce a proton:

3. On the other hand, CN– ion is the conjugate base of a weak acid (HCN). Hence, it has sufficient basic character to abstract a proton from an H2O molecule to form OH–ions:

4. The formation of OH– ions increases the concentration of OH– ions in the solution and makes the solution basic (pH > 7). For the same reason, other salts obtained from weak acids and strong bases form basic aqueous solutions.
Hydrolysis of salts obtained from strong acids and weak bases
A salt of this type undergoes hydrolysis in its aqueous solution because its cation reacts with water to form H3O+ ions. As a result, the concentration of H3O+ ions in the solution becomes higher than that of OH– ions. This makes the solution acidic (pH < 7). Since the cation of such a salt takes part in hydrolysis, this type of hydrolysis is called cationic hydrolysis.
Hydrolysis of salts obtained from strong acids and weak bases Explanation:
Ammonium chloride (NH4Cl) is formed by the reaction of HCl (a strong acid) with NH3 (a weak base).
1. NH4Cl almost completely dissociates in its aqueous solution to produce,
NH4 +(aq) and Cl–(aq) ions
[NH4Cl(aq)→NH4+(aq) + Cl– (aq)]. H2O also ionislightly to produce equal number of
2H2O(l) ⇌ H3O+(aq) + OH–(aq)
2. Cl– ion is the conjugate. the base of strong acid :
So, Cl– ions fail and hence, is a very weak Bronsted base. So, Cl- ion fails to react with H2O to produce OH– ions
Class 11 Chemistry pH and pH Scale Summary

3. NH4+ ion is the conjugate acid of a weak base, NH3, and has sufficient acidic character to donate a proton to H2O molecules. Thus, NH4+ ion reacts with water to form unionised molecules of NH3 and H3O+ ions
NH4+(aq) + H2O(l) ⇌ NH3(aq) + H3O+(aq)]
4. The formation of HaO+ ions increases the concentration of H3O+ ions in the solution and makes the solution acidic (pH < 7). For the same reason, other salts obtained from strong acids & weak bases form acidic aqueous solutions.
Aqueous solution of FeCI3 [or Fe(NO2)g] is acidic:
Being a strong electrolyte, FeCl3 undergoes complete dissociation in the solution to form
[Fe(H2O)g]3+(aq) & Cl–(aq):
FeCl3(aq) + 6H2O(l) → [Fe(H2O)6]3+(aq) + 3Cl–
Water also ionises slightly to produce equal number of H3O+ ions and OH–(aq) ions
2H2O(l) ⇌ H3O+(aq) + OH–(aq)] . Cl– ion is the conjugate base of strong acid (HCl).
Hence, it is a very weak Bronsted base and fails { to react with water in aqueous solution. Due to the small size and higher charge of Fe3+ ion, its charge density is very high. As a result, the H2O molecules bonded to the Fe3+ ion are polarised and their O—H bonds become very weak.
These O—H bonds are easily dissociated to produce H+ ions, which are accepted by H2O molecules to form H3O+ ions.
Fe(H2O)6]3+(aq) +H2O⇌ Fe(H2O)5OH]2+(aq) +H3O+(aq)
Among the H2O molecules attached to the Fe3+ ion,
The one which releases proton finally gets attached to the Fe3+ ion as OH- ion. For the same reason, aqueous solutions of AICl3, CuSO3, etc., are acidic.
AlCl3(aq) + 6H2O(l) → [Al(H2O)6]3+(aq) + 3Cl–(aq)
[Al(H2O)6]3+(aq) + H2O(l) ⇌ [Al(H2O)5 OH]2++H3O+(aq)
Hydrolysis of salts obtained from weak acids and weak bases
In the case of a salt of this type, both the cation and anion react with water. In reaction with water, the cation forms H3O+ ions, and the anion forms OH– ions. Aqueous solutions of such a salPipay be acidic, basic, or neutral, depending upon the relative acid strength of the cation and base strength of anion. If the strengths of the cation and anions are the same, the solution will be neutral.
On the other hand, if the strength of the cation is greater or less than that of the anion, then the solution will be acidic or basic.
When the aqueous solution of the salt is neutral:
CH3COONH4 is a salt of weak acid, CH3COOH, and a weak base, NH3.
Explanation:
1. Being a strong electrolyte, CH3COONH4 dissociates almost completely in aqueous solution to produce NH4 and CH3COO–(aq) ions:
CH3COONH4O(aq) → CH3COO– (aq) + NH4– (aq).H2O weak electrolyte, also ionizes slightly in solution to produce an equal number of H3O+(aq) and OH– (aq) ions
[2H2O(l) ⇌ H3O+(aq) + OH– (aq)].
2. NH4+ion is the conjugate acid of a weak base (NH3) and CH3COO– is the conjugate base of a weak acid, (CH3COOH). In an aqueous solution, both NH4 and CH3COO– are stronger than H2O (which can act both as a weak Bronsted acid and base). As a result, CH3COO– and NH3 ions react with water to establish the following equilibria:
NH+(aq) + H2O(l) ⇌ NH3(aq) + H3O+(aq)
CH3COO– (aq) + H2O(l) ⇌ CH3COOH(aq) + OH–(aq)
3. At ordinary temperature, both CH3COO– and NH3 ions have the same value of dissociation constants. As a result, they get hydrolyzed to the same extent in aqueous solution.
Therefore, the aqueous solution of CH3COONH4 contains equal concentrations of H3O+ (produced by the hydrolysis of NH4 ions) and OH– (produced by the hydrolysis of CH3COO– ions), and hence, the solution is neutral.
When the aqueous solution of the salt is acidic:
Ammonium formate (HCOONH4 strong electrolyte) is a salt of weak acid, HCOOH, and weakbase, NH3.
Explanation:
1. HCOONH4 dissociates almost completely in its aqueous solution to form HCOO and NH
[HCOONH4(aq) H → HCOO–(aq) + NH4+ (aq)]
H2O, a weak electrolyte, also ionizes slightly to form an equal number of H3O+{aq) and OH-(aq) ions. NH4 and HCOO– react with water to form the given equilibria:
NH4+(aq) + H2O(l) ⇌ NH3(aq) + H3O+ (aq)
HCOO– (aq) +H2O(l) ⇌ HCOOH(aq) + OH–(aq)
2. At ordinary temperature, Ka for NH4+ is larger than Kb for HCOO– i.e., in aqueous solution NH– hydrolyses to a greater extent than HCOO–. The concentration of H3O+ is more than that of OH–. So, the aqueous solution of HCOONH4 is acidic.
When the aqueous solution of the salt is basic:
Ammonium bicarbonate, NH4HCO3 is a salt of a weak acid, HCO3, and weak base, NH3.
Explanation:
1. Strong electrolyte, NH4HCO3 dissociates almost completely solution to form NH+ and HCO3 ions:,
⇒ \(\left[\mathrm{NH}_4 \mathrm{HCO}_3(a q) \rightarrow \mathrm{NH}_4^{+}(a q)+\mathrm{HCO}_3^{-}(a q)\right] \cdot \mathrm{H}_2 \mathrm{O}\)
A weak electrolyte, also Ionises slightly to form an equal number of H3O+ (aq) and OH– ) ions
[2H2O(l)x ⇌ H3O++ OH(aq) ]. NH3 and HCO3–ions react with water to establish die following equilibria.
⇒ \(\\mathrm{NH}_4^{+}(a q)+\mathrm{H}_2 \mathrm{O}(l)\rightleftharpoons \mathrm{NH}_3(a q)+\mathrm{H}_3 \mathrm{O}^{+}(a q)\)
⇒ \(\mathrm{HCO}_3^{-}(a q)+\mathrm{H}_2 \mathrm{O}(l)\rightleftharpoons \mathrm{H}_2 \mathrm{CO}_3(a q)+\mathrm{OH}^{-}(a q)\)
2. At ordinary temperature, Kb for HCO3– Is much greater than Kafor NH4+ This means that in an aqueous solution, HCO3– ions hydrolyze to a greater extent than NH4+ ions. So, the concentration of OH– ions is more than that of H3O+ ions in an aqueous solution of NH4HCO3. Thus, the aqueous solution of NH4HCO3, Is music in nature.
Hydrolysis constant, degree of hydrolysis, and pH of an aqueous solution of a salt
Hydrolysis constant:
In the hydrolysis of a salt, u dynamic equilibrium is established Involving the unhydrolyzed salt and the species formed by hydrolysis. The equilibrium constant corresponding to this equilibrium Is called the hydrolysis constant.

Degree of hydrolysis:
The degree of hydrolysis (ft) of a salt may be defined as the fraction of the total number of moles of that salt hydrolyzed in its aqueous solution at equilibrium. In an aqueous solution, the degree of hydrolysis of salt is ft— it means that out of 1 mol of the salt dissolved in the solution, ft mol undergoes hydrolysis.
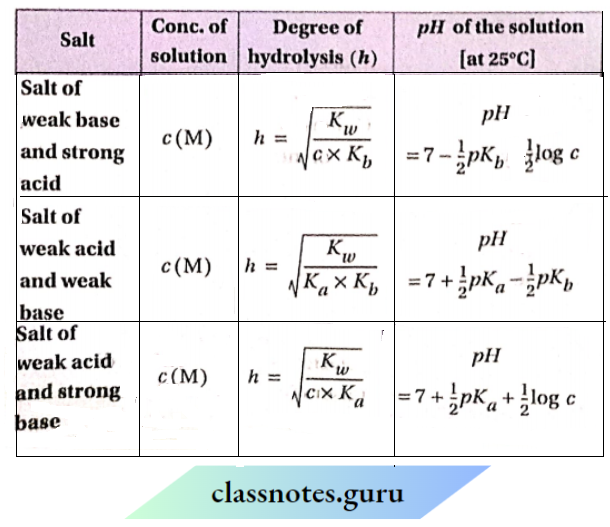
Relation Between Ionisation Constants Of Conjugate Acid-Base
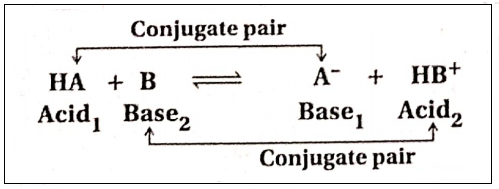
Let’s consider a weak acid, HA. The conjugate base of this acid is A–. Hence, the conjugate acid-base pair is (HA, A–).
In an aqueous solution, HA and A– form the following equilibrium:
⇒ \(\mathrm{HA}(a q)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{A}^{-}(a q)\)
The ionisation constant of HA,
Ka = \(\frac{\left[\mathrm{H}_3 \mathrm{O}^{+}\right] \times\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]}\) ………………………….(1)
pH and pH Scale in Acids and Bases Class 11 Chemistry Notes
In aqueous solution, the base A- reacts with water to form the following equilibrium:
A-(aq) + H2O(l)⇌ HA(aq) + OH-(aq)
The ionisation constant of
⇒ \(\mathrm{A}^{-}, K_b=\frac{[\mathrm{HA}] \times\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{A}^{-}\right]}\) ………………………….(2)
Multiplying equations (1) and (2), we have,
Ka × Kb= [H3O+]’× [OH–]
Again, Kw = [H3O+] × [OH–]
∴ Ka × Kb = Kw
Equation (3) represents the relation between ionization constants of a conjugate acid-base. This equation applies to any conjugate acid-base pair in aqueous solutions
at 25°C,Kw = 10-14
Therefore, at this temperature, Ka × Kb = 10-14
Therefore, at a given temperature, we know the value of Kw and the ionization constant of any one member of the conjugate acid-base pair, then the ionization constant of the other can be determined by applying equation (4)
Examples:
1. At 25°C, Ka (CH3COOH) = 1.8 × 10-5
So, at 25°C, Kb (CH3COO– ) \(=\frac{10^{-14}}{K_a}\)
=\(\frac{10^{-14}}{1.8 \times 10^{-5}}\)
2. At 25°C, Kb (NH3) = 1.8 ×10-5
⇒ \(\text { So, at } 25^{\circ} \mathrm{C}, K_a\left(\mathrm{NH}_4^{+}\right)=\frac{10^{-14}}{K_a}\)
=\(\frac{10^{-14}}{1.8 \times 10^{-5}}=5.5 \times 10^{-10}\)
pH Scale and Its Calculation Class 11 Chemistry Notes
Derivation of the relation, pKa + pKb = pK w:
We know that, Ka × Kb = Kw. Taking negative logarithms on both sides
we have, -log10 (Ka × Kb) = -log10 Kw
or, -log10 Ka– log10 Kb = -log10 Kw
∴ PKa+PKb = PKw
At 25°C, pKw = 14.
So, at 25°C, pKa + pKb = 14
Hence, if the pKa of the acid (or pKb of the base) in a conjugate acid-base pair is known, then the pKb of the base (or pKa of the acid) can be determined by using equation (5)