Drugs Action On Central Nervous System Introduction
The drugs fall under this category having common effects as depression of neuronal activity in the Central Nervous System (CNS), i.e. the brain and spinal cord. In most of the cases the entire brain is intended as target organ, whereas in a few cases skeletal muscle or the spinal cord is targeted. Various classes of the drugs falling under CNS depressant are anxiolytics, sedatives and hypnotics, general anaesthetics, anticonvulsants and antipsychotics.
The Anxiolytic drugs are used is in the treatment of the anxiety disorders, which are conditions characterized by excessive or inappropriate anxiety. The major use of sedative is to calm the patient, whereas hypnotic drugs are used in the treatment of insomnias which are failures to get adequate sleep. The clinical significance of general anaesthetic agents is to produce unconsciousness and loss of perception to pain during surgical procedures.
Anticonvulsant drugs are mainly used to prevent or lessen the sudden excessive electrical activity in brain neurons that is a characteristic of the convulsions or epilepsies. Antipsychotics are used in the disorders like psychoses, most notably the schizophrenias.
Sedatives And Hypnotics Introduction
Hypnotics are used to induce sleep resembling the natural one. They are central nervous system depressants; and produce drowsiness and sound sleep. They find importance in the management of insomnia (sleeplessness) and also as a preanesthetic medication. They find similarity with sedatives.
A sedative drugs induce sedation by reducing irritability or excitement and are central nervous system depressants. Sedative drugs serve to calm or relieve anxiety, restlessness and emotional tension. Whilst, hypnotic drugs are to initiate and/or to sustain a quality sleep in the management of insomnia.
These drugs produce dose-dependent effects highlighting their anxiolytic effect and to that to produce sound sleep. Hence, they are collectively known as sedative-hypnotic drugs. Sedative-hypnotic drugs may produce dependence, and that optimal treatment is to use the lowest effective dose for the shortest therapeutic time period necessary, with gradual discontinuation. They are habit-forming and chronic use is known to disturb the human sleep pattern.
Insomnia is a sleep disorder highlighted with trouble sleeping or sleeplessness which may either no quality or quantity of sound sleep. Insomnia may be indicated with difficulty in falling asleep or staying asleep for the definite duration. Insomnia is followed by daytime sleepiness with low energy, irritability, and also a depressed mood.
Read and Learn More Medicinal Chemistry III Notes
Insomnia can be transient, short term or long term, lasting for days or weeks, or lasting for more than a month. Insomnia can occur independently or as a result of another problem like psychological stress, chronic pain, heart failure, hyperthyroidism, heartburn, restless leg syndrome, menopause and even dependence for caffeine, nicotine, and alcohol.
In addition to benzodiazepines, barbiturates, and a miscellaneous group, many drugs belonging to other pharmacological classes may possess one or more of the anxiolytic, sedative and hypnotic activities.
Mechanism Of Action Of Sedatives And Hypnotics
Gama Amino Butyric Acid (GABA) Activity Enhancers / GABA Receptor Modulators:
The GABAA receptor is one of the two ligand-gated ion channels responsible for mediating the effects of GABA, the major inhibitory neurotransmitter in the brain. The GABAergic stimulation in the brain via the activation of the GABAA also known as benzodiazepine receptors or referred as chloride channel complex, allows increased chloride conductance, thereby resulting in CNS depressant effect. The agonist drugs like barbiturates, benzodiazepines; bind to an allosteric site on the postsynaptic GABAA receptor complex that increase chloride conductance.
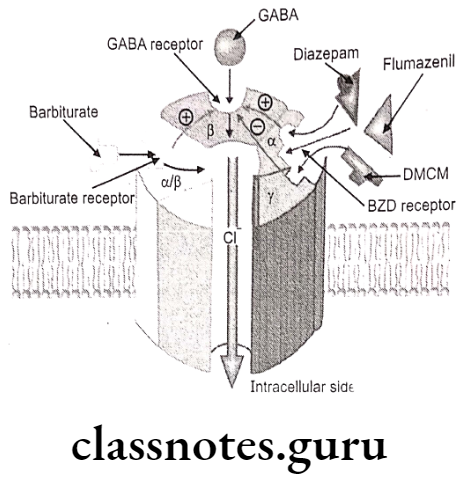
Classification Of Hypnotics And Sedatives
An arbitrary classification of hypnotics and sedatives is as follows:
- Benzodiazepine derivatives eg. Chlordiazepoxide, Diazepam, Oxazepam, Chlorazepate, Lorazepam, Alprazolam, Zolpidem.
- Barbiturate derivatives e.g. Barbital, Phenobarbital, Mephobarbital, Amobarbital, Butabarbital, Pentobarbital, Secobarbital.
- Long acting (Duration of action more than 6 hours) e.g. Barbital, Phenobarbital, Mephobarbital.
- Intermediate acting (Duration of action of 3-6 hours) e.g. Amobarbital, Butabarbital.
- Short acting (Duration of action of less than 3 hours) e.g. Pentobarbital, Secobarbital.
- Miscellaneous:
- Amide and Imide derivatives e.g. Glutethmide.
- Alcohol and their carbamate derivatives e.g. Meprobomate, Ethchlorvynol.
- Aldehyde and their derivatives e.g. Triclofos sodium, Paraldehyde.
Benzodiazepines
The benzodiazepines are lipophilic in nature and are in non-ionized form and thus well absorbed from the GI tract. The benzodiazepine derivatives with 3-hydroxyl function are polar compounds and are absorbed slowly in comparison to the other lipophilic derivatives.
Due to higher lipophilic nature, these drugs are bound to plasma proteins; but do not compete with other protein bound drugs. The benzodiazepine derivatives are effectively distributed because of its more lipophilic nature.
Structure-Activity Relationship Of Benzodiazepines:
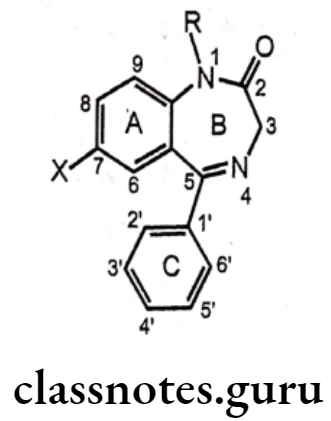
Benzodiazepine derivatives are chemically 5-aryl-1,4-benzodiazepine with a carboxamide function in the seven-member diazepine ring moiety. The SAR study has been classified into three fragments of the 5-aryl-1,4-benzodiazepine nucleus.
Ring A-Aryl or Heteroaryl Moiety
- The primary fragment of the aryl or heteroaryl moiety as the ring A, the aromatic or heteroaromatic ring A is prerequisite for the activity. The moiety exhibits л-л stacking with the aromatic amino acid residues of the GABAA receptor.
- Heteroaromatic moiety derivatives are more potent than aromatic moiety derivatives. An electronegative substituent like -Cl, -NO2 or -CF3 function with electron withdrawing ability at position 7 is required for activity, and better electro-negativity directly correlates with higher activity..
- Any substitution at positions 6, 8, and 9 leads to loss of activity.
Ring B-1,4-benzodiazepine Moiety
- The 1,4-benzodiazepine moiety represents the ring B; the second fragment of the SAR study.
- The saturation of the double bond at positions 4 and 5 (N=C) or shifting of the same to the positions 3 and 4 results in decrease in the activity.
- Any alkyl substitution irrespective of the higher or lower function at the position 3 leads to decrease in the activity.
- Substitution with a hydroxyl (-OH) function at position 3 does not affect the activity of the molecule, but the polar function is readily converted to the glucuronide conjugate and is excreted in urine and thus is short-lived, resulting in decreased duration of action. It highlighted the importance of derivatives with short activity and helpful to induce sleep.
- The absence of the hydroxyl group at position 3 (no substitution) indicates compounds with better duration of action. These derivatives are pharmacokinetically important, derivatives lacking a hydroxyl group at position 3 are non-polar in nature, and are metabolized to the pharmacologically active 3-hydroxylated metabolite in the hepatic CYP450 system slowly. The active 3-hydroxyl metabolites have longer half- lives resulting in increased duration of action.
- The nitrogen at position 1 and the carbonyl (C=O) function at position 2 are important for activity.
- The N-alkyl side chains are tolerated, usually a lower alkyl function.
- A proton-accepting group, i.e. carbonyl (C=O) function at position 2 is required and interacts with histidine residue at the receptor core (a proton donor) in benzodiazepine binding site of GABAA receptor.
- A triazole or imidazole rings are also equally effective, since they are also capable accepting a proton from the histidine residue at the receptor core and exhibiting the H-bonding. The triazole or imidazole moiety can be fused on positions 1 and 2, and eventually lead to the increase in the activity. Derivatives like triazolam and alprazolam highlight drugs with a fused triazolo ring.
- While, Midazolam is a derivative indicated with a fused imidazole ring. Interestingly, an electron-attracting group at position 7 is not required for activity in few of these derivatives.
- These triazole or imidazole rings derivatives are short acting as they are rapidly metabolized by a-hydroxylation of the methyl substituent on the triazolo or imidazolo moiety similar to benzylic oxidation via CYP450 mixed oxidase function. The resulting active a-hydroxylated metabolites are inactivated by glucuronidation. These derivatives are also metabolized by 3-hydroxylation of the benzodiazepine ring.
Ring C-Phenyl Substituent
- A phenyl ring C at position 5 promotes activity.
- Substitution on the phenyl group at ortho (2′) or diortho (2′,6′) positions with electron-withdrawing groups results in increased activity.
- Whilst, substitution on the para (4′) results in profound decrease in activity.
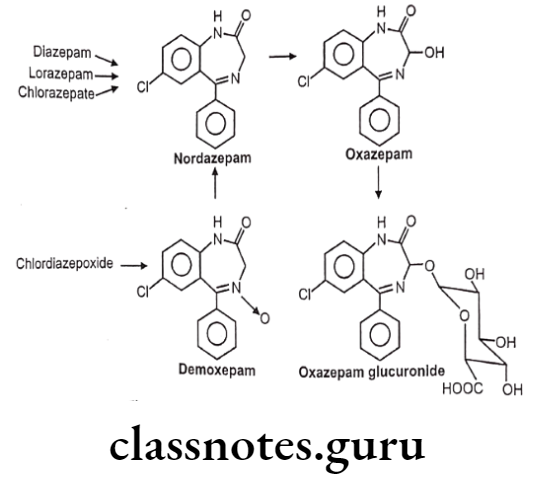
Metabolism of Benzodiazepines:
The benzodiazepines are extensively metabolized via CYP450 mixed function oxidase system, majorly CYP4503A4 and CYP2C19; some of the metabolites of selective benzodiazepines are active and few also have longer half-lives indicating their increased duration of action.
The CYP3A4 inhibitors like ketoconazole or food like grapefruit juice inhibits their metabolism and can affect their duration of action and equally toxicity. The benzodiazepine derivatives have lower abuse potential and a much greater safety margin than that of the barbiturates.
Chlordiazepoxide:
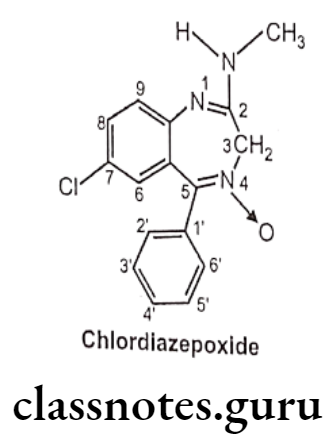
- Chlordiazepoxide is chemically, 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzo- diazepine-4-oxide.
- It is a sedative and hypnotic drug under the benzodiazepine class and the first benzodiazepine to be synthesized.
- It is a long-acting drug and even its metabolite is active and has a long half-life.
- It enhances GABAA receptor activity and results in an increased binding of the inhibitory neurotransmitter GABA to the GABAA receptor leading to inhibitory effects on the Central Nervous System.
Uses:
- Chlordiazepoxide is used in the management of epilepsy, anxiety, insomnia and alcohol or drug abuse withdrawal symptoms.
Diazepam:
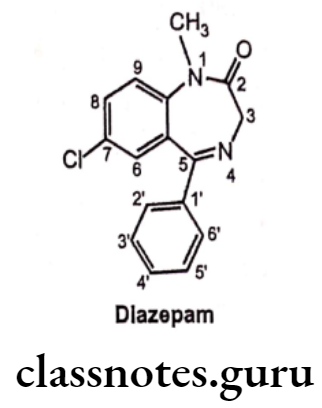
- Diazepam is chemically, 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzo- diazepin-2-one.
- It is a sedative and hypnotic drug under the benzodiazepine class and produces a calming effect.
- It acts by increasing the effect of the neurotransmitter GABA. It enhances GABAA receptor activity leading to Central Nervous System depression.
Uses:
- Diazepam is most frequently prescribed medications in the world under the class of benzodiazepines.
- It is used in the management of epilepsy, anxiety, insomnia, muscle spasms, seizures, trouble sleeping, restless legs syndrome and alcohol or drug abuse withdrawal symptoms.
Oxazepam:
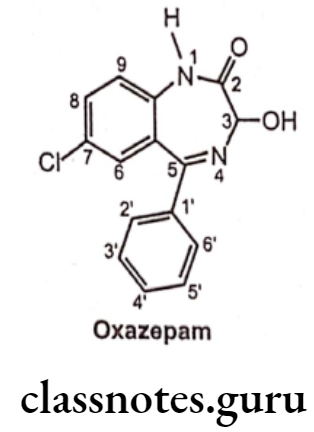
- Oxazepam is chemically, 7-Chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1, 4-benzo- diazepin-2-one.
- Oxazepam acts on benzodiazepine receptors, resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the Central Nervous System. Oxazepam exists as a racemic mixture, and has no therapeutic benefit to the administration of a single enantiomer over the racemic mixture.
- It is a short acting benzodiazepine with a slow onset of action. It is an example for an active metabolite formed during the metabolism of diazepam and other benzodiazepine drugs.
- It is a 3-hydroxy benzodiazepine derivative highlighting its slow absorption and short action, and safer than other benzodiazepines in patients with impaired liver function as it does not require hepatic oxidation, and is simply metabolized by glucuronidation. So oxazepam is less likely to accumulate and cause adverse reactions.
Uses:
- Oxazepam is used in the management of anxiety, insomnia and in the control of symptoms of alcohol withdrawal syndrome.
Clorazepate:
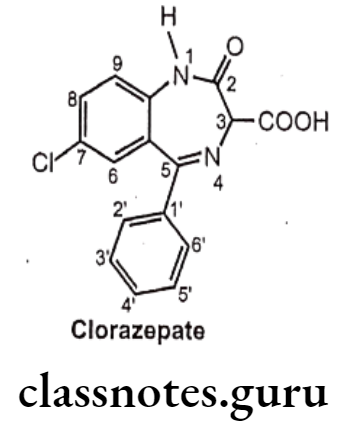
- Clorazepate is chemically, 7-chloro-2,3-dihydro-2-oxo-5-phenyl-1H-1,4-benzo- diazepine-3-carboxylic acid.
- It acts on benzodiazepine receptors, resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the Central Nervous System.
- Clorazepate is an example for prodrug, the parent drug undergoes decarboxylation in the acidic environment of the GIT to form nordiazepam; a non-polar and active metabolite with a half-life of more than forty hours.
- The metabolite desmethyldiazepam is responsible for its therapeutic effects. The desmethyldiazepam is further metabolized to oxazepam which also possesses activity.
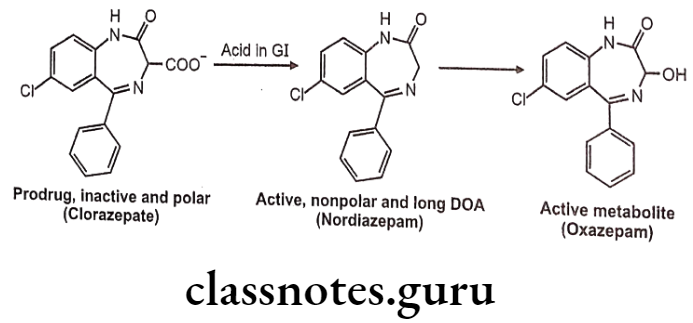
Uses:
- It is a long acting benzodiazepine medication, and finds importance as anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant.
Lorazepam:
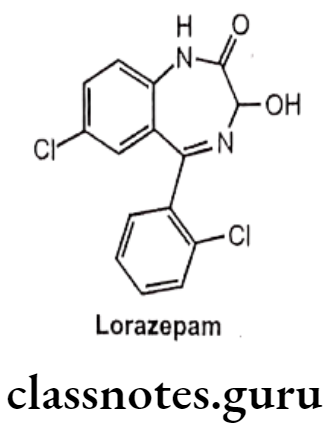
- Lorazepam is chemically, 7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2H- 1,4-benzodiazepin-2-one.
- It acts on benzodiazepine receptors, resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the Central Nervous System.
Uses:
- Lorazepam is a benzodiazepine medication, used to treat anxiety, insomnia, seizures, and chemotherapy-induced nausea and vomiting.
- It is also finds importance as preoperative medication.
Alprazolam:
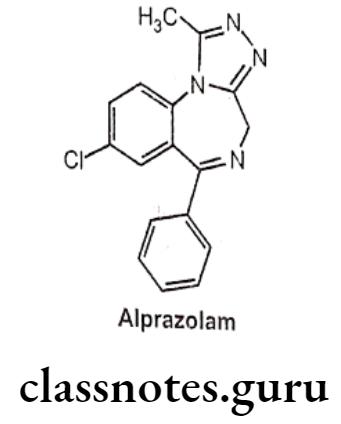
- Alprazolam is chemically, 8-chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4] benzodiazepine.
- It acts on benzodiazepine receptors, resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the Central Nervous System.
Uses:
- Alprazolam is a potent, short-acting benzodiazepine drug, and finds importance as an anxiolytic, sedative and hypnotic.
- It is commonly used for the treatment of generalized anxiety disorder or social anxiety disorder.
Zolpidem:
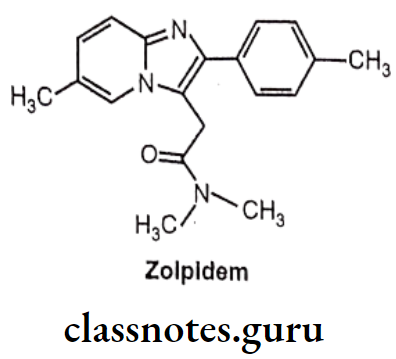
- Zolpidem is chemically, N,N,6-Trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3- acetamide.
- It acts on benzodiazepine receptors, resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the Central Nervous System.
Uses:
- Zolpidem is a non-benzodiazepine and hypnotic of the imidazopyridine class, a medication primarily used for the short term treatment of sleeping problems.
Barbiturates
The barbiturates were previously used extensively as sedative-hypnotic drugs. Now, they have been replaced by the safer benzodiazepine. Barbiturates act by binding to an allosteric recognition site on GABAA receptors that positively modulate the effect of the GABAA. They bind at different binding sites unlike benzodiazepines, and have increased duration of the GABA-gated chloride channel openings.
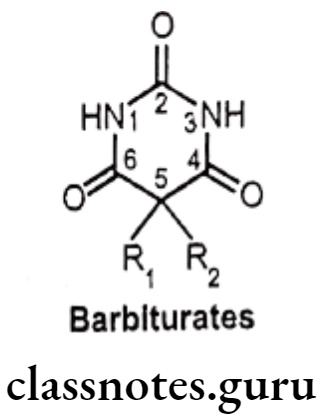
- The barbiturates are chemically 5,5-disubstituted-2,4,6-trioxohexahydropyrimidine or 5,5-disubstituted barbituric acids.
- The structure of barbituric acids indicates their acidic character.
- The derivatives without methyl substituents on the nitrogen exhibit a pKa’s of about
- 7.6; and with a methyl substituent they have pKa’s of about 8.4.
- The free acids have poor water solubility and good lipid solubility.
- The 2-thiobarbiturates also have greater lipophilicity, as the sulfur atom increases lipid solubility.
- Sodium salts of the barbiturates are readily prepared and are water soluble.
Structure-Activity Relationship Of Barbiturates:

- The barbituric acid molety Le. 24,6-trioxohexahydropyrimidine lacks any kind of depressant activity.
- The disubstitution at position 5 of barbituric acid, where both the hydrogens are needed to be replaced by an akyl/aryl group for CNS depression activity.
- The activity is marked due to the replacement of both the hydrogens at position 5, as even if one of the hydrogen is available at position 5, the molecule 2,4,6-trioxohexahydropyrimidine or 5-substituted-24,6-trioxohexahydropyrimidine exhibits tautomerization resulting in a highly acidic trihydroxypyrimidine close to a pka value 4. Thus the compound is largely in the anionic form at physiological pH, with little nonionic lipid-soluble nature available to cross the blood-brain barrier.
- The substitution with alkyls function of both the hydrogens at position 5 of the molecule confers activity with a rapid onset, but a decrease in duration of action.
- This is attributed to the increasing lipophilicity, as an increase in lipophilicity leads to quick or rapid onset of action, and this may be attributed to an ability to penetrate the BBB. An increase in lipophilicity also enhances the hypnotic potency. Redistribution of the molecules confers to decreased concentration of the molecule in CNS and partitioning out of the brain to other sites results in decreased duration of action.
- The short duration of action may also be attributed to its ability to penetrate liver microsomes and biotransformed inactive metabolite.
- The substitution at position 5 with an alkyl group can be of increasing carbon skeleton up to about 5 to 6 carbon moiety for optimal activity. An increase beyond 7 to 9 carbon moiety leads to decrease in depression activity and precipitation of convulsion.
- Substitution of branched cyclic or unsaturated carbon moiety leads to derivatives with short duration of action. These derivatives are oxidative metabolized to form hydroxyl function polar metabolite and eliminated rapidly via conjugation.
- Substitution of lower alkyl group like ethyl and a phenyl moiety at position 5 results in compounds with lower lipophilicity and leads to derivatives with slower onset of action, but relatively longer duration of action.
- There is an inverse correlation between the total number of carbon atoms substituted on the position 5 and the duration of action.
- This can be better understood based on the character of the substituent taken into account.
- The relatively polar character of a phenyl substituent results in long acting barbiturate.
- An alkyl substituent (3-4 carbon aliphatic chain), branching of alkyls, presence of an isolated double or triple bond substituted derivatives result in compounds with intermediate to short active.
- Substitution at position 1 on nitrogens with lower alkyl group results with increased lipophilicity of the molecule leading to increased activity.
- Di-substitution on both the nitrogen at position 1 and 3 results in inactive compounds, this is attributed to the pharmacokinetic parameter, due to the inability to from sodium salt of the barbituric acid and completely water insoluble derivative.
- Therefore following derivatives are only active:
- 5,5-disubstituted barbituric acid.
- 1,5,5-trisubstituted barbituric acid.
- 5,5-disubstituted thio-barbituric acid.
- While derivatives given below are inactive:
- Non-substituted barbituric acid.
- 5-monosubstituted barbituric acid.
- 1,3-disubstituted barbituric acid.
- 1,3,5,5-tetrasubstituted barbituric acid.
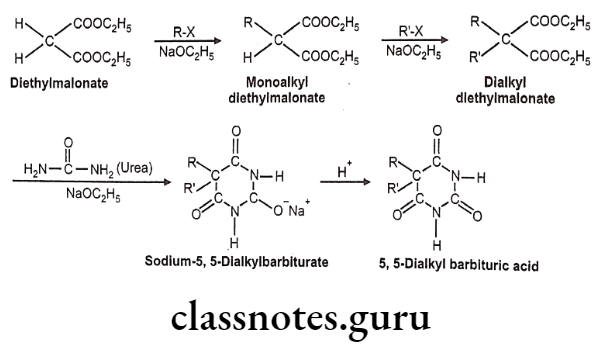
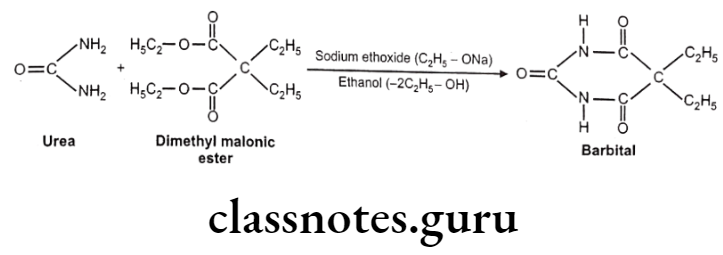
Synthesis of Barbiturates:
The barbiturates are 5,5-disubstituted barbituric acids. The following scheme shows how the 5.5-dialkyl compounds are synthesized.
Metabolism of Barbiturates:
The substituent at position 5 can influence the ease of oxidative metabolism by effects on bond strengths as well as by influencing partitioning. The absorption from the GI tract of the barbiturates is optimal and exhibits substantial plasma proteins binding. The metabolism pattern of the compounds with low lipophilicity is usually excreted intact in the urine, while highly lipophilic compounds are excreted after metabolism to polar metabolites.
With the increase in the lipophilicity, generally the rate of metabolism increases, except for compounds having extremely high lipophilicity like thiopental, which tend to depotize and are thus relatively unavailable for metabolism.
Metabolism generally follows an oxidative biotransformation. N-methylation decreases duration of action, by increasing the concentration of the lipid-soluble free barbituric acid. 2-thiobarbiturates have a very short duration of action because its lipophilicity is extremely high, promoting depotization.
Side-effects of Barbiturates:
The common side effects of barbiturates include headache, drowsiness, dizziness, ataxia, respiratory depression, hypersensitivity reactions, paradoxical excitement and confusion.
Long Acting Barbiturates (Duration of action > 6 hours):
Barbital:
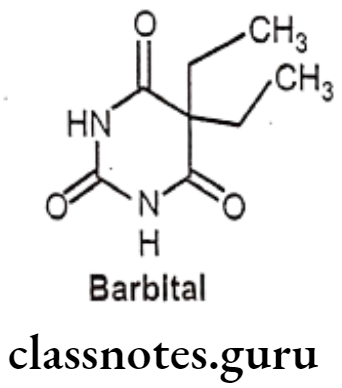
- Barbital is chemically, 5,5-diethyl-2,4,6(1H,3H,5H)-pyrimidinetrione.
- It is also known as barbitone which is chemically 5,5-diethylbarbituric acid, the first commercially available barbiturate.
- It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in inhibitory effects on the Central Nervous System.
Uses:
- Barbital is used as a hypnotic and antiepileptic..
Phenobarbital:
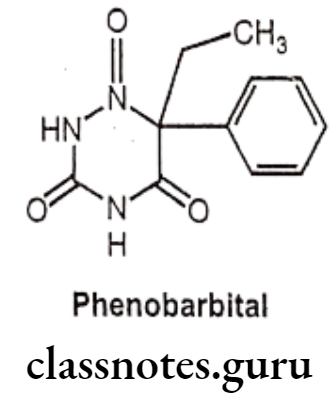
- Phenobarbital is chemically, 5-ethyl-5-phenylbarbituric acid.
- It is a long acting barbiturate derivative and also known as Phenobarbitone, which is the oldest, but still used as anti-epileptic medication.
- It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in increased influx of chloride ions leading to decreased excitability.
- It also produces blockade of excitatory glutamate signaling.
- Phenobarbital is a CYP450 inducer and hence dosing of other drugs is needed to be monitored.
Uses:
- Phenobarbital is used as a sedative, hypnotic and also as an anticonvulsant for both generalized tonic-clonic and partial seizures.
- It is occasionally used to treat drug withdrawal, as a pre anaesthetic medication, Crigler-Najjar syndrome and Gilbert syndrome patients to aid in the conjugation of bilirubin.
Mephobarbital:
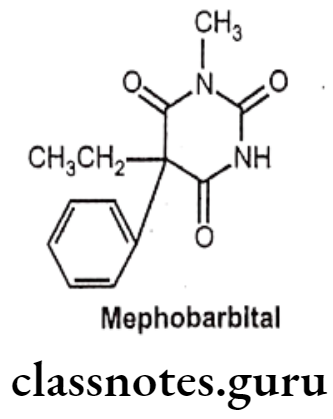
- Mephobarbital is chemically, 3-methyl-5-ethyl-5-phenylbarbituric acid.
- It is a long acting barbiturate derivative, as the parent drug, it undergoes metabolically N-demethylated to phenobarbital and responsible for its activity.
- It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in inhibitory effects on the Central Nervous System.
Uses:
- Mephobarbital is used as a hypnotic and as an anticonvulsant.
Intermediate Acting Barbiturates (Duration of action = 3-6 hours)
Amobarbital:
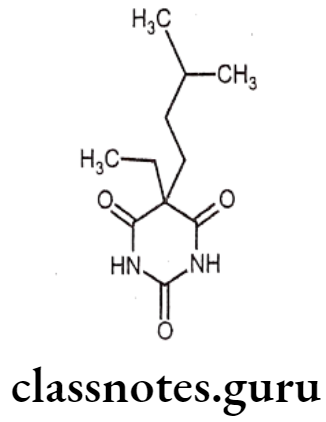
- Amobarbital is chemically, 5-ethyl-5-(3-methylbutyl)-2,4,6-(1H,3H,5H)-pyrimidine- trione.
- It is also known as amylobarbitone, which is an intermediate acting barbiturate with moderate duration of action of 4-5 hours.
- It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in increased influx of chloride ions leading to decreased excitability.
Uses:
- Amobarbital is used as sedative and hypnotic.
Butabarbital:
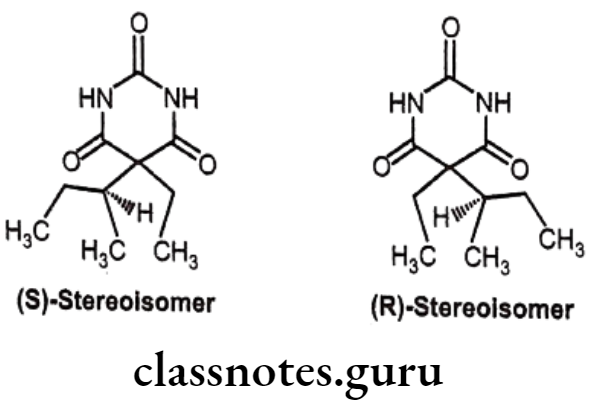
- Butabarbital is chemically, 5-ethyl-5-(1-methylpropyl)-2,4,6(1H,3H,SH)-pyrimidine- trione.
- It is an intermediate acting barbiturate with moderate duration of action of 3-6 hours. It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in increased influx of chloride ions leading to decreased excitability.
- It has a particularly fast onset of effects and short duration of action, has importance in management of severe insomnia and relieving anxiety before surgical procedures.
Uses:
- Butabarbital is used as sedative and hypnotic.
Short Acting Barbiturates (Duration of action < 3 hours)
Pentobarbital:

- Pentobarbital is chemically, 5-ethyl-5-(1-methylbutyl)-2,4,6(1H,3H,5H)-pyrimidine-trione.
- It is a short acting barbiturate with duration of action less than 3 hrs.
- It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in increased influx of chloride ions leading to decreased excitability.
Uses:
- Pentobarbital is used as sedative and hypnotic.
- It also finds importance as a preanesthetic medication and in control of convulsions in emergencies.
- It has an application in reducing intracranial pressure in Reye’s syndrome, traumatic brain injury and induction of coma in cerebral ischemia patients.
- It is also used as a veterinary anaesthetic agent.
Secobarbital:
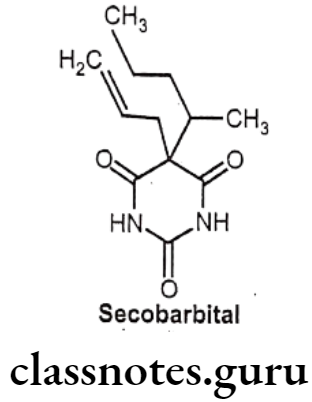
- Secobarbital is chemically, 5-(1-methylbutyl)-5-(2-propenyl)-2,4,6(1H,3H,5H)-pyrimidinetrione.
- It is a short acting barbiturate derivative.
- It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in increased influx of chloride ions leading to decreased’ excitability.
Uses:
- Secobarbital is used as an anaesthetic, anticonvulsant, anxiolytic, sedative, and hypnotic drug.
Miscellaneous Sedatives and Hypnotic Derivatives
A wide range of chemical structures (e.g. imides, amides, alcohols) can produce sedation and hypnosis resembling those produced by the barbiturates. Despite this apparent structural diversity, the compounds have generally similar structural characteristics and chemical properties.
Amide and Imide Derivatives:
Glutethimide:
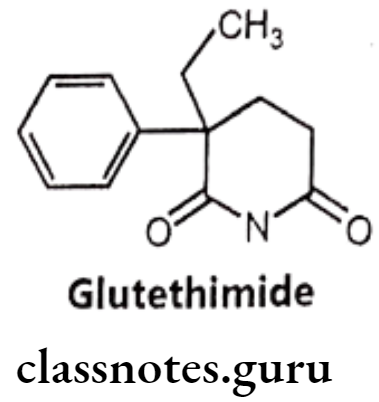
- Glutethimide is chemically, 2-ethyl-2-phenyl glutarimide.
- It is one of the most active non-barbiturate hypnotics that is structurally similar to the barbiturates. Glutethimide is highly lipophilic and undergoes extensive oxidative metabolism with a half-life period of approximately 10 hours.
Uses:
- It is used as a hypnotic and sedative drug.
Alcohols and their Carbamate Derivatives:
Meprobamate:
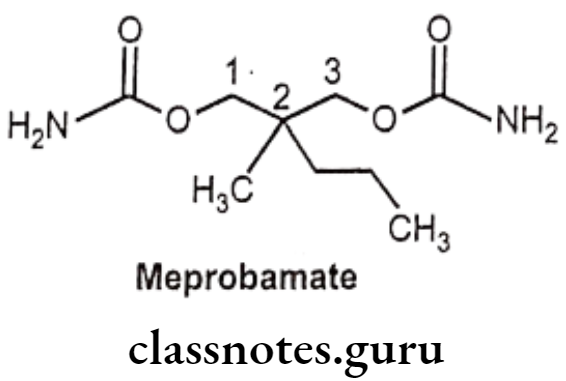
- Meprobamate is chemically, 2-methyl-2-propyl-1,3-propanediol dicarbamate.
- Meprobamate does not act through GABA system.
- It has inter-neuronal blocking properties at the level of the spinal cord, and responsible for its skeletal muscle relaxation.
Uses:
- Meprobamate is indicated as an antianxiety agent and also as a sedative and hypnotic agent.
- It is also effective against absence seizures and also a centrally acting skeletal muscle relaxant.
Ethchlorvynol:
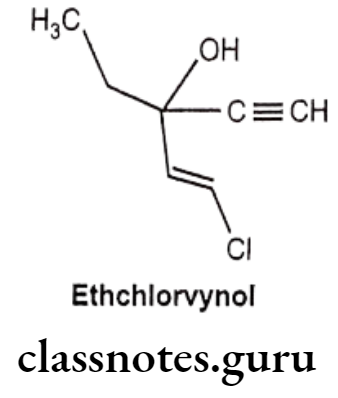
- Ethchlorvynol is chemically, 1-chloro-3-ethyl-1-penten-4-yn-3-ol.
- It is a mild sedative and hypnotic with a quick onset and short duration of action. It is highly lipophilic and extensively metabolized to its secondary alcohol.
- It is highly habit forming and extremely physically addictive.
- A mechanism of action is similar to the benzodiazepines and barbiturates.
- It increases the activity of the inhibitory neurotransmitter GABA to the GABAA receptor which results in increased influx of chloride ions leading to decreased excitability.
Uses:
- Ethchlorvynol is used as a mild sedative and hypnotic drug.
Aldehydes and Their Derivatives
Triclofos sodium:
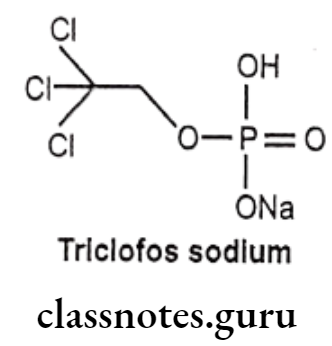
- Triclofos sodium is chemically, sodium-2,2,2-trichloroethyl hydrogen phosphate.
- It is a prodrug which is metabolized in the liver into the active drug trichloro ethanol.
- As trichloroethanol may cause liver damage, therefore triclofos should not be used for extended periods.
- The half-life of triclofos is long and it may cause drowsiness the next day.
Uses:
- Triclofos sodium is a sedative hypnotic drug used seldom for treating insomnia, as it caused dependence and only be used for the short term to relief of severe insomnia.
Paraldehyde:
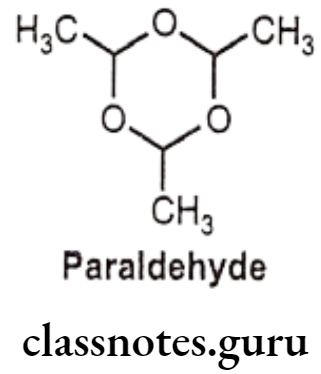
- Paraldehyde is chemically, 2,4,6-Trimethyl-1,3,5-trioxane.
- It, also known as paracetaldehyde, is the cyclic trimer of acetaldehyde, a liquid with a strong characteristic odour and an unpleasant taste. These properties limit its use as medicine.
Uses:
- Paraldehyde is a CNS depressant and an anticonvulsant, hypnotic and sedative.
- It was also included in some cough medicines as an expectorant.
- Paraldehyde is the oldest as it was introduced into clinical practice in 1882 and one of the safest hypnotics and was given to psychiatric and geriatric patients up to the 1960s.
- As an anti-seizure, it was used in the treatment of convulsions to treat status epilepticus.
- Industrially paraldehyde is used in resin manufacture and also as a preservative.
Synthesis
Diazepam
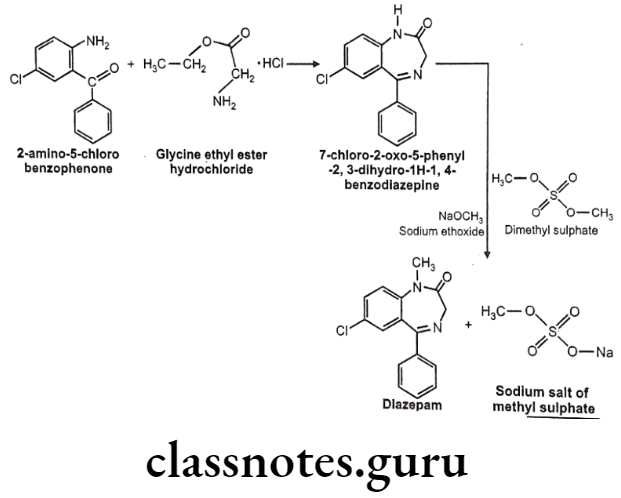
Barbital
Multiple Choice Questions:
Question 1. Hypnotic drugs are used to treat:
- Psychosis
- Sleep disorders
- Narcolepsy
- Parkinsonian disorders
Answer. 2. Sleep disorders
Question 2. 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepine-2-one is IUPAC name of
- Oxazepam
- Nitrazepam
- Diazepam
- Chlordiazepoxide
Answer. 3. Diazepam
Question 3. The IUPAC name of glutethimide is:
- 3-Ethyl-3-phenyl-2,6-piperdine dione
- p-Sulphonamido chloroimido benzoic acid
- 3-(5-Nitrofurfurylideneamino)-oxazolidin-2-one
- 2-(2-Fluorobiphenyl-4-yl)propionic acid
Answer. 1. 3-Ethyl-3-phenyl-2,6-piperdine dione
Question 4. Which of the following chemical agents are used in the treatment of insomnia?
- Benzodiazepines
- Imidazopyridines
- Barbiturates
- All of the above
Answer. 4. All of the above
Question 5. Oxazepam is psychoneurotic has lower side effect due to:
- Geometric hydroxylation
- Ring oxidation
- Conjugation of 3-OH group
- M-demethylation
Answer. 3. Conjugation of 3-OH group
Question 6. Select a hypnotic drug, which is a benzodiazepine derivative:
- Zolpidem
- Chlorazepate
- Secobarbital
- Phenobarbitone
Answer. 2. Chlorazepate
Question 7. In benzodiazepine ring, some positions cannot be substituted to retain the depressant activity:
- Position 6, 8 and 9
- Position 4, 6 and 8
- Position 3, 6 and 8
- Position 2, 3 and 8
Answer. 1. Position 6, 8 and 9
Question 8. Introduction of -OH group at position 3 of benzodiazepine causes:
- Increase in activity
- Loss of activity
- Lowering of activity
- None of above
Answer. 3. Lowering of activity
Question 9. Tick a hypnotic agent – a barbituric acid derivative:
- Flurazepam
- Zaleplon
- Thiopental
- Triazolam
Answer. 3. Thiopental
Question 10. Substitution at 8 and 9 position in a benzodiazepine ring causes:
- Loss in the activity
- Increase in the activity
- Decrease in the activity
- None of above
Answer. 3. Decrease in the activity
Question 11. IUPAC name of Diazepam is:
- 7-chloro-1-methyl-5-phenyl-1,4-benzodiazepine-2-one
- 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepine-2-one
- 7-chloro-1-methyl-5-phenyl-1,4-benzodiazepine
- 6-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepine-2-one
Answer. 2. 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepine-2-one
Question 12. Select a hypnotic drug, which is an imidazopyridine derivative:
- Pentobarbital
- Temazepam
- Zolpidem
- Chloral hydrate
Answer. 3. Zolpidem
Question 13. 2-amino-5-chlorobenzophenone is the convenient starting material for the synthesis of:
- Nitrazepam
- Diazepam
- Chloramphenicol
- Trimethoprim
Answer. 2. Diazepam
Question 14. Which of the following barbiturates is an ultra-short-acting drug?
- Secobarbital
- Amobarbital
- Thiopental
- Phenobarbital
Answer. 3. Thiopental
Question 15. Indicate the mechanism of barbiturate action (at hypnotic doses):
- Increasing the duration of the GABA-gated Cl- channel openings
- Directly activating the chloride channels
- Increasing the frequency of Cl-channel opening events
- All of the above
Answer. 1. Increasing the duration of the GABA-gated Cl- channel openings
Question 16. Alprazolam apart from 1,4-benzodiazepine ring also contains:
- Pyrole ring
- Pyrazole ring
- Triazole ring
- Furan ring
Answer. 3. Triazole ring
Question 17. Which of the following agents is preferred in the treatment of insomnia?
- Barbiturates
- Hypnotic benzodiazepines
- Ethanol
- Phenothiazide
Answer. 2. Hypnotic benzodiazepines
Question 18. Barbiturates are being replaced by hypnotic benzodiazepines because of:
- Low therapeutic index
- Suppression in REM sleep
- High potential of physical dependence and abuse
- All of the above
Answer. 4. All of the above
Question 19. The onset and duration of action of barbiturate are affected by:
- Number of carbon atoms situated on the 5-position
- Substitution of nitrogen atom at position-1
- Number of aromatic groups
- None of the above
Answer. 1. Number of carbon atoms situated on the 5-position
Question 20. Indicate the main claim for an ideal hypnotic agent:
- Rapid onset and sufficient duration of action
- Minor effects on sleep patterns
- Minimal “hangover” effects
- All of the above
Answer. 4. All of the above
Question 21. Sedative action of barbiturate is due to substitution at Cs, it is due to:
- High lipophilicity of group at Cs position
- Electronic withdrawing effect
- Steric effect
- Metal chelation
Answer. 1. High lipophilicity of group at Cs position
Question 22. Which of the following hypnotic drugs is used intravenously as anesthesia?
- Thiopental
- Phenobarbital
- Flurazepam
- Zolpidem
Answer. 1. Thiopental
Antipsychotics
Introduction
The term ‘psychosis’ used to describe a type of mental health issue that seriously affects the way that a person thinks or feels and where the person can lose contact with reality due to a disturbance in the functioning of the brain.
This type of mental health problem can happen to anyone and is much more common than most people realize. Sometimes people can have a one-off episode and after that they get better and never experience psychosis again. Other people may have episodes that come and go and they can be well for long periods in between.
There are two broad classes of functional psychotic disorders i.e. schizophrenia and bipolar disorder. Schizophrenia is a chronic condition with exacerbations, but always with some background symptoms. Bipolar disorder is generally an intermittent condition with the expectation of full recovery between episodes.
Symptoms of schizophrenia are grouped into two categories:
- Positive symptoms: Hallucinations and delusions.
- Negative symptoms: Social withdrawal and lack of energy and motivation that are similar to those found in depression.
Psychosis is characterized by
- Loss of connectedness with reality.
- Feeling of very anxious or agitated.
- Have very low or high moods.
- Persons may develop false ideas or beliefs about reality (delusions).
- Persons may have false perceptions (hallucinations).
- Persons also experience flaws in the ways they think (thought disorders).
- Persons may think that people are against them and they may hear voices or sounds that aren’t real.
- Poor physical health.
- Significantly impairs work, family and social functioning.
The drugs that increase the dopaminergic activity like levodopa (a precursor), amphetamine (release of dopamine) and apomorphine (a direct dopamine receptor agonist) may aggrevate schizophrenia or produce psychosis in some patients.
Antipsychotic drugs are used mainly for the treatment of psychosis. Antipsychotic drugs diminish the underlying thought disorder associated with schizophrenia. As these agents often have a calming effect in agitated psychotic patients, they are also called as major tranquilizers and due to its lessen reactivity to emotional stimuli, with little effect on consciousness, these drugs are also known as neuroleptics.
Antipsychotic drugs are derived from several chemical groups. These drugs may be broadly classified as first-generation or typical or conventional agents (phenothiazines and older non-phenothiazines, such as haloperidol, with similar pharmacologic actions, clinical uses, and adverse effects), and second-generation or atypical agents, which are also called as newer non-phenothiazines.
Mechanism Of Action Of Antipsychotic Drugs
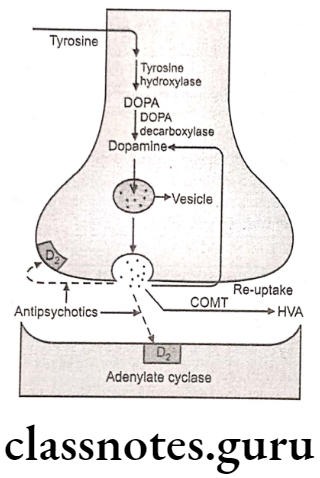
Many antipsychotic drugs strongly block post-synaptic D2 receptors in CNS, especially in the mesolimbic-frontal limbic system and block the action of dopamine.
The most frequent uses of these agents are in manic disorders and the schizophrenias. In the manic disorders, the agents may block dopamine, (3,4-dihydroxyphenethylamine) at limbic D2 and D3 receptors, reducing euphoria, delusional thinking and hyperactivity.
In the chronic idiopathic psychoses (schizophrenias), both conventional (typical) and newer (atypical) antipsychotics appear to act to benefit positive symptoms by blocking dopamine at D2 and D3 limbic receptors. The activity of the atypical agents against negative symptoms may be due to blocking of serotonine2A receptor (5-HT2A).
Classification Of Antipsychotic Drugs
Antipsychotic drugs are classified into following categories:
- Phenothiazines: Promazine hydrochloride, Chlorpromazine Triflupromazine, Thioridazine hydrochloride, Piperacetazine Prochlorperazine meleate, Trifluoperazine hydrochloride. hydrochloride, hydrochloride,
- Ring Analogues of Phenothiazines: Chlorprothixene, Thiothixene, Loxapine succinate, Clozapine.
- Fluoro buterophenones: Haloperidol, Droperidol, Risperidone.
- Beta amino ketones: Molindone hydrochloride.
- Benzamides: Sulpieride.
Phenothiazines
Many potentially useful phenothiazine derivatives have been synthesized and evaluated pharmacologically. Three sub-families of phenothiazine derivatives based on side chain. substitutions were found to be active against psychotic disorder.
Substitution with aliphatic derivatives (e.g., promazine, chlorpromazine and triflupromazine) and piperidine derivatives (e.g., thioridazine) are less potent, whereas substitutions with piperazine derivatives (e.g., prochlorperazine and trifluoperazine) are more potent and effective in small doses. The piperazine derivatives are more selective in their pharmacological activity.
Structure-Activity Relationship of Phenothiazines
Phenothiazines are fused tricyclic (heterocyclic) system, chemically constituted by both lipophilic and hydrophilic groups. Structural features are associated with activity. The general structure of phenothiazine antipsychotic drugs is as follows,
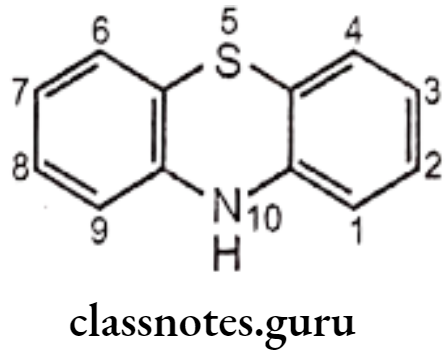
- Position 2 in phenothiazine nucleus was found to be best position for substitution.
- Substitution of electron withdrawing group (e.g. Chlorine) at position 2, increases the antipsychotic activity. (The importance of this substitution is formation of hydrogen bond between the hydrogen atoms of the protonated amino group of the side chain with an electron pair of an electron withdrawing group at position 2 substituent, to develop a dopamine like arrangement.)
- Substitution at the position 3 can improve activity over non-substituted compounds, but not as significantly as substitution at the 2 position.
- Substitution at positions 1 and 4 has a deleterious effect on antipsychotic activity.
- The sulfur atom at position 5 is in a position analogous with that of p-hydroxyl of dopamine leads to assign a receptor-binding function.
- A substituent at position 4 at phenothiazine nucleus might interfere with receptor binding by the sulfur atom.
- Nitrogen-containing side-chain substituent at position 10 at phenothiazine nucleus is required for antipsychotic activity;
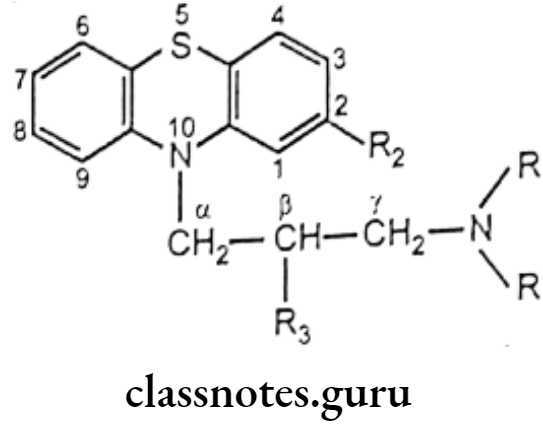
- The ring nitrogen and side-chain nitrogen must be separated by a three carbon chain.
- Shortening or lengthening the chain results into drastically decreased antipsychotic activity. (The three-atom chain length may be necessary to bring the protonated amino nitrogen into proximity with the 2-substituent.).
- Decrease in size from a dimethylamino group to a monomethylamino group at side chain greatly decreases activity.
- Substitution on the side chain with a large group (e.g. phenyl) decreases antipsychotic activity.
- Branching with polar groups such as methyl branching on the B-position has a variable effect on antipsychotic activity.
- The side chains are either aliphatic, piperazine, or piperidine derivatives. Substitution with piperazine side chains leads to greatest potency as well as pharmacological selectivity.
Phenothiazine Derivatives:
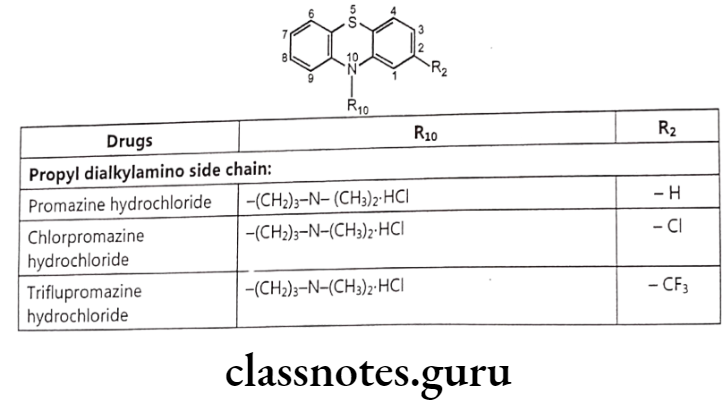
Promazine Hydrochloride:
- Promazine hydrochloride is chemically, N,N-dimethyl-3-phenothiazin-10-yl-propan- 1-amine; hydrochloride.
- Promazine hydrochloride blocks postsynaptic dopamine receptors D1 and D2, mesolimbic receptors and decreases stimulation of psychotic effects, such as hallucinations and delusions.
- It also blocks medullary chemoreceptor trigger zone (CTZ), of vomiting center and thus acts as antiemetic.
- It also blocks alpha-adrenergic receptors and exhibits strong anticholinergic activity.
Uses:
- It is primarily used as antipsychotic agent in short-term treatment of disturbed behaviour.
- It is also used as antiemetic.
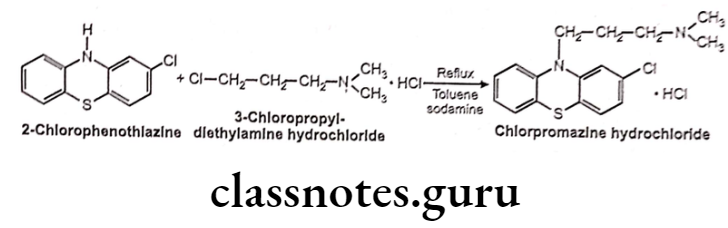
Chlorpromazine Hydrochloride:
- Chlorpromazine hydrochloride is chemically, N, N-dimethyl-3(2-chloropheno- thiazine)-10-yl)-propan-1-amine; hydrochloride.
- It exerts its antipsychotic effect by blocking postsynaptic dopamine receptors in cortical and limbic areas of the brain and thus preventing the excess of dopamine in the brain. Thereby it decreases stimulation of psychotic effects, such as hallucinations ,and delusions.
- It also blocks dopamine receptors in the chemical trigger zone (CTZ) in the brain, thereby relieving nausea and vomiting.
Uses:
- Chlorpromazine hydrochloride acts as an antipsychotic agent used to treat hallucinations and delusions.
- It is also used as antiemetic and used in the treatment of uncontrollable hiccup.
- It also has significant sedative and hypotensive properties, possibly reflecting central and peripheral noradrenergic blocking activity, respectively.
Triflupromazine Hydrochloride:
- Triflupromazine hydrochloride is chemically, N,N-dimethyl-3-[2-trifluoromethyl) phenothiazine-10-yl]propan-1-amine; hydrochloride.
- It is analogous with chlorpromazine hydrochloride, only changes of -CF3 in place of – Cl at position 2 leads to increase in antipsychotic activity.
Uses:
- Triflupromazine hydrochloride is used as antipsychotic drug and is used to treat hallucinations and delusions.
- It has lower sedative and hypotensive properties compared to chlorpromazine hydrochloride.
Thioridazine Hydrochloride:
- Thioridazine hydrochloride is chemically, 10-[2-(1-methylpiperidin-2-yl)ethyl]-2- methylsulfanylphenothiazine; hydrochloride.
- It is a piperidine sub-group of the phenothiazine block to mesolimbic postsynaptic dopamine receptor D2 and decreases dopamine activity, thereby it decreases stimulation of psychotic effects, such as hallucinations and delusions.
- It also binds to serotonin 5-HT2 receptors, resulting in decreased serotonin activity.
Uses:
- Thioridazine hydrochloride is a phenothiazine derivative used in the treatment of pshycotic disorder including schizophrenia.
- It has sedative and hypotensive activity in common with chlorpromazine and less antiemetic activity.
- Piperacetazine Hydrochloride:
- Piperacetazine hydrochloride is chemically, 1-(10-(3-[4-(2-hydroxyethyl)-1- piperidinyl]propyl)-10H-phenothiazin-2-yl)ethanone; hydrochloride.
- It is an antipsychotic prodrug, most notably used for schizophrenia.
Prochlorperazine Meleate:
- Prochlorperazine meleate is chemically, 2-chloro-10-[3-(4-methylpiperazin-1-yl)- propyl]phenothiazine, meleate.
- It is piperazine phenothiazine derivative and it blocks the postsynaptic dopamine D2-receptor in the chemoreceptor trigger zone (CTZ) of the brain and may prevent emesis.
- It also blocks a-adrenergic receptors and results in sedation, muscle relaxation, and hypotension.
Uses:
- Prochlorperazine maleate is a phenothiazine antipsychotic drug.
- It is principally used in the treatment of nausea vomiting and vertigo.
- It also has antihistaminic and anticholinergic activities.
- It is used mainly for its antiemetic effect and not for its antipsychotic effect.
Trifluoperazine Hydrochloride:
- Trifluoperazine hydrochloride is chemically,10-(3-(4-methylpiperazin-1-yl)propyl]-2- (trifluoromethyl) phenothiazine; hydrochloride.
- It blocks central dopamine receptors and is used to treat delusions and hallucinations caused by an excess of dopamine.
- It also blocks the postsynaptic dopamine D2-receptor in the chemoreceptor trigger zone (CTZ) of the brain and may prevent emesis.
- It also blocks central adrenergic receptors and leads to anxiolytic effects.
Uses:
- Trifluoperazine is piperazine phenothiazine derivative and antipsychotic agent that is no longer commonly used in clinical practice.
- It also possesses anxiolytic, and antiemetic activities.
Ring Analogues of Phenothiazines
These agents have structural resemblance to that of the phenothiazine antipsychotics. Most of the drugs also have similar clinical properties and uses as that of phenothiazines.
Thioxanthene Derivative:
The thioxanthene system differs from the phenothiazine system by replacement of the N-H moiety with a carbon atom doubly bonded to the propylidene side chain with the substituent in the 2 position. These compounds (Chlorprothixene and Thiothixene) are very similar in pharmacological activities as that of phenothiazines.
Chlorprothixene:
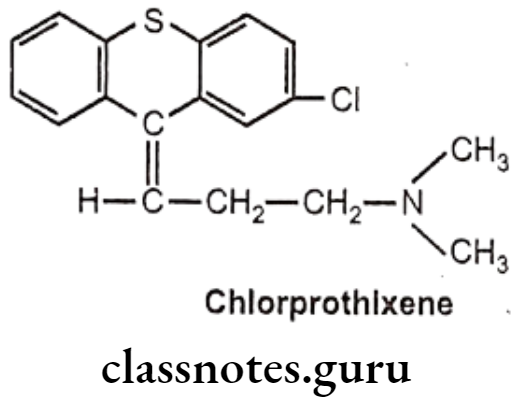
- Chlorprothixene is chemically, 3-(2-chlorothioxanthen-9-ylidene)-N,N-dimethyl- propan-1-amine.
- It is a tertiary amine typical antipsychotic drug of thioxanthenes class.
- It produces its antipsychotic action by blocking the 5-HT2 D1, D2 and D3 receptors.
- It also possesses histamine (H1), muscarinic and a-1 adrenergic receptors blocking action.
Uses:
- Chlorprothixene is used as first generation antipsychotic.
- It also has non-narcotic analgesic, antiemetic, sedative and cholinergic antagonist activity.
Thiothixene:
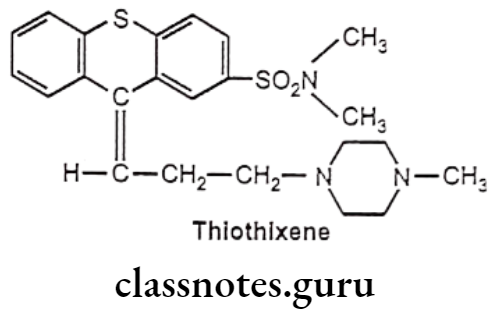
- Thiothixene is chemically, N,N-dimethyl-9-[3-(4-methylpiperazin-1-yl)propylidene] thioxanthene-2-sulfonamide.
- It is a thioxanthene derivative. It produces antipsychotic activity by blocking postsynaptic dopamine receptors in the mesolimbic system.
- It also blocks medullary chemoreceptor trigger zone leading to decreased stimulation of the vomiting center.
Uses:
- Thiothixene is used as an antipsychotic agent.
- It is also used as antiemetics.
Dibenzoxazepine Derivative:
Loxapine Succinate:
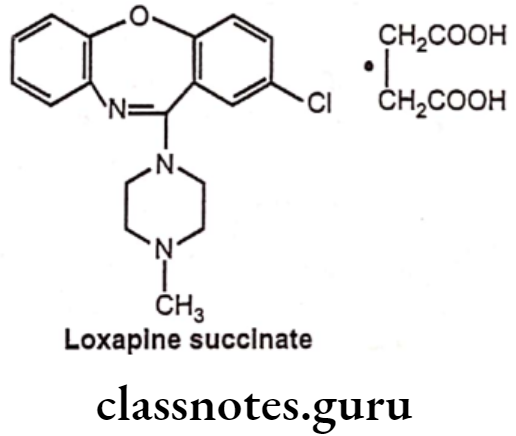
- Loxapine succinate is chemically 8-chloro-6-(4-methylpiperazin-1-yl)benzo[b] [1,4]benzoxazepine, butanedioic acid.
- It produces its action by blocking the dopamine receptors at postsynaptic receptor sites in the limbic system, cortical system and basal ganglia, thereby reducing the hallucinations and delusions.
Uses:
- Loxapine succinate is an antipsychotic agent used in schizophrenia.
- It also possesses antiemetic, sedative, anticholinergic and antiadrenergic actions.
Dibenzodiazepine Derivative:
Clozapine:
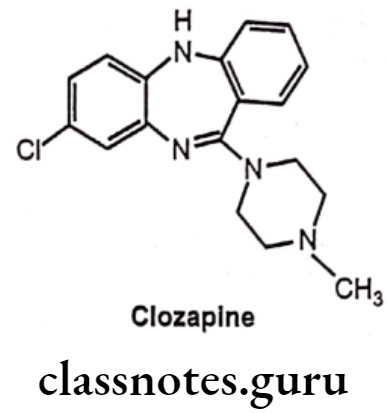
- Clozapine is chemically 3-chloro-6-(4-methylpiperazin-1-yl)-5H-benzo[b][1,4] benzo- diazepine.
- It is an important atypical antipsychotic.
- It weakly blocks D2 receptors and relieves schizophrenic symptoms such as hallucinations, delusions and dementia.
- It also has serotonin (bind with 5-HT2A/2c receptor) receptors antagonist activity.
- It has notably low production of extra pyramidal symptoms (EPS) and reduction of negative symptoms.
Uses:
- Clozapine is an important atypical antipsychotic.
- Its use is restricted because of a relatively high frequency of agranulocytosis as a severe side effect.
Fluorobutyrophenones
Many of the fluorobutyrophenones possess antipsychotic activity. The general structural features for antipsychotic activity are as follows:
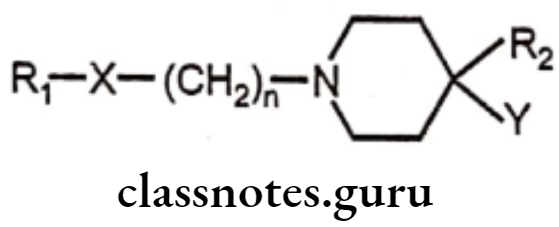
Structure Activity Relationship:
- Aromatic ring with para fluoro substitution at R1 is required for optimum activity.
- Carbonyl group (–) at ‘X’ is required for optimal activity, although the other groups like C(H)OH and C(H)aryl also have good antipsychotic activity.
- Carbon chain length with 3 carbon atoms is required for optimal activity, whereas longer or shorter chain length decreases the activity.
- The aliphatic amino nitrogen incorporated into a cyclic form is required for highest activity.
- R2 must be an aromatic ring attached directly or occasionally separated by one intervening atom to the 4th position for optimal activity.
- The ‘Y’ group can vary with different groups required to assist antipsychotic activity, Example: -OH group in haloperidol.
Haloperidol:
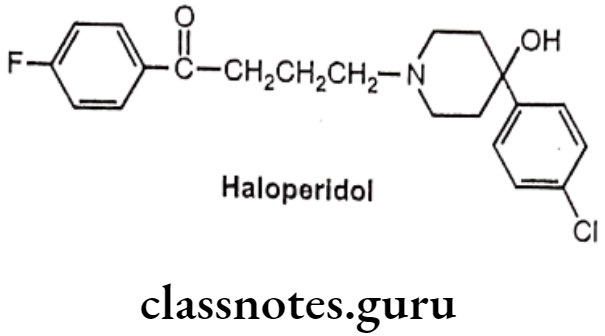
- Haloperidol is butyrophenone, chemically it is, 4-[4-(4-chlorophenyl)-4-hydroxy- piperidin-1-yl)-1-(4-fluorophenyl)butan-1-one.
- It competitively blocks postsynaptic dopamine receptors in the mesolimbic system of the brain and so is used to treat delusion and hallucination.
- It has strong affinity for D2 and D3 receptors.
- It also blocks dopamine receptors in the chemoreceptive trigger zone (CTZ) and leads to its anti-emetic effect.
Uses:
- Haloperidol is a typical antipsychotic drug.
- It is a potent antipsychotic useful in schizophrenia and in psychoses associated with brain damage.
- It also possesses neuroleptic and antiemetic activities.
Droperidol:
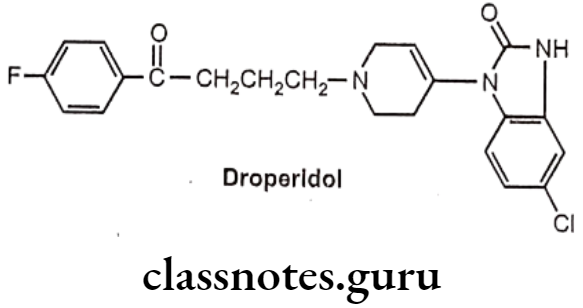
- Droperidol is butyrophenone, chemically it is, 3-[1-[4-(4-fluorophenyl)-4-oxobutyl]- 3,6-dihydro-2H-pyridin-4-yl]-1H-benzimidazol-2-one.
- It has general properties similar to those of haloperidol.
- It acts by blocking dopamine D2 receptors.
- It blocks dopamine receptors in the chemoreceptor trigger zone (CTZ) and leads to its anti-emetic effect.
- It also acts on postsynaptic GABA receptors in the CNS and increases the inhibitory effect of GABA which leads to sedative and anti-anxiety activities.
Uses:
- Droperidol is used as preanaesthetic neuroleptics.
- It is used in combination with an opioid analgesic agent fentanyl preanaesthetically.
- It also possesses anti-emetic, sedative and anti-anxiety properties.
Risperidone:
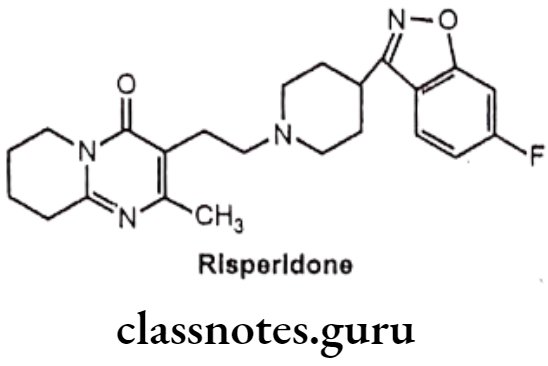
- Risperidone is a benzisoxazole derivative, chemically it is, 3-[2-[4-(6-fluoro-1,2- benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]- pyrimidin-4-one.
- Risperidone selectively antagonizes serotonin 5-HT2 receptor.
- It also binds with the limbic dopamine D2 receptor which leads to antipsychotic activity.
Uses:
- Risperidone acts as an atypical antipsychotic agent.
- It is reported to decrease the negative (e.g., withdrawal, apathy) as well as the positive (e.g., delusions, hallucination) symptoms of schizophrenia.
Beta Amino Ketones
Molindone Hydrochloride:
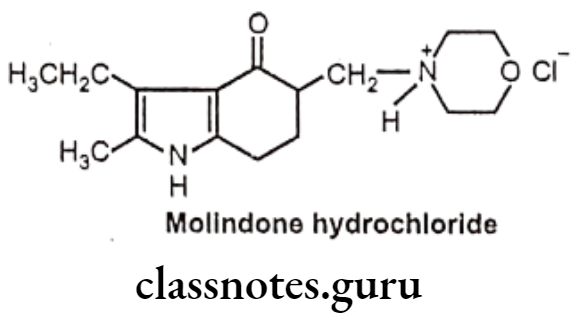
- Molindone hydrochloride is chemically 3-ethyl-2-methyl-5-(morpholin-4-ylmethyl)- 1,5,6,7-tetrahydroindol-4-one;hydrochloride.
- It exerts its effect by blocking dopamine receptors (D2 and D3) in the reticular activating and limbic systems, thereby decreasing dopamine excess in the brain. It also has moderate affinity for cholinergic and alpha-adrenergic receptors.
Uses:
- Molindone is a conventional antipsychotic used in the therapy of schizophrenia and other psychoses.
- It is useful in the treatment of the aggressive type of undersocialized conduct disorder.
Benzamides
Sulpieride:
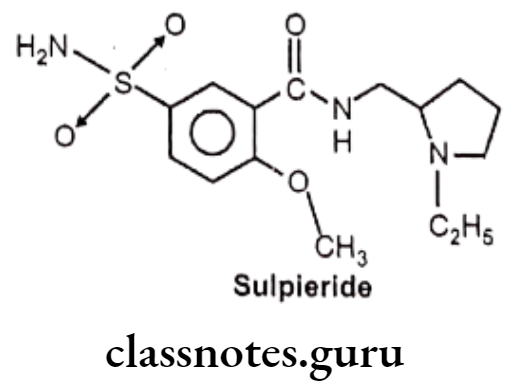
- Sulpieride is benzamide derivative, chemically N-[(1-ethylpyrrolidin-2-yl)methyl]-2- methoxy-5-sulfamoylbenzamide.
- It is more selective and acts primarily as a dopamine D2 antagonist.
Uses:
- Sulpieride is used therapeutically as an antipsychotic.
- It is also used as antidepressant and as a digestive aid.
Synthesis
Chlorpromazine Hydrochloride
Multiple Choice Questions:
Question 1. Neuroleptics are used to treat:
- Neurosis
- Psychosis
- Narcolepsy
- Parkinsonian disorders
Answer. 2. Psychosis
Question 2. 5H-dibenz [b,f]azepine-5-carboxamide is chemical name for:
- Imipramine
- Carbamazepine
- Carbapenam
- Diazepam
Answer. 2. Carbamazepine
Question 3. Most antipsychotic drugs:
- Strongly block postsynaptic D2 receptor
- Stimulate postsynaptic D2 receptor
- Block NMDA receptor
- Stimulate 5-HT receptor
Answer. 1. Strongly block postsynaptic D2 receptor
Question 4. In phenothiazine tranquillizing agents, replacement of C-2 hydrogen by chlorine:
- Decreases activity
- Increases activity
- Do not affect activity
- Leads to penetration to CNS
Answer. 2. Increases activity
Question 5. Which of the following antipsychotic drugs is typical?
- Clozapine
- Quetiapine
- Haloperidol
- Olanzapine
Answer. 3. Haloperidol
Question 6. Chlorpromazine is derivative of 2-chlorphenothiazine which can be prepared from starting material:
- 2-Chlorophenothiazine
- 2- Nitrophenothiazine
- 2-Aminophenothiazine
- 2-Trifluoromethyl phenothiazine
Answer. 1. 2-Chlorophenothiazine
Question 7. Indicate the atypical antipsychotic drug:
- Haloperidol
- Clozapine
- Thioridazine
- Thiothixene
Answer. 2. Clozapine
Question 8. Which of the following antipsychotic drugs has high affinity for D4 and 5-HT2 receptors?
- Clozapine
- Fluphenazine
- Thioridazine
- Haloperidole
Answer. 1. Clozapine
Question 9. Choose the correct chemical name for chlorpromazine hydrochloride:
- [3-(2-chlorophenothiazine-10-yl)propyl]diethyl amine hydrochloride
- [2-(3-chlorophenothiazine-10-yl)propyl]dimethyl amine hydrochloride
- [3-(2-chlorophenothiazine-10-yl)propyl]dimethyl amine hydrochloride
- [3-(3-chlorophenothiazine-10-yl)propyl]dimethyl amine hydrochloride
Answer. 3. [3-(2-chlorophenothiazine-10-yl)propyl]dimethyl amine hydrochloride
Question 10. Indicate the antipsychotic drug, which is a phenothiazine aliphatic derivative:
- Thiothixene
- Risperidone
- Chlorpromazine
- Clozapine
Answer. 3. Chlorpromazine
Question 11. Indicate the antipsychotic drug, which is a butyrophenone derivative:
- Droperidol
- Thioridazine
- Sertindole
- Fluphenazine
Answer. 1. Droperidol
Question 12. Droperidol belongs to the class of:
- Carbamates
- Xanthanes
- Butyrophenone
- Phenothiazine
Answer. 3. Butyrophenone
Question 13. Indicate the antipsychotic drug, which is a thioxanthene derivative:
- Haloperidol
- Clozapine
- Chlorpromazine
- Thiothixene
Answer. 4. Thiothixene
Question 14. Indicate the antipsychotic agent – a dibenzodiazepine derivative:
- Fluphenazine
- Clozapine
- Risperidone
- Droperidol
Answer. 2. Clozapine
Question 15. Indicate the antipsychotic drug having significant peripheral a-adrenergic blocking activity:
- Haloperidol
- Chlorpromazine
- Clozapine
- Risperidone
Answer. 2. Chlorpromazine
Question 16. Indicate the antipsychotic drug having a muscarinic-cholinergic blocking activity:
- Chlorpromazine
- Clorprothixene
- Risperidone
- Haloperidol
Answer. 1. Chlorpromazine
Question 17. Which of the following antipsychotic agents is preferable in patients with coronary and cerebrovascular disease?
- Chlorpromazine
- Fluphenazine
- Haloperidol
- Perphenazine
Answer. 3. Haloperidol
Question 18. Haloperidol is a major tranquilizer, is belongs to the class of:
- Carbamates
- Propanediol
- Butyrophenone
- Phenothiazine
Answer. 3. Butyrophenone
Question 19. The mechanism of haloperidol antipsychotic action is:
- Blocking D, receptors
- Central alpha-adrenergic blocking
- Inhibition of norepinephrine uptake mechanisms
- All of the above
Answer. 4. All of the above
Question 20. Which of the following antipsychotic drugs has high affinity for D, and 5-HT2 receptors?
- Droperidol
- Clozapine
- Thlothixene
- Risperidone
Answer. 4. Risperidone
Anticonvulsants
Introduction
The word ‘epilepsy’ comes from the Greek word epilambanein (to seize). Epilepsy is not a contagious disease or psychological disorder; it can develop at any age. It is said to be common neuralgic disorder found in approximately 0.7% of the population of any age. Epilepsy is a group of chronic CNS disorders due to sudden and transitory seizures of abnormal motor and sensory neurons resulting into a repeated neuronal discharge.
It is a physical condition which causes burst of hyperactivity in brain characterized by chronic, recurrent, paroxysmal changes in neuralgic function. This hyperactivity produces ‘seizures’ which vary from one person to another in frequency and form. It is seen as a sudden abnormal function of the body, often with loss of consciousness, an excess of muscular activity, or sometimes loss of it, or an abnormal sensation. A seizure may last for few seconds to few minutes.
The terms epilepsy or convulsion or seizure are often used interchangeably and basically have the same meaning.
Epilepsy is said to be ‘symptomatic epilepsy’ which develops after a particular identifiable events such as asphyxia, head injury, meningitis, birth trauma or brain injury during pregnancy whereas, epilepsy is called as ‘idiopathic epilepsy’ which develops without any identifiable cause.
Classification Of Different Types Of Epilepsy (Seizure)
A brief classification of different types of epilepsy is important as it facilitates accurate diagnosis and drug selection for precise treatment against seizure disorder.
The major classification is as follows:
Generalized Seizures:
Generalized seizures essentially involve the entire brain and do not have an apparent local onset. Two major types of generalized seizures are as follows:
The generalized tonic-clonic seizure (grand mal)
It is the most common type of epilepsy in which the person often experiences an aura (feeling something around the body, fear and discomfort). It proceeds by a series of bilateral muscular jerks followed by loss of consciousness, which in turn is followed by a series of tonic and then clonic spasms. The seizures generally last from 2 to 5 minutes.
The non-convulsive seizures or absence seizures (petit mal)
This type of epilepsy is most frequently found in children. It consists of a sudden brief loss of consciousness, characterized by blank spells or loss of speech. This seizure is for very short period of time which usually last from 1 to 30 seconds even many persons are not aware that they have had a seizure. In petil mall epilepsy there is no motor activity or some minor clonic motor activity exists.
Partial (or focal) Seizures:
Partial seizures have a focus (i.e., begin locally). Major types of focal (partial) epilepsy are simple focal (Jacksonian motor epilepsy) and complex (psychomotor or temporal lobe) focal seizures.
Simple focal (Jacksonian motor epilepsy) seizures:
Jacksonian epilepsy is rare and usually associated with lesion of a certain part of the brain (cerebral cortex). It is characterized by focal or local clonic type convulsions of localized muscle groups (for example, thumb, big toe, and so forth). The patient does not loss consciousness and therefore be able to tell what happened. The seizures normally last from 1 to 2 minutes.
Complex (Psychomotor or temporal lobe) focal seizures:
Psychomotor epilepsy is uncommon and is characterized by certain abnormal types of behaviour (for example, extensive swallowing or chewing). It mostly occurs in children through adolescence. The individual may experience an aura followed by confusion and bizarre (very strange or unusual behaviour) with perceptual alterations, such as hallucinations or a strong sense of fear. The seizures normally last from 1 to 2 minutes. The seizure may be misdiagnosed as a psychotic episode and difficult to treat.
Status Epilepticus:
A status epilepticus occurs whenever a seizure persists for at least 30 minutes or it repeated so frequently that recovery between attacks does not occur. It is a dangerous condition which may result in brain damage (cerebral necrosis) with severe morbidity or death. A status may be the patient’s first epileptic event or may be precipitated by suddenly discontinuing anticonvulsant therapy.
Each of the epilepsy types is characterized by a typical abnormal pattern in the Electroencephalography (EEG) which gives sudden excessive electrical activity in the brain.
Anticonvulsant Drugs
The proper diagnosis of seizure type is essential to the selection of an appropriate anticonvulsant drug. If used for the wrong seizure type, some anticonvulsant drugs actually increase seizure activity.
The ‘anticonvulsants’ are also called as ‘antiepileptic drugs’ and ‘antiseizure drugs’ that are used regularly to control and manage the neurological disorder caused by excessive nerve cell discharge in the brain. Generally the term anticonvulsant is used for an agent that blocks experimentally produced seizures in laboratory animals whereas; antiepileptic drugs are used medically to control the epilepsies.
For many years, treatment options for epilepsy were limited. However, over the last decade, many new pharmacological therapies have been introduced, and several more are in development.
Classification of Anticonvulsant Drugs:
The anticonvulsants are classified as follows:
- Barbiturates: Phenobarbital, Methabarbital.
- Hydantoins: Phenytoin, Mephenytoin, Ethotoin.
- Oxazolidinediones: Trimethadione, Paramethadione.
- Succinimides: Phensuximide, Methsuximide, Ethosuximide.
- Urea and monoacylureas: Phenacemide, Carbamazepine.
- Benzodiazepine: Clonazepam, diazepam.
- Miscellaneous: Primidone, Valproic acid, Gabapentin, Felbamate, Tiagabine, Lamotrigine, Zonisamide.
Mechanism of Action of Anticonvulsants:
The mode of action of anticonvulsants is not clear. However, it is believed that the anticonvulsants suppress seizures by depressing the cerebral (motor) cortex of the brain.
Gama amino butyric acid (GABA) is the major inhibitory neurotransmitter in the brain. Several of the anticonvulsant medicines work through the GABA system. A major mode of action of anticonvulsants (Benzodiazepine and barbiturate) can be positive allosteric modulation of GABAA receptors. The mechanism of action of barbiturates is based on their structure.
Phenobarbital acts by blocking voltage gated sodium channel, whereas calcium-T channel is blocked by 5,5-dialkyl substituted barbiturates members. Oxazolidine-2.4-diones and succinimides act via calcium T-type channel block. The major mode of action for phenytoin, carbamazepine, valproic acid, felbamate, lamoirigine and zonisarnide are reported to act by blocking voltage-gated sodium channel.
Structure – Activity Relationship of Anticonvulsants:
Several major groups of anticonvulsant drugs viz., Barbiturates, Hydantoins, Oxazolidine diones, Succinimides and Acetylureas have the common structural features as shown below. These groups have in common a similar heterocyclic ring structure with variety of substituents. Very small changes in structure can dramatically alter the mechanism of action and clinical properties of the compound.
![]()
- Both R and R’ should be hydrocarbon radicals.
- If both R and R’ are lower alkyls, then it is more active against absence seizure (petit mal) and not active against generalized tonic-clonic (grand mal) or partial seizures.
- If one of the hydrocarbon substituents is an aryl group, then the activity increases towards generalized tonic-clonic and partial seizures and no action against absence seizure.
Barbiturates
Phenobarbital:
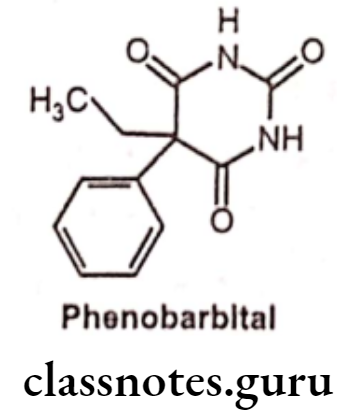
- Phenobarbital is chemically 5-ethyl-5-phenyl-1,3-diazinane-2,4,6-trione.
- It is the drug of choice and is used virtually in all the three types of epileptic seizures viz, grand mal, petit mal and psychomotor.
- It binds to an allosteric site on the GABAA receptor, and it enhances the GABA receptor mediated current by prolonging the opening of the chloride channels. It leads to membrane hyperpolarization and ultimately synaptic inhibition and decreased neuronal excitability.
- It also blocks excitatory responses induced by glutamate,
- It has a long half-life time and therefore it will take a few weeks before reaching a therapeutic and effective level,
- The main side-effects of phenobarbitone are drowsiness, especially during the first week of treatment.
Uses:
- Phenobarbital is a barbiturate that is widely used as a sedative and an antiseizure medication.
- It is used in idiopathic generalized epilepsies.
- It is also reasonably effective in other generalized seizures and in partial seizures.
Methabarbital:
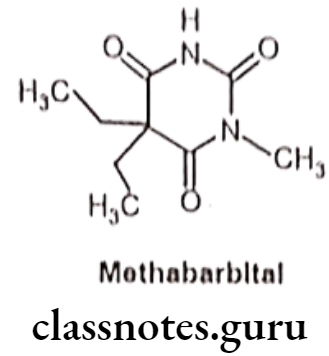
- Methabarbital is chemically, 5,5-diethyl-1-methyl-1,3-diazinane-2,4,6-trione.
- It is mostly demethylated to barbital in vivo. Also it possesses more sedating property than Phenobarbital.
- It could be safely recommended for grand mal seizures.
- It binds at a distinct binding site associated with a Clionopore at the GABA receptor, increasing the duration of time for which the Clionopore is open.
Uses:
- Methabarbital has similar properties to phenobarbital and is used in the treatment of epilepsy.
- It is also used for the treatment of short term insomnia.
- It belongs to Central Nervous System depressants group that induce drowsiness and relieve tension or nervousness.
Hydantoins
- The hydantoins are close structural relatives with barbiturate, only differing in lacking the 6-oxo group. They possess imidazoline-2, 4-dione heterocyclic system.
- Absence of one carbonyl group leads to weaker organic acids than the barbiturates. Hydantoins show their effect by activating Na*- K* dependent and Ca** dependent ATPase and increase Na* transport.
- Hydantoins are more active against partial and generalized tonic-clonic seizure rather than absence seizure.
- All of the clinically useful hydantoins used in treatment of generalized tonic-clonic seizure, possess an aryl substituent on the 5 position.
- Hydantoins with lower alkyl substituents are not active against absence seizure.
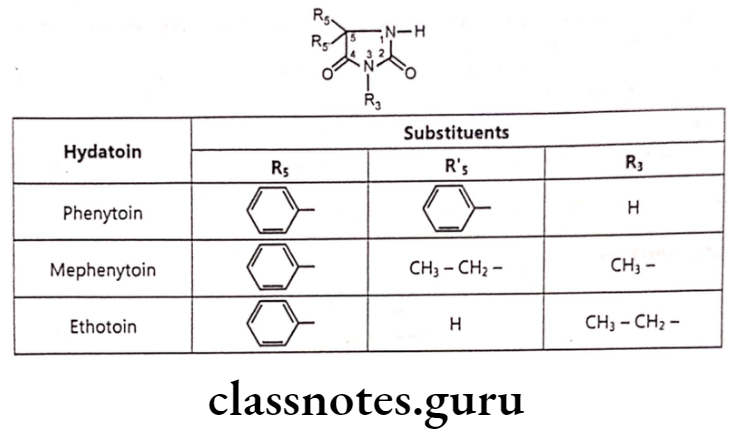
Phenytoin:
- Phenytoin is chemically, 5,5-diphenylimidazolidine-2,4-dione.
- Phenytoin potentially acts by promoting sodium efflux from neurons located in the motor cortex. This inhibits neuronal firing and results in the stabilization of neuronal membranes, thereby preventing the spread of seizure activity at the motor cortex.
- It acts as a anticonvulsant by blocking sodium channel and decreases presynaptic glutamic acid release. It also reduces glutamate-induced ischemic damage to neuron.
Uses:
- Phenytoin is an anticonvulsant that is used to treat a wide variety of seizures.
- It is useful against all types of seizures except absence seizure.
- It is also used as an anti-arrhythmic and a muscle relaxant.
Mephenytoin
- Mephenytoin is chemically, 5-ethyl-3-methyl-5-phenylimidazolidine-2,4-dione.
- It has similar mechanism of action as that of phenytoin.
- It is found to be relatively more toxic, therefore it is exclusively given to such patients who do not response to other treatments.
Uses:
- Mephenytoin is more active against partial and generalized tonic-clonic seizure.
- The adverse effect such as dermatitis, agranulocytosis or hepatitis associated with mephenytoin is higher than phenytoin.
Ethotoin
- Ethotoin is chemically, 3-ethyl-5-phenylimidazolidine-2,4-dione.
- It is recommended for the patients who are hypersensitive to phenytoin.
- It has similar mechanism of action as that of phenytoin.
- The adverse effect and toxicity are generally less severe than those associated with phenytoin, but it appears to be less effective.
Uses:
- Ethotoin is used against generalized seizures.
Oxazolidinediones
- Oxazolidine-2,4-diones are analogous to hydantoins, only replacement of the -NH group at position-1 with an oxygen atom.
- These drugs are more active against absence seizures, provided that branched atom of these compounds is substituted with lower alkyls.
- Aryl substituted oxazolidine-2,4-diones have shown activity against generalized tonic-clonic seizures.
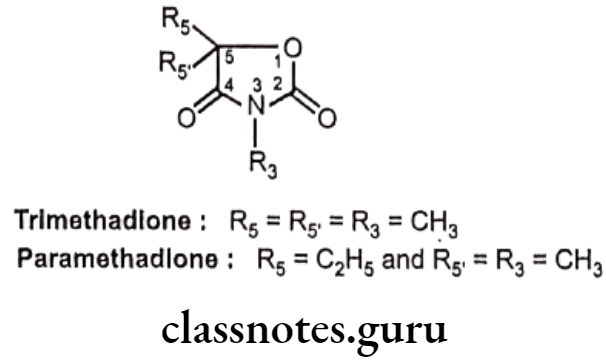
Trimethadione
- Trimethadione is chemically, 3,5,5-trimethyl-1,3-oxazolidine-2,4-dione.
- It undergoes metabolism N-demethylation to active metabolite dimethadione. • Dimethadione blocks calcium-T channel and reduces T-type calcium currents in thalamic neurons, thereby stabilizing neuronal membranes.
Uses:
- Trimethadione is an anticonvulsant effective in absence seizures.
- It’s clinical use is limited because of dermatological and hematological toxicities.
Paramethadione:
- Paramethadione is chemically 5-ethyl-3,5-dimethyl-1,3-oxazolidine-2,4-dione.
- It blocks calcium-T channel and reduces T-type calcium currents in thalamic neurons. This inhibits corticothalamic transmission and raises the threshold for repetitive activity in the thalamus.
Uses:
- Paramethadione is an anticonvulsant effective in absence seizures.
Succinimides
As oxazolidine diones are toxic, an extensive search was carried out to replace them with less toxic drugs. Substitution of ring oxygen in the oxazolidine diones with a -CH2 group gave the antiseizure succinimides. The precise mechanism of action of succinimides is unknown, but it is proposed to act by decreasing the activity of T- type calcium channel. These drugs are used in the treatment of absence (petit mal epilepsy) seizure.
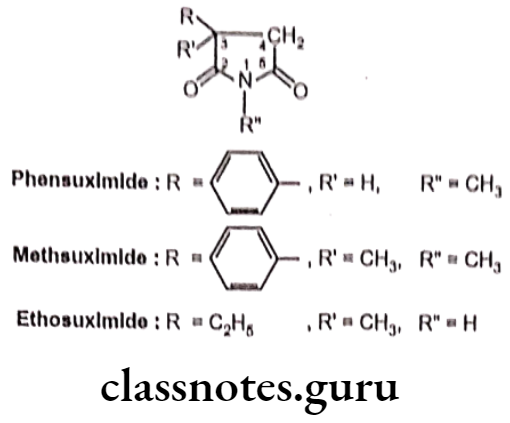
Phensuximide:
- Phensuximide is chemically 1-methyl-3-phenylpyrrolidine-2,5-dione.
- It suppresses the paroxysmal three cycles per second spike and wave EEG pattern associated with lapses of consciousness in absence (petit mal) seizures.
- N-demethylation occurs to yield the putative active metabolite.
Uses:
- Phensuximide occasionally is used for the treatment of absence seizures.
- The phenyl substituent leads to be active against generalized tonic-clonic and partial seizures.
- It also had some activity against maximal electroshock seizure.
Methsuximide:
- Methsuximide is chemically, 1,3-dimethyl-3-phenylpyrrolidine-2,5-dione.
- It increases the seizure threshold and suppresses the paroxysmal three cycles per second spike and wave ECG pattern seen with absence (petit mal) seizures.
- It is generally considered as more toxic than ethosuximide.
Uses:
- Methsuximide is used primarily for the treatment of absence seizures and complex partial seizure.
- It also had some activity against maximal electroshock seizure.
Ethosuximide:
- Ethosuximide is chemically, 3-ethyl-3-methylpyrrolidine-2,5-dione.
- It acts by blocking calcium T channel of the thalamic neurons. This results in decrease in burst firing of thalamocortical neurons, which stabilize the nerve activity in the brain and prevents seizures.
- It is more active and less toxic than trimethadione.
Uses:
- Ethosuximide is used in the treatment of absence (petit mal) seizures.
- With other antiepileptic drugs it can be used to treat other types of epilepsy.
Urea and Monoacylureas
Phenacemide:
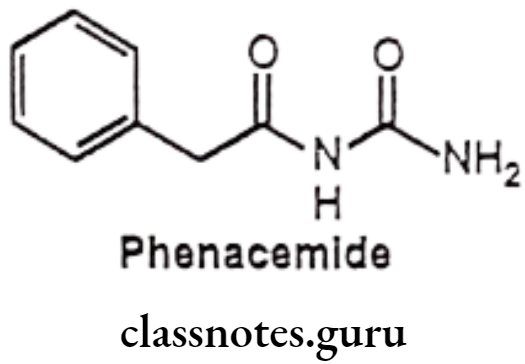
- Phenacemide is chemically, N-carbamoyl-2-phenylacetamide.
- It acts on the Central Nervous System to reduce the number and severity of seizures.
- It binds to and blocks neuronal Na* channels or voltage sensitive Ca** channels.
- It blocks or suppresses neuronal depolarization and hyper synchronization.
Uses:
- Phenacemide is used to control certain seizures in the treatment of epilepsy.
Carbamazepine:
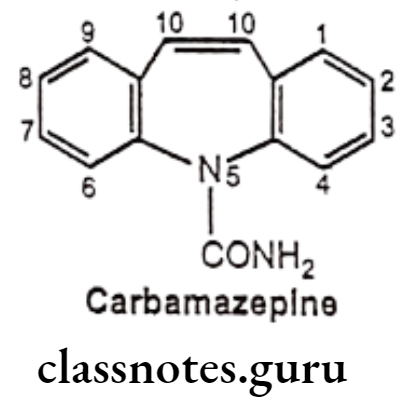
- Carbamazepine is chemically 5H-dibenz[b, f] azepine-5-carboxamide.
- It is chemically related to tricyclic antidepressants (TCA) with anticonvulsant and analgesic properties.
- It exerts its anticonvulsant activity by reducing polysynaptic responses and blocking post-tetanic potentiation.
Uses:
- Carbamazepine is used as an anticonvulsant to control grand mal and psychomotor or focal seizures.
Benzodiazepines
Clonazepam:
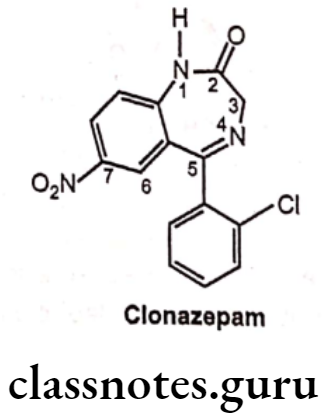
- Clonazepam is chemically, 5-(2-chlorophenyl)-7-nitro-1,3-dihydro-1,4-benzo-diazepin-2-one.
- It is a synthetic benzodiazepine derivative.
- It produces its anticonvulsant activity by acting selectively at benzodiazepine allosteric binding sites on GABAA receptors and enhances GABA receptor responses.
Uses:
- Clonazepam is an anticonvulsant used for several types of seizures such as myotonic or atonic seizures, photosensitive epilepsy and absence (petit mal) seizures.
- It is reasonably effective in generalized tonic-clonic (grand mal) or partial seizures.
Diazepam:
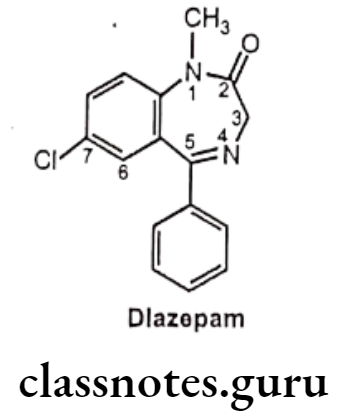
- Diazepam is a benzodiazepine derivative, chemically it is 7-chloro-1-methyl-5- phenyl-3H-1,4-benzodiazepin-2-one.
- It binds with GABA receptor located in the limbic system and the hypothalamus and inhibits the activities of GABA. This leads to open chloride channel increases influx of chloride ions into the neuron, results into membrane hyper polarization and a decrease in neuronal excitability.
Uses:
- Diazepam is the drug of first choice for the treatment of status epilepticus and administered by IV rout.
- It is also useful in treating generalized tonic-clonic (grand mal) seizure.
- It is also used as anti-anxiety and as hypno-sedative.
Miscellaneous
Primidone:
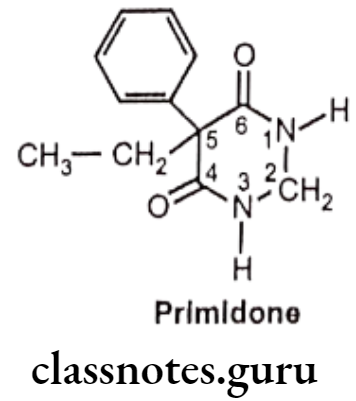
- Primidone is chemically, 5-ethyl-5-phenyl-1,3-diazinane-4,6-dione.
- It is an analog of phenobarbital with antiepileptic property.
- It’s mode of action is similar to phenobarbital, i.e. activation of GABAA and increased frequency of opening of the chloride channel within the receptor complex. This leads to an alteration in the electrical activity of the nerve cell membrane results in to hyper polarization and prevention of partial and tonic-clonic seizures.
- It produces its anti-seizure properties as such and through partially metabolism to phenobarbital and phenylethyl malonamide (PEMA), which may also contribute to its activity. Adverse effects are reported to be more frequent than with phenobarbital.
Uses:
- The efficacy of primidone is against all types of seizures except absence seizure.
Valproic acid:
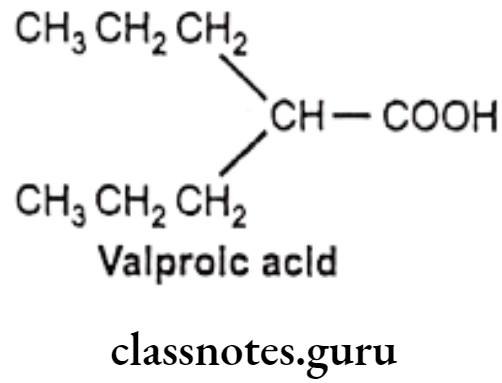
- Valproic acid is chemically, 2-propylpentanoic acid.
- It is synthetic derivative of propylpentanoic acid.
- It may act by increasing GABA levels in the brain or by blocking voltage dependent sodium channels and disorganized electrical activity.
- As this drug undergoes extensive dissociation at physiological pH produce poor partitioning across BBB, hence less potent compared to other drugs.
Uses:
- Valproic acid has anticonvulsant properties and is used in the treatment of grand mal epilepsy, petit mal epilepsy and complex partial seizure.
- It is also used as a mood stabilizer.
- It possesses antineoplastic and antiangiogenesis activities.
Gabapentin:
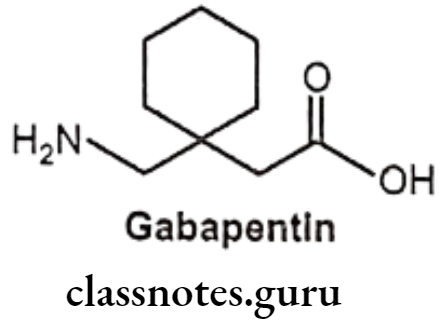
- Gabapentin is chemically, 1-(aminomethyl) cyclohexaneacetic acid.
- It is synthetic GABA-mimetic analogue capable of penetrating the CNS.
- It is a water-soluble amino acid, act by altering the metabolism or release of GABA and decreased CNS disorganized electrical activity.
- It raises brain GABA levels in patients with epilepsy.
- It also acts by binding with calcium channels.
Uses:
- Gabapentin is used in refractory partial seizures and generalized tonic-clonic seizures.
- It also approved for the treatment of postherpetic neuralgia.
Felbamate:
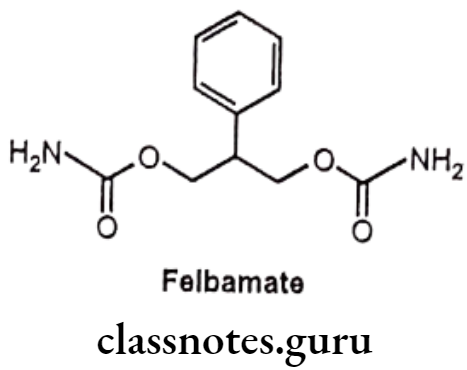
- Felbamate is chemically 2-phenylpropane-1,3-diyl dicarbamate.
- It acts by interaction with strychnine-insensitive glycine recognition site of the N-methyl-D-aspartate (NMDA) receptor ionophore complex. Hence blocks the effect of excitatory amino acid and suppresses the neuronal disorganized electrical activity. It possesses GABA receptor blocking action and produces inhibitory effect on neuronal excitatory.
- It also acts by blocking sodium channels.
Uses:
- Felbamate is used in combination with other antiepileptic medications against generalized tonic-clonic and complex partial seizures.
- As it is associated with a serious risk of aplastic anaemia and acute liver failure, its use is restricted.
Tiagabine:
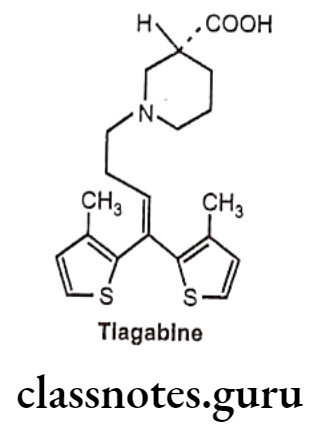
- Tiagabine is chemically, (3R)-1-[4,4-bis(3-methylthiophen-2-yl)but-3-enyl]piperidine- 3-carboxylic acid.
- It is a nipecotic acid derivative with an improved ability to cross the blood-brain barrier.
- It acts by blocking GABA receptor and inhibits GABA uptake by glial cells.
Uses:
- Tiagabine is used largely as an adjunctive agent in therapy of partial seizures in adults or children.
Lamotrigine:
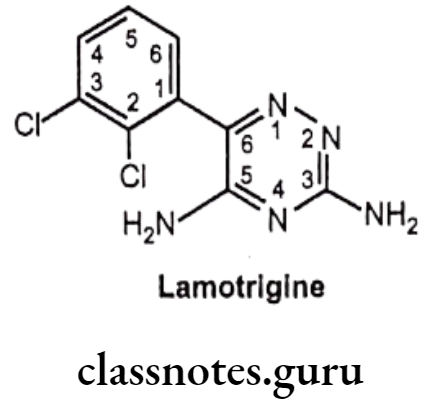
- Lamotrigine is a triazine compound and is chemically, 6-(2,3-dichlorophenyl)-1,2,4- triazine-3, 5-diamine.
- It enhances the action of an inhibitory neurotransmitter, GABA, and reduces the pain- related transmission of signals along nerve fibers.
- It acts by blocking sodium channels and preventing excitatory neurotransmitters (glutamate) release and inhibits serotonin reuptake.
- It reduces neuronal cell death in ischemia by reducing glutamate release.
Uses:
- Lamotrigine is an anti-epileptic agent and mood stabilizer.
- It is effective against refractory partial seizures.
- It also has analgesic property.
Zonisamide:
- Zonisamide is chemically, 1,2-benzisoxazole-3-methanesulfonamide.
- It is sulfonamide derivative and acts by blocking sodium channel and calcium-T channels, thereby stabilizing neuronal membranes and decreased CNS disorganized electrical activity..
Uses:
- Zonisamide is new generation anticonvulsant used in combination with other antiepileptic drugs for use in the treatment of partial seizure.
Synthesis
Phenytoin
Carbamazepine
Ethosuximide
Multiple Choice Questions:
Question 1. The mechanism of action of antiseizure drugs is:
- Enhancement of GABAergic (inhibitory) transmission
- Diminution of excitatory (usually glutamatergic) transmission
- Modification of ionic conductance
- All of the above mechanisms
Answer. 4. All of the above mechanisms
Question 2. Ethusuximide is an a-disubstituted derivative of succinimide. It contains:
- Two methyl groups
- Two ethyl groups
- One methyl and one ethyl group
- One methyl and one propyl group
Answer. 3. One methyl and one ethyl group
Question 3. Valproic acid is:
- 2-ethylpentoic acid
- 2-methylpentoic acid
- 2-propylpentoic acid
- 2-butylpentoic acid
Answer. 3. 2-propylpentoic acid
Question 4. Which of the following antiseizure drugs produces enhancement of GABA-mediated inhibition?
- Ethosuximide
- Carbamazepine
- Phenobarbital
- Lamotrigine
Answer. 3. Phenobarbital
Question 5. Which of the following antiseizure drugs produces a voltage-dependent inactivation of sodium channels?
- Lamotrigine
- Carbamazepin
- Phenytoin
- All of the above
Answer. 4. All of the above
Question 6. The drug for partial and generalized tonic-clonic seizures is:
- Carbamazepine
- Valproate
- Phenytoin
- All of the above
Answer. 4. All of the above
Question 7. Indicate an anti-absence drug:
- Valproate
- Phenobarbital
- Carbamazepine
- Phenytoin
Answer. 1. Valproate
Question 8. The drug against myoclonic seizures is:
- Primidone
- Carbamazepine
- Clonazepam
- Phenytoin
Answer. 3. Clonazepam
Question 9. The anticonvulsant drug belongs to category benzodiazepines:
- Primidone
- Carbamazepine
- Clonazepam
- Phenytoin
Answer. 3. Clonazepam
Question 10. The anticonvulsant drug belongs to category hydantoin:
- Primidone
- Carbamazepine
- Clonazepam
- Phenytoin
Answer. 4. Phenytoin
Question 11. The most effective drug for stopping generalized tonic-clonic status epilepticus in adults is:
- Lamotrigine
- Ethosuximide
- Diazepam
- Zonisamide
Answer. 3. Diazepam
Question 12. Select the appropriate consideration for phenytoin:
- It blocks sodium channels
- It binds to an allosteric regulatory site on the GABA-BZ receptor and prolongs the openings of the Cl-channels
- It effects on Ca2+ currents, reducing the low-threshold (T-type) current
- It inhibits GABA-transaminase, which catalyzes the breakdown of GABA Unit 4 | 4.52
Answer. 1. It blocks sodium channels
Question 13. Phenytoin is used in the treatment of:
- Petit mal epilepsy
- Grand mal epilepsy
- Myoclonic seizures
- All of the above
Answer. 1. Petit mal epilepsy
Question 14. The drug of choice for partial seizures is:
- Carbamazepine
- Ethosuximide
- Diazepam
- Lamotrigine
Answer. 1. Carbamazepine
Question 15. The mechanism of action of carbamazepine appears to be similar to that of:
- Benzodiazepines
- Valproate
- Phenytoin
- Ethosuximide
Answer. 3. Phenytoin
Question 16. Indicate the drug of choice for status epilepticus in infants and children:
- Phenobarbital sodium
- Clonazepam
- Ethosuximide
- Phenytoin
Answer. 1. Phenobarbital sodium
Question 17. Which of the following anticonvulsant drug belongs to category Oxazolidine diones?
- Paramethadione
- Carbamazepine
- Clonazepam
- Phenytoin
Answer. 1. Paramethadione
Question 18. Which of the following anticonvulsant drug belongs to category monoacylurea?
- Paramethadione
- Carbamazepine
- Clonazepam
- Phenytoin
Answer. 2. Carbamazepine
Question 19. The drug of choice in the treatment of petit mal (absence seizures) is:
- Phenytoin
- Ethosuximide
- Phenobarbital
- Carbamazepine
Answer. 2. Ethosuximide
Question 20. Valproate is very effective against:
- Absence seizures
- Myoclonic seizures
- Generalized tonic-clonic seizures
- All of the above
Answer. 4. All of the above
Question 21. Indicate the antiseizure drug – a benzodiazepine receptor agonist:
- Phenobarbital
- Phenytoin
- Carbamazepine
- Clonazepam
Answer. 4. Clonazepam
Question 22. The most dangerous effect of antiseizure drugs after large overdoses is:
- Respiratory depression
- Gastrointestinal irritation
- Alopecia
- Sedation
Answer. 1. Respiratory depression
