Properties Of X-Ray
Important Notes
- Properties of X-rays
- Travel at the rate of speed of light
- Invisible
- Cannot be focused, reflected or reflected
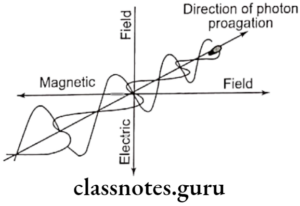
- Effected by magnetic and electrical fields
- They effect photographic plates
- They cast shadows of the object in their paths
- The three mechanisms that explains the interactions of X-rays with matter are
- Coherent scattering
- Photoelectrical absorption
- Compton scattering
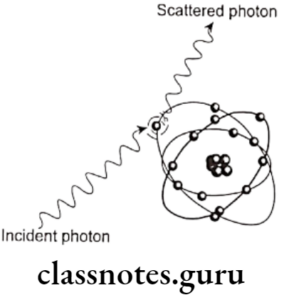
- Thomson Effect/Classical Scattering/Coherent Scattering
- It occurs when a low – energy incident photon passes near an outer electron of an atom
- The incident photon is non absorbed but scattered without loss of energy.
- Energy of scattered photon = Energy of incident photon
- It accounts for about 8% of the total number of interactions
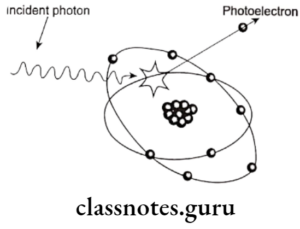
- Photoelectric Absorption.
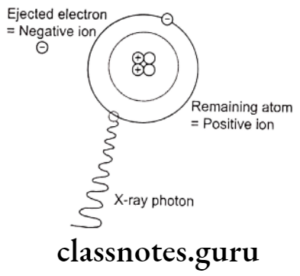
- It occurs when an incident photon collides with a bound electron in an atom.
- The incident photon is absorbed and the electron is expelled from its shell and becomes a photoelectron
- Energy of photoelectron = Energy of incident photon – blinding energy of electron.
- It accounts for about 30% of interactions.
- Compton Scattering.
- It occurs when a photon interacts with an outer electron of an atom.
- The electron receives kinetic energy and recoils from the point of impact.
- The incident photon is scattered from the site of collision, making the atom ionized.
- Approximately 62% of photon undergo Compton scattering.
- Compton scattering is the major source of secondary radiation.
- Inverse square law.
- It states that the intensity of the X-ray beam is inversely proportional to the square of the distance from the source to the film
- Increased distance leads to the divergence of the X-ray beam
Properties of X-rays in radiology
Properties Of X-Ray Short Essays:
Question 1. Properties of X-ray.
Answer.
Properties of X-ray
- Physical Properties:
- It is electromagnetic radiation
- It travel through space
- They travel in a straight line
- X-ray travel with the speed of light
- They cannot be reflected, refracted or deflected
- They show properties of interference, diffraction
- They do not have any mass or weight
- They obey inverse square law
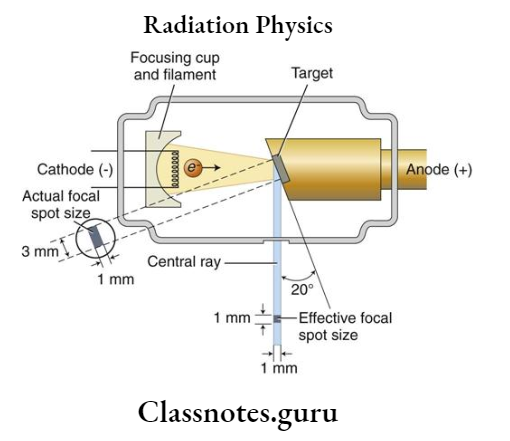
- These are produced by collision of electrons
- Chemical Properties:
- These induce color changes of several substances
- Example: Methylene blue gets bleached
- Cause oxidation of ferrous sulphate to ferric sulphite
- Causes destruction of the fermenting of enzymes
Read And Learn More: Oral Radiology Question and Answers
- Biological Properties:
- Excitation – this property is used in the treatment of malignant tumor
- Germicidal property – This property is used for sterilization oand preservation of food
- Physiochemical Property:
- Causes blackening of photographic paper on paper
- This property is known as photographic effect
Question 2. Interaction of X-ray with matter.
Answer.
Interaction of X-ray with matter
- Coherent scattering:
- Low energy photon passing near atom of outer electron gets scattered without loss of energy
- Incident photon causes vibration of electrons
- This electron radiates energy in the form of another X-ray photon
Significance:- 8% of total X-ray interaction are consist of it
- Effect of it in producing film fog is negligible
- Photoelectric effect:
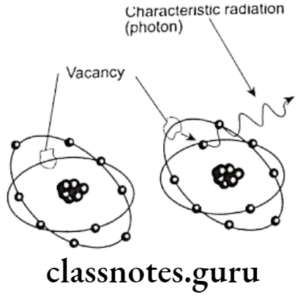
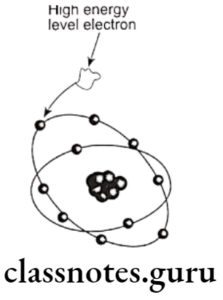
- When the incident photon strikes inner orbital electron, it is ejected as photoelectron
- Vacancy of ineer orbit is filled by electron from higher energy level
- The ejected electrons travel only a short distance
- All of the energy of photons is deposited in the absorber
Significance:- Consists of 30% of total interaction
- It is good for dentist
- But it is bad for patients due to increased absorption
- Compton effect:
- Photon interacts with the outer free electron
- It results in formation of scattered photon of low energy
- As well as ejection of recoil electron
Significance:- Consists of 62% of total interactions
- It is good for patients
- But it is bad for dentist as it causes film fog
Physical properties of X-rays
Properties Of X-Ray Short Answers
Question 1. Inverse Square law.
Answer.
Inverse Square law
- It states that the intensity of the X-ray beam is inversely proportional to the square of the distance from the source to the film
- Increased distance leads to the divergence of the X-ray beam
- Thus number of photons decreases
- As a result, intensity of the X-ray beam diminishes
1 ∝ 1/d2
1 = k/d2, where k is constant
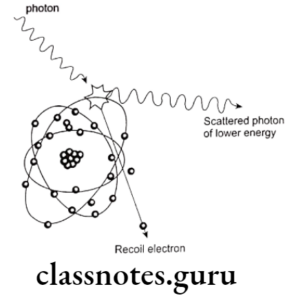
Question 2. Compton effect.
Answer.
Compton effect
- Photon interacts with the outer free electron
- It results in formation of scattered photon of low energy
- As well as ejection of recoil electron
Compton Effect Significance:
- Consists of 62% of total interactions

- It is good for patient
- But it is bad for dentist as it causes film fog
Characteristics of X-ray radiation
Question 3. Uses of X-ray
Answer.
Uses of X-ray
- Used for diagnostic purposes
- Medicolegal use
- For treatment of tumours
- For treatment of skin diseases
- To improve the quality of oil paints
- For crystallography
- For sterilization of instruments
- As detective measure
- Used in the field of engineering
- As spectroscopy
- As photochemistry
- In the field of radiobiology
Question 4. Coherent scattering.
Answer.
Coherent scattering
- Low energy photon passing near atom of outer electron gets scattered without loss of energy
- Incident photon causes vibration of electrons
- This electron radiates energy in the form of another X-ray photon
Coherent Scattering Significance:
- 8% of total X-ray interaction are consist of it
- Effect of it in producing film fog is negligible
Biological effects of X-rays
Properties Of X-Ray Viva Voce
- X-ray have neutral charges
- Velocity of x-ray is equal to that of light