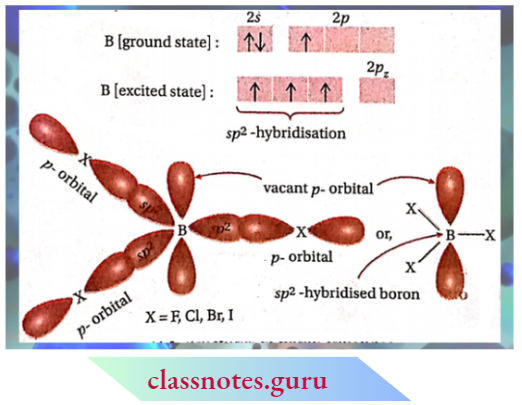
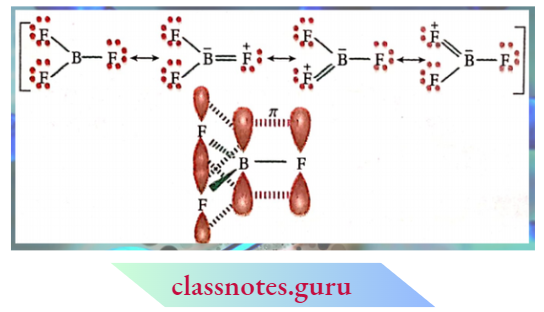
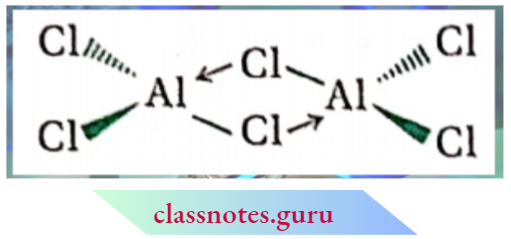
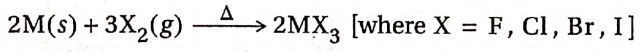CBSE Class 11 Chemistry Notes For Chapter 12 Organic Chemistry Basic Principles And Techniques Classification And Nomenclature
It has been known from ancient times that minerals, plants, and animals are the three major sources of naturally occurring substances. However, very little information was known regarding their chemistry until the beginning of the eighteenth century. In 1675, Lemery classified the natural substances into three classes such as mineral substances, vegetable substances, and animal substances based on the sources from which they were obtained, and it was readily accepted.
- In 1784, it was Lavoisier who first showed that all compounds derived from vegetable and animal sources always contained carbon and hydrogen and sometimes oxygen, nitrogen, sulfur, and even phosphorus. So, there is a dose relationship between the vegetable and animal products.
This led to the classification of natural substances into two categories:
- Organic compounds: All those substances which were obtained from plants and animals, i.e., the substances which were obtained from living organisms and
- Inorganic compounds: All those substances that were obtained from non-living sources, such as rocks and minerals etc. This classification, however, found justification in the fact that in several cases, the same compound could be derived from both vegetable and animal sources.
A detailed investigation of the structure of the organic compounds revealed that almost all of them essentially contain both carbon and hydrogen atoms (hydrocarbon) and some of them also contain the atoms of a few other elements such as nitrogen, oxygen, phosphorus, halogens etc.
Read and Learn More CBSE Class 11 Chemistry Notes
Since these are formed by replacing the hydrogen atoms in the hydrocarbons by these atoms, therefore, these are regarded as derivatives of hydrocarbons. Organic compounds are, therefore, hydrocarbons and their derivatives and the branch of chemistry that deals with the study of these compounds is known as organic chemistry.
Inorganic chemistry, on the other hand, is defined as the chemistry of all elements other than carbon and their compounds
Organic Chemistry Basic Principles and Techniques Class 11 Notes
Tetrahedral Arrangement Of The Four Valencies (Bonds) Of Carbon
In 1859, Kekule proposed that carbon exhibits the normal valency of four units in simple as well as complicated organic molecules and an atom of carbon is joined to other atoms by four covalent bonds which remain in a plane. Also, the angle between any two adjacent bonds is 90°.
- This proposal, however, could not explain some special characteristics of organic compounds, and a change in Kekule’s theory was urgently required.
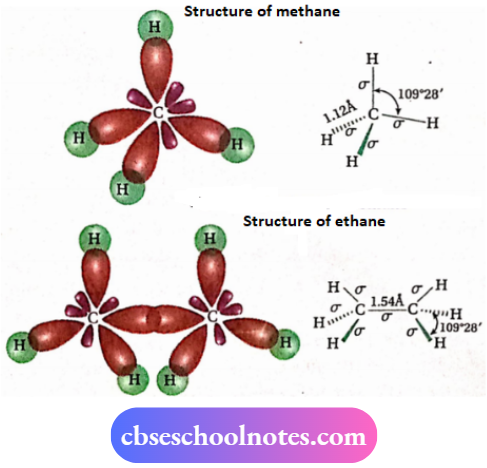
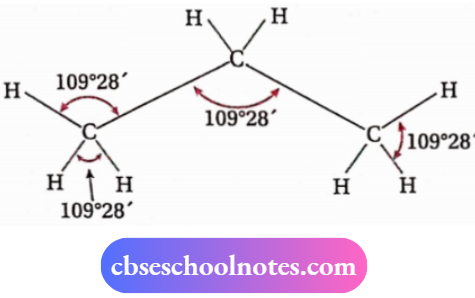
- In 1874, both can’t Hoff and Le Bel predicted that the four bonds of a carbon atom are directed towards the four corners of a regular tetrahedron, i.e., the angle between any two adjacent bonds is I09°28′ (tetrahedral angle).
- This representation is regarded as a tetrahedral model or space model. It was supported by electron diffraction and spectroscopic studies.
- The tetrahedral arrangement of four bonds of carbon laid the foundation for a fascinating field of ‘stereochemistry It is for this reason, that the first Nobel Prize in chemistry was awarded to van’t Hoffin 1901

Explanation for the existence of a large number of organic compounds:
An important and interesting property of a carbon atom is its unique capacity to form bonds with other carbon atoms. This property of forming bonds with atoms of the same element is called catenation. Carbon shows maximum catenation in its group (group 14) in the periodic table.
This is because of the greater strength of the C —C bond as compared to other atoms. For example, the C— C bond is very strong (335 kj- mol-1 ) in comparison to the Si—Si bond (220 kj-mol-1 ) or Ge—Ge bond (167 kj – mol-1). As a result, carbon atoms can link with each other to form either linear chains of various lengths or branched chains and also rings of different sizes.

Again, two or more organic compounds having the same molecular formula may be formed. Compounds having the same formula but different properties are called isomers and the phenomenon is called isomerism.
Catenation and isomerism are the two properties of carbon that are responsible for the existence of a large number of organic compounds. Carbon is also involved in forming multiple bonds with other carbon atoms (C=C, C≡C) and also with oxygen (C—O) and nitrogen atoms (C=N, C≡N). Themultiplebondingis also responsible for the existence of a variety of carbon compounds.
Electronic explanation of the tetra covalency of carbon: Lewis’ theory:
In 1916, G.N. Lewis put forward the electronic concept regarding the formation of bonds. According to him, atoms with similar or almost similar electrochemical nature combine by forming one or more electron pair using odd electron(s) in their valence shells, and in this way, they attain stable electronic configurations (electron octets) of the nearest noble gases.
Carbon exists in group IVA of the second period of the periodic table. In the perspective of its position just between the electropositive and electronegative elements, it can be said that it is neither an electropositive nor an electronegative element. Its ionization enthalpy is sufficiently high and its electron affinity is of moderate magnitude.
Therefore, carbon (ground state electronic configuration: ls²2s²2px¹2py¹) can neither produce C4+ ion by losing 4 electrons from its valence shell nor form C-4- ion by gaining 4 electrons.
Both processes require large amounts of energy which are not ordinarily available during a chemical reaction. Thus, carbon does not usually form electrovalent compounds
Exception:
During the reaction between carbon and highly electropositive sodium, carbon forms the C4- ion thereby producing the electrovalent compound sodium carbide, Na4C ).
According to the electronic configuration, carbon should be bivalent, i.e., it should exhibit a valency of two because of the presence of two odd electrons in 2px– and 2py–orbitals.
To account for tetracovalency, it is believed that during the process of bond formation (an energy-releasing process), the two electrons in the 2s -orbital get unpaired, and one of them is promoted to the empty 2pz -orbital. Therefore, the electronic configuration of carbon in the excited state is ls²2s²2px¹2py¹ 2pz¹) So, carbon can complete its octet by sharing the four odd electrons in its valence shell
With the 4 electrons of the valence shells of the other 4 atoms. Hence, carbon fulfills its octet by covalency and In the formation of covalent compounds, its valency is always 4
Example:
One C -atom forms four electron pairs by combining with four H-atoms and produces one methane (CH4) molecule with consequent completion of its octet According to Lewis’s theory, 1 electron pair stands for 1 covalent single bond, represented by dash sign(-).

Now, two carbon atoms can form 1, 2, or 3 electron pairs by contributing 1, 2, or 3 electrons respectively from each carbon atom and use them equally to form a carbon-carbon single bond (C—C), carbon-carbon double bond (C= C) or carbon-carbon triple bond (C = C) respectively. For example, ethane (CH3 —CH3), ethylene (CH2=CH2), and acetylene (HC = CH) contain one carbon-carbon single, double, and triple bond respectively.

Although Lewis’s theory gives a satisfactory explanation to the tetracovalency of carbon, it fails to offer any idea about how these valencies are oriented in three-dimensional space. Moreover, this concept of covalent bond formation by sharing of electrons is purely a qualitative approach that tells nothing about the attractive forces involved in bond formation
Class 11 Chemistry Organic Chemistry Basic Principles NCERT Notes
Hybridization Of Carbon And Shapes Of Some Simple Organic Molecules
For sp³, sp² and sp -hybridisations of carbon and the shapes ofmethane, ethane, ethylene and acetylene see article no. 4.8 of the chapter “Chemical Bonding and Molecular Structure”. From the knowledge of the state of hybridisation of atoms in an organic molecule, an idea about shapes ofthe molecules can be obtained.
These maybe summarised as follows:
1. Atoms bonded to an sp³ -hybridised carbon atom are tetrahedrally oriented. So, any molecule containing an sp³ -hybridised carbon atom must have threedimensional shape (irrespective of the presence of any sp2 or sp -hybridised carbon atom in it).
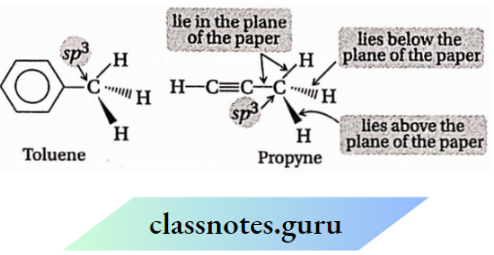
2. The two sp² -hybridized C-atoms and the atoms directly attached to them remain in the same plane. So, if any organic molecule contains only sp² -hybridized C-atoms which are arranged in a chain or cyclic pattern, then the whole molecule becomes planar.
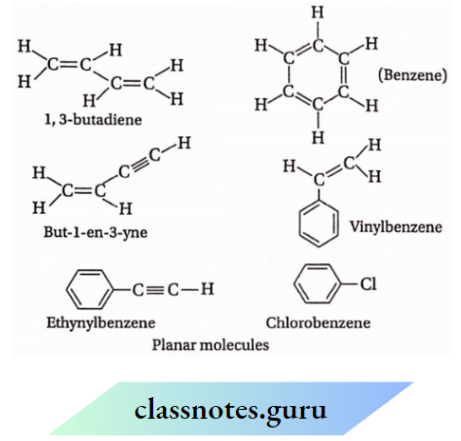
For example: 1,3-butadiene (CH2=CH—CH=CH2) and benzene molecules are planar.
If a molecule contains both sp and sp² – hybridized C-atoms, then the molecule will also be planar. For example, the but-l-en-3-yne (CH2= CH—C= CH) molecule is planar. If sp² or sp -hybridized carbon atoms remain attached to a benzene ring, then the molecule will also be planar. For example, vinylbenzene and ethylbenzene molecules are found to be planar. When an atom is present as a substituent in benzene, even then the molecule becomes planar.
For example: Fluorobenzene, chlorobenzene, bromobenzene etc… are planar molecules.
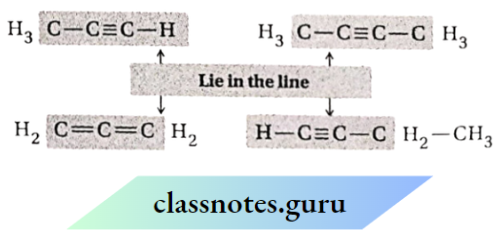
3. The two sp -hybridized carbon atoms and the atoms directly attached to them remain on the same line. So, if a

A molecule contains only sp -hybridized carbon atoms which remain bonded one after another, and then the molecule will be linear in shape. But 1,3-diyne, for example, is a linear molecule.
Structure of some familiar organic compounds:

Prediction of the state of hybridization of a carbon atom from the nature of bonding:
If a carbon atom is bonded to four atoms by four single bonds (i.e., 4 cr -bonds), then that carbon atom is sp³ -hybridized;
- If a carbon atom is bonded to three atoms by two single bonds [i.e., 2 σ – bonds) and one double bond [i.e., 1 σ and 1 π -bond), then that carbon atom is sp² -hybridized; and
- If a carbon atom is bonded to two atoms by one single bond [i.e., one bond) and one triple bond [i.e., one σ -bond and two π bonds) or by two double bonds [i.e., two car and two n bonds), then that carbon atom is sp -sp-hybridized

Basic Principles of Organic Chemistry Class 11 NCERT Notes
Effect Of Hybridisation On Bond Lengths And Bond Strengths
The bond length as well as bond strength of any bond depends upon the size of the hybrid orbitals involved in bond formation.
1. The s -characters of sp3, sp², and sp -hybrid orbitals are 25%, 33.33%, and 50% respectively. As s -the orbital is more closer to the nucleus as compared to p -orbital, more the percentage of s -the character of the hybrid orbital, more it will be attracted by the nucleus, and as a result, its size will
The s -characters of sp³, sp² and sp -hybrid orbitals are 25%, 33.33%, and 50% respectively. As s -orbital is closer to the nucleus as compared to p -orbital, the more the percentage of s -character of the hybrid orbital, the more it will be attracted by the nucleus, and as a result, its size will decrease more.
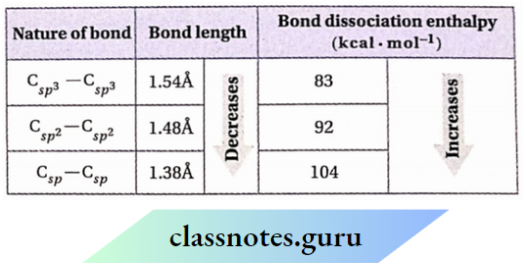
Therefore, the sizes of sp³, sp², and sp hybrid orbitals follow the order: sp < sp² < sp³. Consequently, the bond formed by an sp³ -hybrid orbital is longer than the bond formed by an sp² hybrid formed orbital by an which-hybrid orbital turn is longer than the bond
Example:
The Csp3 bond is longer than the Csp2 —H bond which in turn is longer than the Csp —H bond. Similarly, the Csp3 —Csp3 bond is longer than the Csp2 —Csp2 bond which turns longer than the Csp — Csp bond. For the same reason, the lengths of single, double, and triple bonds follow the order: C —C>C=C>≡ C. The presence and the increase in the number of n -bonds between two carbon atoms are also responsible for the decrease in bond length.
2. Shorter the bond, the greater is its strength i.e., stronger itis. Therefore, a <r -bond formed by sp -hybrid orbitals is stronger than a σ -bond formed by sp² -hybrid orbitals which in turn forms more stronger σ -bond than formed by sp³ -hybrid orbitals. Again, the bond strength increases with an increase in bond multiplicity and so, a triple bond is stronger than a double bond whichin turn is stronger than a single bond
Bond lengths and bond dissociation enthalpies of different bonds:


Different Structural Representations Of Organic Compounds
Lewis Structure Or electron dot Structure
In Lewis structure or electron dot structure, only the electrons of the valence shell of each atom of a molecule is H shown. For example, the electron dot structure of propene is shown at the right side. Since this style of writing is time-consuming and inconvenient, it is not generally followed

Dash formula
The Lewis structure, however, can be simplified by representing each pair ofelectrons involved in forming a covalent bond by a dash(—). In this representation, a single dash represents a single bond, a double dash (=) represents a double bond, and a triple dash (≡) represents a triple bond.
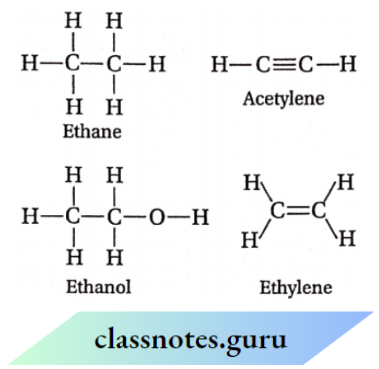
The lone pair of electrons on the heteroatoms e.g., oxygen, nitrogen, halogens, sulfur, etc. May or may not be shown. Thus, ethane, ethylene, acetylene and ethanol can be represented by their structural formulas as given above. Such structural representation is also called complete structural formula or graphic formula.
Condensed structural formula
The complete structural formulas can be further abbreviated by deleting some or all of the covalent bonds and by indicating the number of identical groups attached to an atom by a subscript. Such simplified structural representations of molecules are called condensed structural formulas. Thus, ethane, ethylene, acetylene and ethanol can be represented as: CH3CH3 (ethane); H2C — CH2 (ethylene); HC=CH (acetylene); CH3CH2OH (ethanol)
Similarly, a long chain of carbon atoms such as CH3CH2CH2CH2CH2COOH can be represented as CH3(CH2)4COOH.
Bond-line Structural formula
It is a very simple, short and convenient method of representing the structures of organic molecules. In this representation, only the carbon-carbon bonds are shown by lines drawn in a zig-zag fashion.
The line ends and the intersection of lines represent carbon atoms carrying an appropriate number of H -atoms so that its tetravalency is fulfilled (i’.e., the terminals denote CH3 -groups, and the unsubstituted intersections or line junctions denote CH2 -groups). The heteroatoms and the H-atoms attached to them are, however, specifically shown. A single bond is represented by a single line ( —), a double bond is represented by two parallel lines (= ) and a triple bond is represented by three parallel lines (≡).

In bond-line notation, the cyclic compounds are represented by an appropriate ring or polygon without showing carbon and hydrogen atoms. Each comer of a polygon represents a carbon atom while each side of the polygon represents a carbon-carbon bond.
For example:

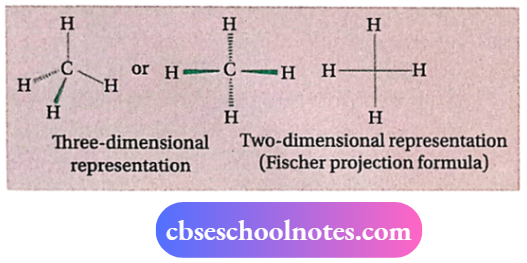
Three-dimensional representation of organic molecules
The three-dimensional structure of organic molecules is quite difficult to represent on paper (two-dimensional). So, certain graphic conventions have been proposed. For example, by using a solid wedge and dashed wedge the threedimensional structure of a molecule from a two-dimensional presentation can be perceived.
In these formulas, the solid wedge (i.e., the thick solid or heavy line) is used to indicate a bond lying above the plane ofthe paper and projecting towards the observer, and the dashed wedge is used to represent a bond lying below the plane ofthe paper and projecting away from the observer. The bonds lying in the plane of the paper are shown by normal or ordinary lines (—).
A three-dimensional representation of a methane molecule, for example, is shown below:

Organic Chemistry Techniques Class 11 Notes
Classification Of Organic Compounds Based On Carbon Skeleton
Due to the existence large number of organic compounds is rather inconvenient to study the chemical nature of these compounds individually. To simplify and systematize the study of organic chemistry, organic compounds have been broadly classified into two categories depending on the nature of their carbon skeleton or structures.
The flow sheet given here is the general classification of organic compounds:

Acyclic or open chain or aliphatic compounds:
The compounds containing open chains of carbon atoms with the appropriate number of H -atoms and functional groups are called acyclic or open-chain compounds. These compounds are also called aliphatic compounds because the earlier members of this class were obtained either from animal or vegetable fats (Greek, aliphatic = fat). Due to the variation in the structure of carbon chains, two types of open-chain compounds have been formed.
These are:
- Unbranched or straight-chain compounds and
- Branched chain compounds.
1. Straight chain compounds
The open chain or aliphatic compounds in which no carbon atom (except the two terminal C -atoms) is attached to more than two carbon atoms are called unbranched or straight chain compounds.
Examples:

2. Branched-chain compounds
The open-chain or aliphatic compounds in which at least one carbon atom is attached to three or more carbon atoms are called branched-chain compounds
Examples:

Cyclic or closed chain or ring compounds
Compounds containing one or more closed chains or rings of atoms in their molecules are called cyclic or closed chain or ring compounds. Depending upon the nature of the atoms present in the ring, the cyclic compounds may be divided into the following two classes
1. Carbocydic or homocyclic compounds:
The compounds containing rings that are made up ofonly carbon atoms are called carboxylic or homocyclic compounds.
These are further divided into two sub-classes as follows:
1. Alicyclic compounds:
The carboxylic compounds which show resemblance in properties with the aliphatic compounds are called alicyclic compounds
Examples:

2. Aromatic compounds: For aromatic compounds, see the aromatic hydrocarbon portion
2. Heterocyclic compounds:
Cyclic compounds containing more heteroatoms (atoms other than C and H i.e., O, N, S, etc.) in their rings are called heterocyclic compounds. Depending upon their chemical behaviour, they are further divided into the following two categories
1. Alicyclic heterocyclic compounds:
Aliphatic cyclic compounds containing one or more heteroatoms in their rings are known as alicyclic heterocyclic compounds
Examples:

2. Aromatic heterocyclic compounds:
Aromatic compounds containing one or more heteroatoms in their molecules are called aromatic heterocyclic compounds.
Examples:

Saturated and unsaturated compounds
1. Saturated compounds:
The organic compounds in which the carbon atoms are linked with each other only by single covalent bonds are called saturated compounds.
Examples: Methane (CH4), ethane (CH3—CH3), ethyl alcohol (CH3 —CH2 —OH), methylamine (CH3 —NH2) etc.
2. Unsaturated compounds:
The organic compounds which contain at least one carbon-carbon double bond or triple bond are called unsaturated compounds
Examples:
Ethylene (H2C= CH2), l-butene(C2H5CH= CH2)
1,3-butadiene (H2C=CH—CH=CH2) etc.
Organic compounds containing unsaturated groups such as are considered saturated compounds. -CHO, -COOH, -COOR etc. But no C =C or C = C For example, propionic acid (CH3CH2COOH) is a saturated compound but acrylic acid (CH2=CH—COOH) is an unsaturated compound.
Functional Groups & Homologous Series
Functional group Definition
A functional group may be defined as an atom or group of atoms present in an organic compound, which is responsible for its characteristic chemical properties.
Generally, compounds having the same functional group have similar properties while compounds with different functional groups have different chemical properties. For this reason, organic compounds are classified into different classes or families based on the functional groups present In the reactions of organic compounds, the organic groups or radicals generally do not suffer any change but the functional groups actively participate:

Some common functional groups present in various organic compounds along with their classes:
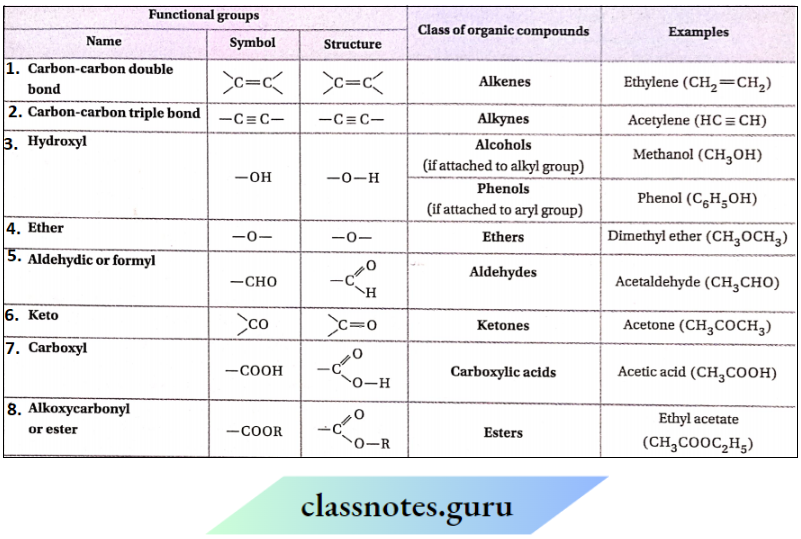

1. Though the functional groups govern the chemical properties, they are also found to influence the physical properties in some cases. For example, alcohols (ROH) due to the presence of —OH group remain associated through intermolecular H -bonding and as a result, the boiling points of alcohols are much higher than that of ethers having similar molecular masses.
2. Organic compounds with 2 or more different functional groups exhibit characteristic properties of all the functional groups present in it. For example, aldol [CH3CH(OH)CH2CHO] exhibits the properties of ; both alcohol and aldehyde.
3. Although different compounds having the same functional group show similarities in their chemical properties, their properties are not identical. For example, formaldehyde (HCHO) and acetaldehyde (CH3CHO) containing the same functional group ( —CHO), do not respond to the same type of reaction. The former participates in the Cannizzaro reaction but not in Aldol condensation reaction while the latter takes part in Aldol condensation reaction but the Cannizzaro reaction
Homologous series
Homologous series definition:
A homologous series is defined as a series or group of similarly constituted organic compounds which have the same functional group and thus similar chemical properties and any two successive members differ in their molecular formula by a CH2– group. Members of such a series are called homologues.
Some homologous series, their general formulas and structures of compounds upto C4

Characteristics of homologous series:
1. All the members of a homologous scries can be represented by the same general formula. For example, CnHn+1 OH is the general formula of alcohols.
2. Same functional group is present in ail members of any homologous series. So, the members of any homologous series have almost identical chemical properties (the phenomenon of such resemblance in properties among the compounds of the same homologous series is called homology). However, with an increase in molecular mass, the chemical reactivity ofthe compounds usually decreases.
3. Any two successive members of a particular series differ in molecular formulary CHHomologous series group or 14 mass units.
The physical properties such as density, melting point, and boiling point of the members of a homologous series increase gradually with the increase in molecular mass. However, solubility and volatility show a declining trend with a rise in molecular mass.
4. The members of a homologous series can be prepared by almost identical methods, known as the general methods of preparation.
Significance of homologous series:
- From the knowledge ofthe method of preparation and the properties of a particular member of a homologous series, the method of preparation and properties of the other members of the same series can easily be predicted.
- Therefore, by dividing the vast number of organic compounds into homologous series followed by the study of the method of preparation, properties, and reactions of a representative member, an overall idea about the whole family can be obtained.
- However, the first member of a series often differs from the other members in the method of preparation and properties.
- For example, the method of preparation and properties of formic acid (HCOOH), the first member ofthe carboxylic acid family, are different from that ofthe other members of the family.
NCERT Solutions Class 11 Chemistry Organic Chemistry Principles
IUPAC Nomenclature Of Aliphatic Organic Compounds
According to the IUPAC system, the name of an organic compound consists of 3 parts:
- Word root
- Suffix and
- Prefix.
1. Word root:
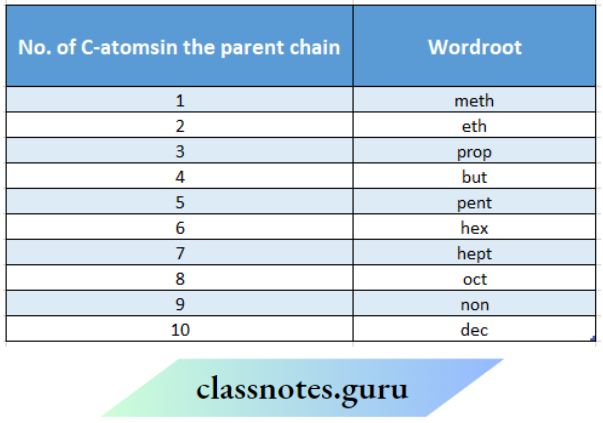
Word root, the basic unit of the name, denotes the number of carbon atoms present in the parent chain (the longest possible continuous chain of carbon atoms including the functional group and multiple bonds) of the organic molecule.
For chains containing up to four carbon atoms, special word roots (based upon the common names of alkanes) such as ‘meth’ for C1, ‘eth’ for C2, ‘prop’ for C3, and ‘but’ for C4 are used where C1, C2, C3 and C4 represent the number of carbon atoms in the chain. For chains of five or more carbon atoms, Greek numerals or number roots are used to represent the word roots.
For example:
‘Pent’ is used for the C5 chain, ‘hex’ is used for C6 chain etc. The general word root for any carbon chain is ‘alk’
2. Suffix
These are of the following two types
1. Primary suffix:
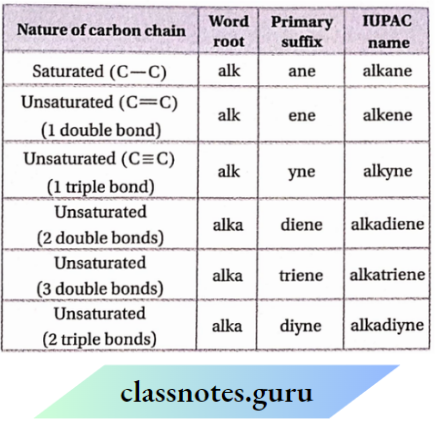
A primary suffix is always added to the word root to indicate whether the carbon chain is saturated or unsaturated. There are three basic primary suffixes. If the carbon atoms are linked only by single covalent bonds (C —C) ‘-ane’ is used.
If there is at least one double bond (C= C) present in the chain, the primary suffix ‘- ene’ and if there is at least one triple bond (C = C) in the chain, the primary suffix ‘-yne’ is used. Hence, the name of
- CH3CH2CH13 is prop + ane = propane and
- CH3CH=CH2 is prop + ene = propene.
If the parent carbon chain contains 2, 3, or more double or triple bonds, numerical suffixes such as ‘di’ (for two), ‘tri’ (for three), ‘tetra’ (for four), etc.
Are added to the primary suffix. If the primary suffix begins with a consonant then an extra ‘a’ is to be added to the word root. For example, the primary suffix used for two double bonds is diene. Now if it is added to the word root ‘but’ for C4 chain, then the name of the compound will be butadiene.
2. Secondary suffix:
A secondary suffix is used to indicate the functional group presents an organic molecule and is to be added to the primary suffix while writing the IUPAC name. Secondary suffixes of some important functional groups are listed:
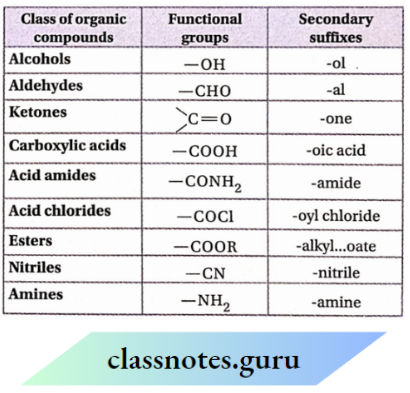
It is to be noted that while adding the secondary suffix to the primary suffix, terminal ‘e’ of the primary suffix [i.e., ‘ane,’ ‘ene’ or ‘yne’) is dropped if the secondary suffix begins with a vowel but is retained if the secondary suffix begins with a consonant.
For example: The name of CH3CH2OH is: eth + ane + ol = ethanol and that of CH3CH2CN is: prop + ane + nitrile = propanenitrile.

3. Prefix
It is a part of the IUPAC name of a compound that appears before the word root. Prefixes are of two types
1. Primaryprefix: A primary prefix is used to differentiate a cyclic compound from an acyclic compound
For Example:
In case of carbocyclic compounds, the primary prefix ‘cyclo’ is used just before the word root. For example, the cyclic hydrocarbon, maybe named as

2. Secondaryprefix:
In the IUPAC system of nomenclature, some groups are not considered functional groups or secondary suffixes. These are treated as substituents and are called secondary prefixes. These are added just before the word root (or the primary prefix in the case of alicyclic compounds) in alphabetical order.
The secondary prefixes of a few substituents are given in the following table:

Therefore, while writing the IUPAC name of an aliphatic organic compound the various parts are to be added in the following sequence: Secondary prefix + Primary prefix+ Wordroot+ Primary suffix + Secondary suffix. It is not necessary that all the parts may be present in a particular compound. However, the word root and primary suffix must be present.
Example:
In case ofthe compound, \(\stackrel{4}{\mathrm{C}} \mathrm{H}_3 \stackrel{3}{\mathrm{C}} \mathrm{HCl} \stackrel{2}{\mathrm{C}} \mathrm{H}_2 \stackrel{1}{\mathrm{C}} \mathrm{H}_2 \mathrm{OH}\), the word roots ‘but’, the primary suffix ‘ane’, the secondary suffix is ‘ol’ and the secondary prefix is ‘chloro’. As the compound is acyclic, the primary prefix is absent. Therefore, the IUPAC name of the compound is chloro (at position C-3 ) + but + an (e is omitted) + ol (at position C-l )- 3-chlorobutan-l-ol.
IUPAC Nomenclature of some organic compounds

Organic Chemistry Basic Principles Class 11 Summary
Common & IUPAC Nomenclature Of Some Important Classes Of Organic Compounds
Saturated hydrocarbons (Alkanes)
In the IUPAC system, saturated acyclic hydrocarbons are called alkanes. IUPAC names of alkanes are obtained by adding the suffix ‘ane’ to the word root indicating the number of C-atoms present in the chain. The first 4 alkanes (CH4 to C4H10) have their special names, i.e., methane, ethane, propane, and butane.
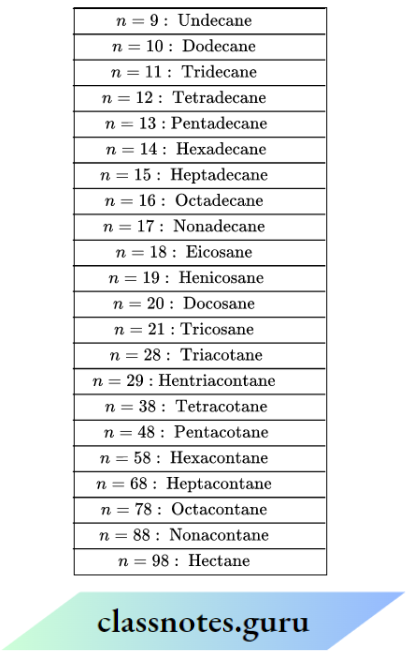
The names of alkanes containing 5 or more C-atoms are obtained by adding prefixes such as ‘pent’ (5), ‘hex’ (6), ‘hept’ (7), ‘Oct’ (8), etc., indicating the number of C-atoms in the molecule to the suffix ‘ane! Although common and IUPAC names of alkanes are the same, the prefix (normal)is added to the common name of alkanes containing 4 or more C-atoms.
IUPAC and common names of first ten members of the alkane family:

Names of some higher members of alkane family (CH3(CH2)n CH3 ,n= 9,10,11,….etc..are as follows:
Alkyl groups
An organic group produced by the removal of one H -atom from an alkane molecule is called an alkyl group or alkyl radical.
For example:
- Removal of one H-atom from methane (CH4) produces methyl group (-CH3),
- An ethyl group ( —C2H5) is formed by the removal of any one of six equivalent H -atoms from ethane (C2H6)

All the H -atoms of alkanes containing more than two carbon atoms are not always equivalent. So, two or more alkyl groups can be derived from these alkanes.
For example:
In the case of propane (CH3CH2CH3), the removal of one hydrogen atom attached to the terminal carbon yields an unbranched propyl group. But when one H -atom linked to the middle carbon is removed, an isopropyl group is obtained.

The alkyl groups are generally represented by the letter R. So, an alkane is represented as R —H. The monovalent alkyl groups have the general formula: CnH2n+1 [n = 1.2,3 etc.]
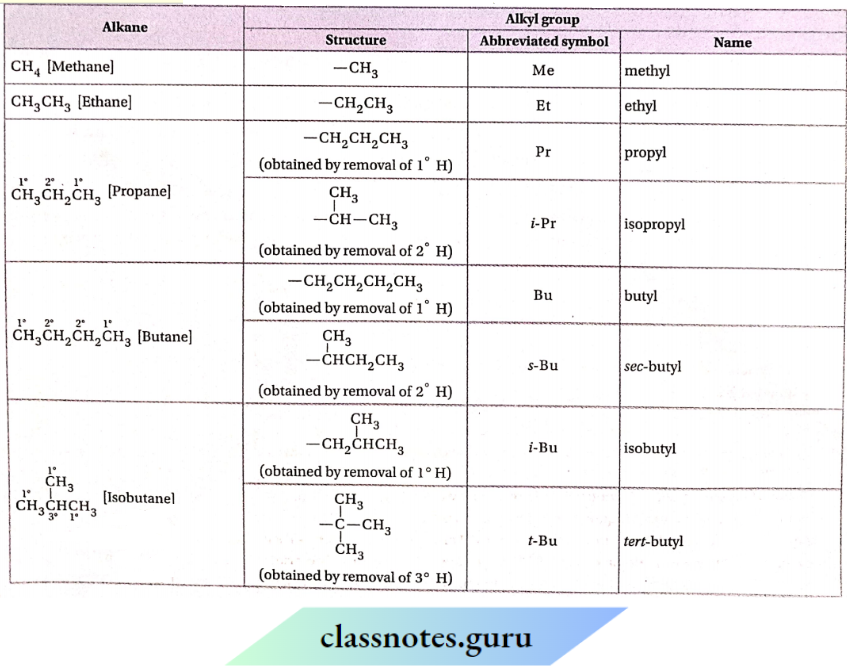
Nomenclature of alkyl groups
The names of the alkyl groups are derived by replacing the suffix ‘ane’ of the corresponding alkane by the suffix ‘yl’

Classification of alkyl groups
1. Primary (1°) alkyl groups:
The removal of one 1° H -atom from an alkane gives a primary or 1° alkyl group. Ethyl (CH3CH2-), isobutyl (Me2CHCH2—), neopentyl (Me3CCH2—), etc. Are some examples of primary alkyl groups. The primary alkyl groups obtained from simple straight-chain alkanes are called normal alkyl groups
In the common nomenclature system, those are designated as n -alkyl group Imt In the IUPAC system, n Is omitted
For example:
In the common or trivial system of nomenclature, CH,CH2CH2– IS written as n -propyl group hut In the IUPAC system, it Is designated as propyl group. The primary alkyl groups In which the second last carbon in the chain is branched to one
The group are named by using the prefix Mso’
For example:
The —CH2CH(CH3)3 group is called the isobutyl group. (As a group, up to isohexyl and as an alkane up to isohexane, the use ofthe ‘iso’ has been accepted by the IUPAC system).
The primary alkyl groups in which the second last carbon in the chain is branched to two —CH3 groups are named by using the prefix ‘nco’.
For example: The —CH2C(CH3 )3 group is called neopentyl group. (As a group, upto neo hexyl and as an alkane upto neohexane, the use of‘neo’ is accepted by die IUPAC system.)

2. Secondary (2°) alkyl group:
Removal of one 2° H-atom from an alkane forms a secondary (2°) alkyl group. In both trivial & IUPAC system,itis written as sec-alkyl (pronounced as secondary alkyl group),
For example: CH3 CH2 CHCH3 is a 2° alkyl group named as a sec-butyl group.
3. Tertiary (3°) alkyl group:
The removal of one 3° H -atom from an alkane leads to the formation of a tertiary or 3° alkyl group. In both the trivial and IUPAC systems, it is written as ferf-alkyl group or f-alkyl group (pronounced as tertiary alkyl group),
For example:—C(CH3 )3 is a tertiary alkyl group whose name is a fert-butyl group or a t-butyl group
Structures and IUPAC names of some alkyl groups

Monovalent radicals derived from unsaturated acyclic hydrocarbons end with ‘-enyl ‘-ynyl,’ ‘-dienyl,’ etc., depending on the nature of the radicals or groups. Positions of double and triple bonds are indicated by numerals where necessary.
The c-atom of any radical containing free valence is always numbered as ‘1’
For example:
CH ≡ C— (ethynyl), \(\stackrel{3}{\mathrm{C}} \mathrm{H} \equiv \stackrel{2}{\mathrm{C}} \stackrel{1}{\mathrm{C}} \mathrm{H}_2\) (prop-2-any)
⇒ \(\stackrel{3}{\mathrm{C}} \mathrm{H}_3 \stackrel{2}{\mathrm{C}} \mathrm{H}=\stackrel{1}{\mathrm{C}} \mathrm{H}-\) (prop-l-enyl) etc. The following trivial names are retained in the IUPAC system: CH2=CH— (vinyl),
CH2=CHCH2— (allyl), etc. The radical (CH2= ) is called ‘methylene’ and (=CH—)is called ‘methine.
The presence of one or more free valency in radicals derived from parent alkenes is indicated by suffixes like monovalent(-yl), divalent (-diyl), trivalent (-triyl) etc.
For example: CH3CH< is ethane-1, 1-diyl; (CH3)2C< is propane-2,2-diyl etc.
Aryl groups:
The organic groups derived from benzene and other benzene derivatives are termed as aryl groups. Aryl groups are generally represented by Ar. The simplest aryl group is phenyl group (—C6H5 ). It can be obtained by removing one hydrogen atom from a molecule of benzene (C6H6 )

Trivial or common system of nomenclature of other classes of compounds:




IUPAC nomenclature of different classes of compounds at a glance:
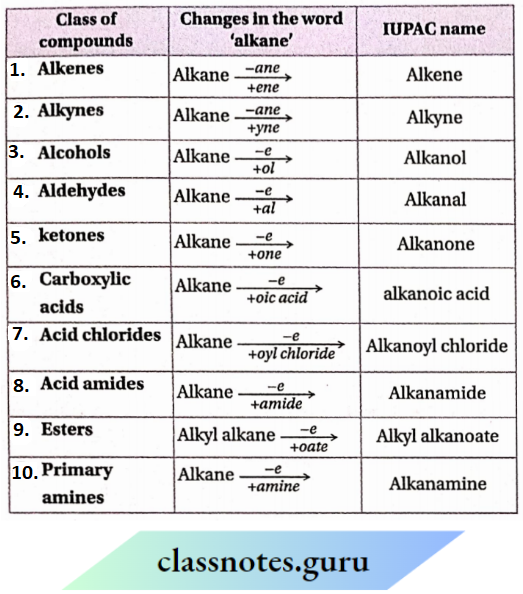
Class 11 Chemistry Organic Chemistry Techniques and Principles
Rules Branched For Iupac Nomenclature Of Chain Alkanes
Longest chain rule
The longest continuous chains of the alkane is to be identified first. It is known as the parent or root chain. The number of carbon atoms in the parent chain determines the word root
It is to be noted that the longest chain may or may not be straight but it must be continuous. All other carbon atoms that are not included in the parent chain are called branched chains side chains or substituents. The branched-chain alkane is, therefore, named as a derivative ofthe parent chain
Example:

The parent chain in the compound (I) contains 6 carbon atoms and the CH3 -group is a side chain or substituent. Therefore, it is to be named as a derivative of hexane. The parent chain in the compound (II) contains 8 C-atoms but is not straight so, it is named as a derivative of octane. CH3 -and C2H6 -groups are the two substituents here. If two chains of equal lengths are possible, then the one with a maximum number of side chains or substituents is to be considered as the parent chain.
Example:
In the compound (III), the parent chain is the horizontal six-carbon chain containing two alkyl substituents ( —CH3 and C2H5) but not the other six-carbon chain containing only one alkyl substituent [(CH3)2CH-]

Lowest number rule
The carbon atoms of the parent chain is to be numbered as 1, 2, 3, 4, . etc. from one end in such a way that the carbon atom carrying the substituent gets the lowest possible number.
Example:
In the following compound, the numbering can be done in two different ways. The numbering of the carbon chain as given in the structure (IVA) is correct because the carbon carrying the substituent gets a lower number i.e., 3. However, the numbering of the carbon chain as given in the structure (IVB) is incorrect because the carbon carrying the substituent gets a higher number i.e., 5.

The number indicating the position of the substituent in the parent chain is called its positional number or locant. Thus, the correct locant for the methyl side chain in the above compoundis 3.
When two substituents are present in the chain, then the lowest set of locant rule is applied. It states that when two or more different sets of locants are possible, then that set of locants will be the lowest which (when compared term by term with other sets, each in order of increasing magnitude) has the lowest term at the first point of difference. This rule is used irrespective ofthe nature ofthe substituent.
Example:
When the carbon atoms of the parent chain of the following compound (V) are numbered from the sides, two sets of locants are obtained. Out of the two sets of locants (2,3) and (3,4), the first set is lower and hence preferred because the first term, i.e., 2 in the first set is lower than the first term, i.e., 3 in the second set

Similarly, for the compound (6):

Out ofthe two sets of officiants (2,2,4) and (2,4,4), the first set is lower and hence preferred as the second term in the first set i.e.,2 is lower than the second term 4in the second set.
According to the latest IUPAC recommendations of nomenclature (1993), the lowest set of locant rules Is preferred even If it violates the lowest sum rule.
For example:
In the case of the following compound, the numbering of the carbon chain from left and right gives two different set of locants with two different sum of locants.

The numbering from the left is correct because the first term CH3 i.e., 2 in the set (2, 7, 8) is lower than the first term i.e., 3 in the set (3, 4, 9), even though the sum of locants is lower when the numbering is done from the right. Thus, the correct name ofthe alkanes 2, 7, 8-trimethyIdecane.
Name of the branched chain alkanes
When there is one alkyl group in the parent chain, its name is to be prefixed to the name of the parent alkane, and its position on the chain is to be indicated by writing before it the number of the carbon atom carrying the substituent.
The name of the substituent is separated from its positional number or locant by a hyphen (-). The final name of the alkane is to be written as one word, i.e., there will be no gap between the name of the substituent and the parent alkane.
Example:

Alphabetical order of the side chains or substituents
When two or more different alkyl groups (side chains or substituents) are present on the parent chain, such groups prefixed by their positional numbers or locants, are to be arranged in alphabetical order irrespective of their positional numbers and written before the name of the parent alkane.
Example:
In the given compound, between ethyl and methyl groups, ethyl comes first in the alphabetical order and therefore, its name is 3-ethyl-2-methylhexane. When a number appears between two substituent groups then hyphens are used on both sides ofthe number

It is to be noted that while deciding the alphabetical order of various alkyl groups, prefixes such as ‘iso’ or‘neo’ are to be considered as a part of the fundamental name of the alkyl group while the prefixes, ‘second ‘tert’ are not
Examples:

Numbering of different alkyl groups at equivalent positions
If two different alkyl groups or substituents are present at equivalent positions, i.e., at the same position from the two ends of the parent chain, then numbering of the chain is to be done in such a way that the alkyl group which comes first in the alphabetical order gets the lower number.
Example:

Naming of same alkyl groups at different positions
When the parent chain contains two or more same alkyl groups at different positions (or at the same position), the positional number of each alkyl group is separated by commas, and suitable prefixes such as ‘di’ (for two), ‘tri’ (for three), ‘tetra’(for four) etc., and then they are to be attached to the name of the alkyl group. When two alkyl groups are attached to the same carbon atom, the positional number or locant is to be written twice. It is to be noted that the prefixes like ‘di’, ‘tri’, ‘tetra’, etc. are not considered while deciding the alphabetical order ofthe alkyl groups

Naming of complex substituents/substituted substituents
1. If the alkyl group on the parent chain is complex, i.e., if it has a branched chain, it is named as a substituted alkyl group by numbering the C-atom of this group attached to the parent chain as 1. The name of the complex substituent is generally enclosed in brackets to avoid any confusion with the numbering of the parent chain.

2. When the same complex substituent occurs more than once on the parent chain, it is indicated by multiplying the prefixes such as “bis’ (for two), ‘tris’ (for three), ‘tetrakis’ (for four), ‘pentakis'(for five), etc
Example:

IUPAC nomenclature of bicyclic compounds
The name of a bicyclic compound in the IUPAC system is based on the name ofthe alkane having the same number of carbons as there in the ring system. The name follows the prefix bicyclo and a set of brackets enclosing numbers indicating the number of carbons in each of the three bridges connecting the bridgehead carbons in order of decreasing size.
For example:
The following bicyclic compounds containing nine and eight carbon atoms are named bicyclo [4.3.0]nonane and bicyclo[3.2.1]octane respectively.

What is wrong with the following names? Draw the structures they represent and write their correct names. (i)1,1-dimethylhexane (iii)3-methyl-5-ethylheptane (iv) 4, A-dimethyl-3-ethylpentane (v) 3, 4,7-trimethyloctane (vi) 3,3-diethyl-2,A,Atrimethylpentane 36. Give the IUPAC name of the following alkane containing complex substituents:
Rules For Iupac Nomenclature Of Unsaturated Hydrocarbons
For naming the compounds containing multiple (double and triple) bonds, the following additional rules are to be applied:
1. The parent chain must contain multiple bonds (double or triple) regardless of the fact whether it denotes the longest continuous chain or not
For example:
In compound (I), the parent chain contains 5 carbon atoms and not 6 carbon atoms since the latter does not include the double bond
Example:

2. While naming a particular member ofthe alkene or alkyne family, the primary suffix ‘ane’ of the corresponding alkanes to be replaced by ‘ene’ or ‘yne’ respectively.
3. The numbering of the parent chain is to be done in such a way that the first C-atom associated with the multiple bond gets the lowest possible number
Examples:
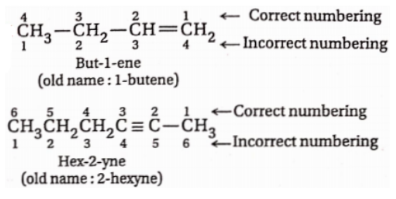
4. If the parent chain contains a side chain, then also the multiple bonds get priority in numbering.
If the parent chain contains 2 or 3 double or triple bonds, then the primary suffix ‘diene’ (or ‘triene’) or ‘diyne’ (or ‘triyne’) are to be used to represent them. In these cases, terminal ‘a’ is also added to the wordroot For numbering, the lowest set oflocants rule is to be followed.
Examples:

5. If the parent chain contains both double and triple bonds, the following points are to be remembered while writing their names:
The unsaturated hydrocarbon is always named as a derivative of alkyne, i.e., the primary suffix ‘ene’ is always to be written before ‘yne’.
In all these cases, the terminal ‘e’ of the one is dropped. The numbering of the parent chain is to be done from that end which is nearer to the double or triple bond, i.e., the lowest set officiants rule is to be followed
Examples:

If the positions of double and triple bonds are identical, i.e., if the set officiants from both sides ofthe chain is the same, then the double bond is always given preference over the triple bond
Example:

6. If the unsaturated hydrocarbon contains a side chain along with double and triple bonds, then the numbering of the parent chain is to be done in such a way that the multiple bonds get the lowest set of locants. However, if the numbering from both ends of the parent chain gives the same set of locants to the multiple bonds, then the locant for the side chain must be minimum.
Examples:

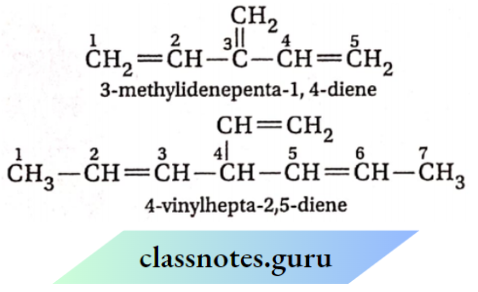
When there are more than two double bonds in the hydrocarbon and it is impossible to include all of the min the parent chain, then the double bond which is not included in the parent chain is treated as a substituent
NCERT Class 11 Chemistry Organic Chemistry Techniques Notes
IUPAC Nomenclature Of Compounds With Functional Groups, Multiple Bonds And Substituents
The following additional rules are to be followed while naming organic compounds containing one functional group, double and triple bonds and substituents:
1. In these compounds, the longest chain of carbon atoms containing the functional group and the maximum number ofdouble and triple bonds are to be considered as the parent chain.It may or maynot be the longest possible carbon chain.
For example:
In the following compound (I), the parent chain containing the functional group and the double bond has 6 carbon atoms while the longest possible carbon chain has 7 carbon atoms.
Example:

2. The parent chain is to be numbered in such a way that the functional group gets the lowest locant, even if it violets the lowest set oflocants rule for substituents. For example, in the following compound, the lowest locant for the functional group >C= O is 3 and not 5
Example:

3. If the organic compound contains a terminal functional group such as —CHO, —COOH, —COCl, —CONH2, —COOR, —C =N, etc., The numbering of the parent chain must be started from the functional group, we., it is always given number but the number is usually omitted from the final name ofthe compound
Example:

4. If a compound contains two or more similar functional groups, numerical prefixes [di, tri, tetra etc.) are used to indicate their numbers, and the terminal ‘e’ of the primary suffix (ane, ene or yne) is retained while writing the name.
Examples:

5. If an organic compound contains more than two similar terminal functional groups and all of them are directly attached to the parent chain, then none of them are considered as a part of the parent chain. Special suffixes such as carboxylic acid (for —COOH), carbaldehyde (for—CHO), carbonitrile (for —C=N), carboxamide (for —CONH2) etc. are used to name these
Examples:

IUPAC Nomenclature Of Compounds With Pentane-2,4-Dione Two Different Functional Groups
When an organic compound contains two or more different functional groups, then one of the functional groups is to be selected as the principal functional group while all other functional groups (also called secondary 7 functional groups) are to be treated as substituents. The choice of principal group is made on the basis ofthe following order of preference:
—COOH>—SO3H >— COOCO — >— COX (X=halogen) >—CONH2> —C≡ N>—CHO>C= O> —OH> —SH>—NH2
All the remaining groups such as halo (fluoro, chiro, bromo, and iodo), nitroso (—NO), nitro (—NO2), alkoxy (—OR), alkyl (—R), aryl {Example: C6H3), etc., are always treated as substituents or simply as prefixes.
Suffixes and prefixes of some Important functional groups [with decreasing priority

Polyfunctional compounds are named as follows:
- The chain containing the principal functional group, the maximum number of secondary functional groups, and multiple (double or triple) bonds, if any, is to be considered as the principal chain in the compound.
- The principal chain is to be numbered in such a way that the principal functional group gets the lowest possible number followed by double bond, triple bond, and substituents.
- The prefixes for the secondary functional groups and other substituents are to be placed in alphabetical order before the word root. If two or more identical secondary functional groups are present, these are to be indicated by using di, tri, tetra, etc. as prefixes
- The principal functional group is to be written after the word root and the compound is to be named as a member of that particular class of compound.
Examples of some functional compounds:

IUPAC nomenclature of other classes of compounds (According to 1993 Recommendations)




Structural Formulas Of Organic Compounds From Their Iupac Names
- To write the structure of an organic compound if its IUPAC name is given, the given steps are to be foin (parent chain) is to be selected from the word root ofthe IUPAC name ofthe compound
- The parent chain is to be numbered from either end.
- If the name of the compound contains primary suffix ‘ene’ or ‘yne\itis placed at the indicatedposition along the chain. Iv] Name and position of the functional group (secondary suffix) is to be identified from the IUPAC name andit is to be placed atits rightpositionin the carbon chain.
- The names and positions of other prefixes, if any, are to be identified from the IUPAC name and to be attached at proper positionin the carbon chain.
- Finally, the required number ofH- atoms are added, wherever
necessary, to satisfy tetra covalency of each C-atom
Examples 1: Letus write the structural formula of 5-hydroxy- 2-methylhex-3-enoic acid.
Step 1: The word ‘hex’ indicates that the parent chain contains 6 carbon atoms: C —C —C—C —C—C
Step 2: Numbering ofthe carbon chain 6 5 is done as indicated: \(\stackrel{6}{\mathrm{C}}-\stackrel{5}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}}\)
Step 3: TheIUPACname of the compound has the primary C suffix ‘ene’ at position 3. Therefore, C-3 and C-4 ofthe parent chain are linked by a double bond. \(\stackrel{6}{\mathrm{C}}-\stackrel{5}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}=\stackrel{3}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}}\)
Step 4: The secondary suffixes ‘oic acid’. Therefore, the carbon atom of the —COOH group is indicated as 1. \(\stackrel{6}{\mathrm{C}}-\stackrel{5}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}=\stackrel{3}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}} \mathrm{OOH}\)
Step 5: The prefixes ‘hydroxy’ and ‘methyl’ are attached at the positions 5 and 2 respectively

Step 6: A required number of H- atoms are added to various carbon atoms to get the final structure ofthe compound.

2. IUPAC name: But-2-en-l-o
Step 1: C —C —C—C —C—C
Step 2: \(\stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}}\)
Step 3: \(\stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}=\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}}\)
Step 4: \(\stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}=\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}}-\mathrm{OH}\)
Step 5: \(\mathrm{H}_3 \stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}} \mathrm{H}=\stackrel{2}{\mathrm{C}} \mathrm{H}-\stackrel{1}{\mathrm{C}} \mathrm{H}_2 \mathrm{OH}\)
3. UPAC name: 3-amino-4-methylpentanoic acid
Step 1: C —C —C—C —C—C
Step 2: \(\stackrel{5}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}}\)
Step 3: \(\stackrel{5}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}} \mathrm{OOH}\)
Step 4:

Step 5:

4. IUPAC name: 3-ethyl-4, 5-dimethyl hex-l-yn-3-o
Step 1: C —C —C—C —C—C
Step 2: \(\stackrel{1}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}-\stackrel{5}{\mathrm{C}}-\stackrel{6}{\mathrm{C}}\)
Step 3: \(\stackrel{1}{\mathrm{C}} \equiv \stackrel{2}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}-\stackrel{5}{\mathrm{C}}-\stackrel{6}{\mathrm{C}}\)
Step 4:

Step 5:

Step 6:

Class 11 Chemistry Organic Chemistry Basic Principles And Techniques Organic Chemistry Isomerism And Organic Reaction Mechanism Introduction
Organic compounds tend to exist as isomers. There are two or more compounds which have the same molecular formula (or molecular mass) but different physical and chemical properties, i.e., these are isomers of each other. This phenomenon is known as isomerism.
Again, organic compounds being covalent normally participate in molecular reactions. The mechanism of a reaction is the path followed by the substrate and reagent (the reacting species) while forming the products and in fact, it explains how the bonds in the reacting molecules break and new bonds in the product molecules are formed. To understand the mechanisms of organic reactions, some fundamental concepts are to be conceived. In this chapter, the isomerism in organic compounds and some basic concepts of organic reaction mechanisms have been discussed.
Isomerism In Organic Compounds
The property of isomerism in organic compounds is due to different sequence of bonding of their atoms or due to different arrangements of their atoms or groups in space when the sequence of bonding is same
Isomerism in organic compounds Definition:
The phenomenon of the existence of two or more compounds possessing the same molecular formula but different physical and chemical properties is known as isomerism. Such compounds are called isomers

Example:
Two compounds having the same molecular formula, C2H5O but with completely different physical and chemical properties are found to exist. One compound is a liquid which boils at 78°C and reacts with metallic sodium to liberate hydrogen gas and the other compound is a gas which boils at -24°C and does not react with sodium. Therefore, these two compounds are entirely different in nature.
The first compound is ethyl alcohol belonging to the class of compounds known as alcohols and the second compound is dimethyl ether belonging to the class of compounds known as ethers. Because of the difference in their structures, they are completely different in their properties.
These two compounds are, therefore, isomers and the phenomenon of the existence of these two compounds of identical molecular formulas but belonging to different families is known as isomerism
CH3— CH2— OH( Ethyl alcohol)
CH3— O—CH3( Dimethyl Ether)
Structural Isomerism
The compounds having the same molecular formula but different structures or molecular constitutions, i.e., different atom-to-atom bonding sequences or atomic connectivity are called structural or constitutional isomers and the phenomenon Is known as structural isomerism. Structural isomerism can be further subdivided into five different categories. Besides, tautomerism is also considered structural Isomerism.
1. Chain Isomerism
Chain Isomerism Definition:
Two or more compounds (belonging to the same family) having the same molecular formula but different carbon skeletons are called chain or nuclear isomers and the phenomenon Is called chain or nuclear isomerism
Example:
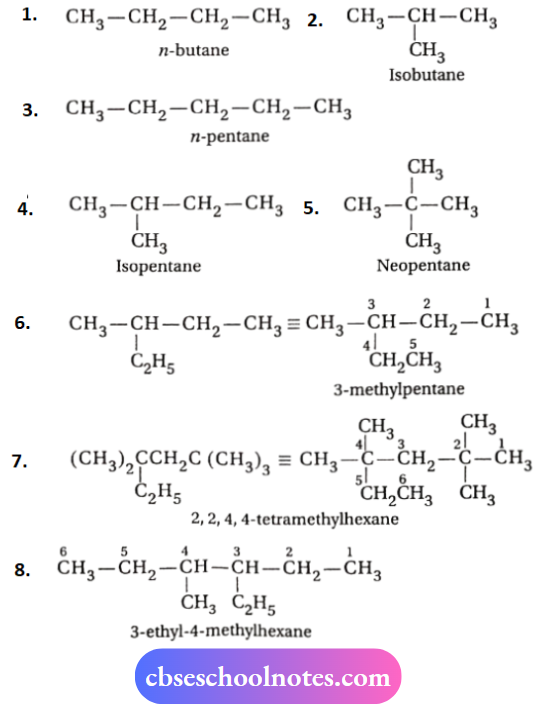
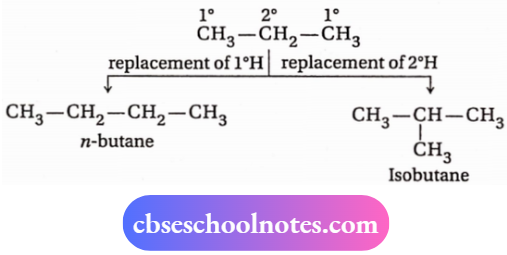
1. n -butane and isobutane are two chain isomers because they have die same molecular formula (C4H10) but differ in their carbon skeletons.

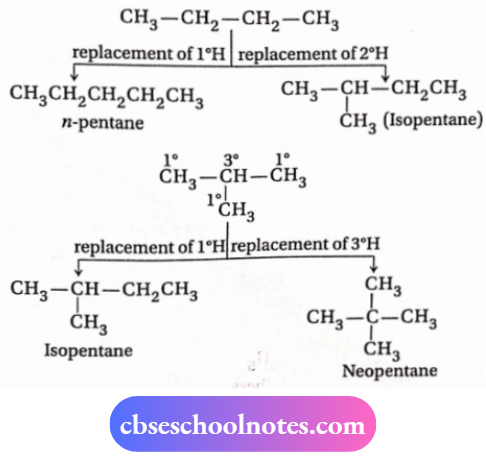
2. n-pentane, isopentane and neopentane are the three yJJJj function, group isomerism Chain isomers because they (molecular formula C5H12) differ in their carbon skeleton

2. Position isomerism
Position isomerism Definition:
When two or more compounds having the same structure of the carbon chain, i.e., the same carbon skeleton, differ in the position of substituent, multiple (double or triple) bond or functional group, these are called position isomers and this phenomenon is called position isomerism
Examples:
1. n -propyl alcohol and isopropyl alcohol are two position isomers. They have the same molecular formula (C3H8O) and have identical carbon skeletons. But in n propyl alcohol, hydroxy (-OH) group is at the terminal C-atom of the chain while in isopropyl alcohol the hydroxy (-OH) group is attached to the middle C -atom.

3. Functional group isomerism
Functional, group isomerism Definition:
Two or more compounds having the same molecular formula but different functional groups [i.e., belonging to different families) are called functional isomers and this phenomenon is called functional group isomerism or functional isomerism
1. Alcohols & ethers (CnH2n+ 2O) exhibit functional group isomerism. Ethyl alcohol and dimethyl ether having the same molecular formula, C3H6O represent two functional isomers. The functional group of ether is divalent oxygen (— O —) while the functional group of alcohol is the alcoholic hydroxy (— OH) group.

2. Aldehydes, ketones, unsaturated alcohols and unsaturated ethers exhibit functional group isomerism. The molecular formula, C3H6O for example, represents the following two functional isomers:

3. Carboxylic acid and esters (CnH2nO2) exhibit functional group isomerism. For example, the molecular formula C2H4O2 represents the following two functional isomers
CH -COOH (Acetic acid) (Func. gr: —COOH)
H – COOCH(Methyl formate)( Func. gr:—COOCH3)
4. 1°, 2° and 3° amines (CnH3nN) exhibit functional group isomerism. The molecular formula C3H9N, for example, represents
The following three functional isomers:
CH3CH2CH2NH2 Propan-l-amine (1° amine)
CH3CH2NHCH3 N-methylhexanamine (2° amine)
(CH3)3N [N,N-dimethylethanolamine (3° amine)]
5. Aromatic alcohols, phenols and ethers exhibit functional group isomerism. For example, the molecular formula, C7H8O represents the following three functional isomers,

6. Dienes, alienes & alkynes (CnH2n-2) exhibit functional group isomerism. The molecular formula C5H8, for example, represents four functional group isomers

7. Cyanides & isocyanides (CnH2n-1N) exhibit functional group isomerism. The molecular formula C3H5N, for example, represents two functional group isomers:
CH3CH2CN – Propanenitrile
CH3CH2NC – Ethyl isocyanide
5. Metamerism
Metamerism Definition:
When two ‘or more compounds having the same molecular formula but different numbers of carbon atoms (or alkyl groups) on either side of the functional groups such as —O —S—, — NH—, —CO — etc. are called metamers and the phenomenon is called metamerism. Metamerism occurs among the members of the same homologous series
Examples: The molecular formula, C4H10O represents the following metamers of ether family:

Examples of some metamers:

6. Ring-chain isomerism
Ring-chain isomerism Definition:
Compounds having the same molecular formula but possessing open-chain and closed-chain structures are called ringchain isomers and the phenomenon is called ring-chain isomerism.
Examples:
1. Propene and cyclopropane are ring-chain isomers with the molecular formula C3H6

2. Propyne and cyclopropene are ring-chain isomers with the molecular formula

7. Tautomerism
This is a special kind of functional group isomerism involving dynamic equilibrium between the isomers.
Tautomerism Definition:
The functional group isomerism which arises due to the reversible transfer of a group or atom from one polyvalent atom to the other within the same molecule witth necessary rearrangement of linkages and the resulting isomers exist in dynamic equilibrium with each other is called tautomerism. The interconvertible isomers are called tautomers or tautomerides. Tautomerism is also called desmotropism
Conditions for tautomerism:
- There must be at least one or H -atom present concerning each functional group in the compound.
- The compound must contain an electronegative atom bonded by a double or triple bond e.g., C=O, N=O, C=NH etc.
Keto-enol tautomerism:
In this type of tautomerism, one form is the keto-form containing a keto group >C=O ) while the other form is the enol-form containing an enolic group >C=C—OH). The term ‘enol’ comes from ‘ene’ of the double bond and ‘ol’ of the hydroxy (—OH) group. Ketoenol tautomerism is possible only for those carbonyl compounds which contain at least one a -H -atom
Example:
Ethyl acetoacetate is an important example of this type. Tautomeric equilibrium generally (if no other factors ! operate) favours the structure in which the H-atom is bonded to the C -atom rather than the more electronegative O -atom, i.e., equilibrium favours the weaker acid. However, in this case, because of conjugation and intramolecular H-bonding the percentage of enol-form is much higher (8%) as compared ! to that of acetone where no such factors operate

Keto and enol-forms are, in reality, two dynamic structural isomers. These isomers are always interconvertible and . one H-atom is shifted from one C-atom to an O-atom and vice-versa to establish the equilibrium.
Factors affecting the percentage of enol content:
- Intramolecular H-bonding (chelation) stabilises the enol and thus increases the amount of enol-form.
- Conjugation resulting in resonance stability of the enol-form helps to increase the enol content.
- Polar protic solvents usually decrease the percentage of enol form because the more polar keto-form becomes relatively more stabilised in this medium
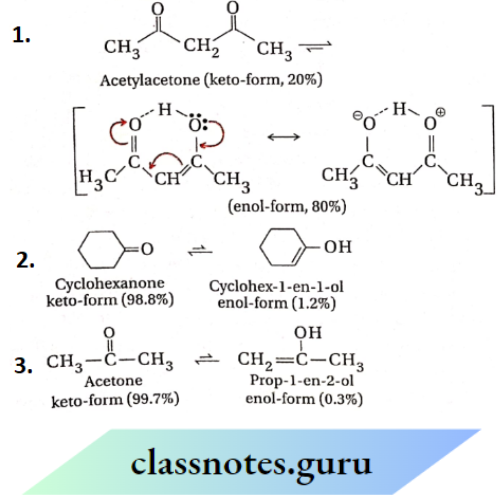
The percentage of the enol-form is greater because of its much higher stability caused by strong intramolecular H-bonding and effective resonance.
1. The following compounds do not exhibit keto-enol tautomerism due to lack of a -H-atom

2. Keto-enol actually involves interconversion of  group and a tautomerism —C=C(OH) group.
group and a tautomerism —C=C(OH) group.

The sum of bond energies of the is 347.9 kcal-mol-1 and that of the —C=C(OH) group is 336.0 kcal. mol-1 . So, the keto form is thermodynamically more stable than the enol form in the absence of other factors which can stabilise the enol form.
3. Pseudotropism: Tautomerism in which there is practically; no existence of one tautomer is called pseudotropism. For example

Nitro-acinitro tautomerism:

Nitroso-oximino tautomerism:
CH3—CH2—N Nitrosoetane(nitroso form) ⇌ CH3 —CH—N — OH(Ethanal oxime (oximino form)
Organic Chemistry Techniques for Class 11 Chemistry
Stereoisomerism
Stereoisomerism Definition:
The isomers having the same structure formula, i.e., er same atom-to-atom bonding sequence or connectivity which differ in the relative arrangement of atoms or groups in space are called stereoisomers and the phenomenon is called stereoisomerism. It is the specific directional property of covalent bonds in threedimensional space which gives rise to stereoisomerism.
Stereoisomerism is broadly classified into two categories:
- Configurational isomerism
- Conformational isomerism.
The isomerism that arises due to different spatial arrangements of groups or atoms (which are not interconvertible) in the same structural isomer is called configurational isomerism.
Configurational isomerism can be subdivided into two types:
- Optical isomerism and
- Geometrical isomerism.
1. Optical isomerism
Optical isomerism Definition:
If two molecules having the same atom-to-atom bonding sequence or connectivity (i.e., the same constitution) are mirror images of each other and are non-superimposable, then these are called enantiomers. Enantiomers rotate the plane polarised light to an equal degree but in opposite directions. Because of their effect on plane polarised light, separate enantiomers are said to be optically active and because of this property, they are called optical isomers and the phenomenon is called optical isomerism
Example:
As shown in the figure, lactic acid or 2-hydroxypropanoic acid (CH3CHOHCOOH) exists as a pair of enantiomers. In (-)-lactic acid, the sequence of occurrence of the groups: OH->COOH→CH3 appears in a clockwise direction but in (+)-lactic acid, it appears in an anticlockwise direction when viewed along the C — Ii bond axis from the side opposite to that of the H-atom. Both the enantiomers rotate the plane of plane polarised light to the same extent but in opposite directions. Enantiomer which rotates the plane of polarised light towards the right is called dextrorotatory or d- or (+)-lactic acid and which rotates the plane of polarised light towards the left is called laevorotatory or l- or (—)-lactic acid

Compounds which can rotate the plane of plane polarised light are called optically active compounds and those which cannot are called optically inactive compounds. Both the enantiomers of lactic acid are optically active.
Symmetric and asymmetric molecules:
If a molecule has at least one of the following elements of symmetry:
- Plane of symmetry,
- Centre of symmetry
- Alternating axis of symmetry
Then it is a symmetric or achiral molecule. Symmetric molecules are optically inactive. However, if a molecule has none of these symmetry elements, it is called an asymmetric or chiral molecule. Asymmetric molecules are optically active.
A symmetric molecule is superimposable on its mirror image while an asymmetric molecule is not. Therefore, whether a molecule is symmetric or asymmetric may also be verified by constructing models of the molecule & its mirror image and then placing one model on the other. If they are found to be superimposable, then the molecule must be symmetric; if not, then the molecule is asymmetric.
Example:
The two mirror-image forms of lead-acid are asymmetric and so, they are optically active. In our daily lives, we come across many things which are related to mirror images and do not get superimposed,
For example: Our left hand is the mirror image of our right hand but the left hand and the right hand are non-superimposable. So, the glove of the left hand do not fit in the right hand
Plane of symmetry or Sigma plane (σ ):
The plane of symmetry (σ) is defined as an imaginary plane that bisects a molecule in such a way that one half of the molecule is the mirror image of the other half (the plane acting as a mirror). The plane is also called a mirror plane.

Example: Meso-tartaric acid has a plane of symmetry.
Centre of symmetry or Centre of inversion (i):
A centre of symmetry is a point from which lines, when drawn on one side and produced an equal distance on the other side, will meet identical points (i.e., atoms) in the molecule

Asymmetric carbon atom and optical activity:
When a carbon atom of an organic molecule is attached to four different atoms or groups (Cabde), then that carbon atom is called an asymmetric carbon atom or chiral carbon. It is also called a stereogenic centre. Molecules containing only one asymmetric carbon atom are asymmetric and optically active. If a molecule contains more than one asymmetric carbon atom, it may be asymmetric or symmetric, i.e., it may be optically active or inactive.
Example:
(+) tartaric acid having two asymmetric C-atoms is optically active but meso-tartaric acid is optically inactive.

The presence of asymmetric carbon atoms is not essential for exhibiting optical activity:
Some of the substituted allenes (abC=C=Cab) are found to be optically active, even though they contain no asymmetric carbon atom. The reason for their optical activity is the overall asymmetry of their molecules.
Example: The two mirror images of 1,3-dichloro propadiene are overall asymmetric and so, they are optically active

Therefore, the presence of asymmetric C-atom in a molecule is not essential for exhibiting optical activity. If the structure is overall asymmetric, then the molecule will be optically active.
Conditions necessary for optical activity:
- The compound is non-superimposable on its mirror image, or
- It contains only one asymmetric C-atom or
- Plane, centre and alternating axis of symmetry are absent in the molecule.
E. Eliel in his book, has mentioned a molecule which does not contain any element of symmetry, yet it is not optically active. He further stated that principally possibility of the existence of such molecules cannot be discarded outright. Hence, the necessary and sufficient condition for the optical activity of any compound is the non-superimposability ofthe molecule on its mirror image
- Meso-compounds: If a compound having more than one chiral centre is found to be optically inactive, then it is called a meso-compound. Forexample, meso-tartaric acid.
- Enantiomers: Stereoisomers that are not superimposable on each other but related to each other as mirror images are called enantiomers. Thus (+) & (-)-lactic acid form a pair of enantiomers. Similarly, stereoisomers I & II of 3- bromopentan-2- ol form a pair of enantiomers. Enantiomers are optically active molecules having equal but opposite specific rotations. All other physical and chemical properties of enantiomers are the same.
- Diastereoisomers: Stereoisomers that are not mirror images of each other are called diastereoisomers. Stereoisomers I & III of 3-bromopentan-2-ol are diastereoisomers of each other
Calculation of no. of stereoisomers:
No. of stereoisomers, both optically active and inactive (meso-form) can be obtained from the number of chiral centres present in the molecule

Examples:
1. 3-bromopentan-2-ol (CH3CHOHCHBrCH2CH3) has two dissimilar chiral centres. Therefore, it has 2² = 4 optically active stereoisomers and no optically inactive meso-isomer. Fischer projections of these four stereoisomers are shown below

(I, II) and (III, IV) represent two pairs of enantiomers and they are optically active. Each of (I, III), (I, IV), (II, III), and (II, IV) represents a pair of diastereoisomers.
2. 2,3-dibromobutane (CH3CHBrCHBrCH3) has 2 similar chiral centers and can be divided into two mirror-image halves. Therefore, it can have 2(2-1)_ = 21 = 2 optically active isomers and 2(2-2)/2_ = 20 = 1 optically inactive meso-isomer. Fischer projections of these 3 stereoisomers are shown below

V and VI represent a pair of enantiomers and are optically active. VII represents an optically inactive meso-isomer having a plane of symmetry. Each of (V, VII) and (VI, VII) represents a pair of diastereoisomers.
3.  or 3-bromopentane- 2,4-diol has three chiral centres and can be divided into two mirror-image halves by passing a plane through the central carbon atom. Therefore, it can have 2(3-1)-2(3-1)//2 = 4-2 = 2 optically active isomers and 2(3-1)/2 = 21 = 2 optically inactive mesoisomers.
or 3-bromopentane- 2,4-diol has three chiral centres and can be divided into two mirror-image halves by passing a plane through the central carbon atom. Therefore, it can have 2(3-1)-2(3-1)//2 = 4-2 = 2 optically active isomers and 2(3-1)/2 = 21 = 2 optically inactive mesoisomers.
Fischer projections of these 4 stereoisomers are as follows:

IX and X represent a pair of enantiomers and are optically active. XI and XII represent two optically inactive meso-isomers and both of them have a plane ofsymmetry (cr -plane). Each of (DC, XI), (X, XI), (IX, XII), (X, XII) and (XI, XII) represents a pair of diastereoisomers.
Fischer projection formula:
Fischer developed a twodimensional plane projection formula for three-dimensional molecules. Fischer projection uses a cross to represent the stereocentre and the four bonds attached to it Stereocentre lies at the centre of the cross but is not explicitly shown. Horizontal bonds point towards the observer (i.e:, bonds inclined upwards), while the verticle bonds are directed away from the observer (i.e., bonds are inclined downwards). As per the IUPAC convention, the number-1 carbon atom is placed at the top of the vertical line. For example,

Racemic modification:
The racemic modification is an equimolecular mixture of a pair of enantiomers. The racemic modification is optically inactive due to external compensation, i.e., optical rotation caused by one enantiomer is compensated by the opposite rotation produced by the other. A racemic modification is indicated by writing (d, l ) or (±) before the name of the compound.
Example: (±)-or(d, l )-2-hydroxypropanoic acid or lactic acid. R/S nomenclature of optical isomers:
2. Geometrical or cis-trans isomerism
Geometrical Definition:
Isomers which have the same structural formula, i.e., the same atom-to-atom bonding sequence or connectivity but have different spatial arrangements of atoms or groups around the double bond or a ring system are called geometrical isomers and the phenomenon is called geometrical isomerism.
A π-bond prevents free rotation of the carbon atoms of a double bond concerning each other. Due to this restricted rotation, the relative positions of the atoms or groups attached to the doubly bonded carbon atoms get fixed. As a result of this, many substituted alkenes can exist in two distinctly isomeric forms which differ from each other only in the relative positions of the atoms or groups in space around the double bond
The isomer in which the similar atoms or groups lie on the same side of the double bond or a ring system is called the cis-isomer while the isomer in which the similar atoms or groups lie on the opposite sides of the double bond or a ring system is called the trans-isomer. Because of this, geometrical isomerism is also called cis-trans isomerism
The two geometrical or cis-trans isomers are stereoisomers which are not mirror images of each other. Therefore, these are diastereoisomers of each other.
E/Z nomenclature of geometrical isomers:
In this system of nomenclature, each ofthe two atoms or groups attached to each doubly bonded carbon atom are assigned according to their priority based on Cahn-Ingold-Prelog rules or simply CIP rules. If the atoms or groups of higher priority are on the same side of the double bond, the isomer is designated as Z (Zusammen in German means together) and if the groups or atoms of higher priority are on the opposite sides ofthe double bond, the isomer is designated as E (Entgegen in German means opposite).
Example: In l-bromo-2-chloropropene, for C l, Br > H (in priority) and for C -2, Cl > CH3 (in priority).

Geometrical isomers of three types of olefins:

Like the compounds containing a C-C double bond, compounds containing
1. Carbon-Nitrogen double bond (C= N)
Example: Oxime, Hydrazone etc.],
2. Nitrogen-Nitrogen double bond (N=N)
Example: azo, diazo compounds etc.] and some alicyclic compounds exhibit cis-trans or geometrical isomerism

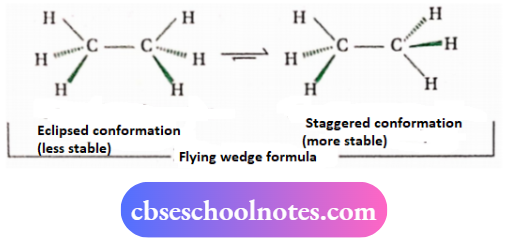
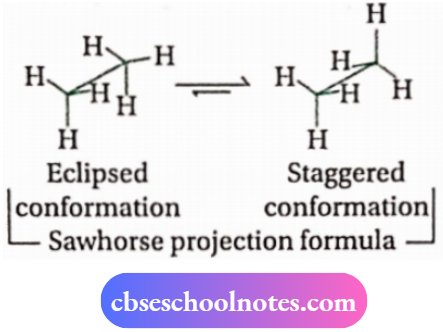
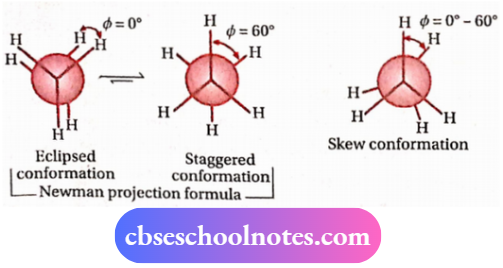
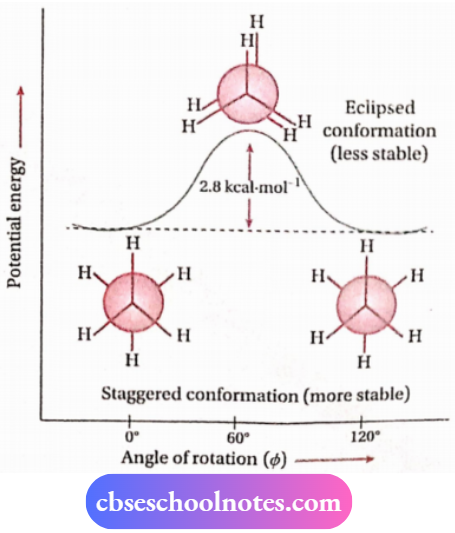
3. Conformational isomerism
Distinction between cis and trans-isomers:
The cis and trans isomers can be distinguished with the help of certain physical characteristics as follows:
1. Melting point:
The molecules of the trans-isomer of a compound is relatively more symmetrical than those of the os-isomer and hence remain closely packed in the crystal lattice. As a consequence, the melting point of the trans-isomer is usually higher than that of the corresponding cis-isomer

2. Solubility:
In general, a cis-isomer is found to be relatively more soluble in a particular solvent because the molecules of the cis-isomer, being less symmetrical, are weakly held in the crystal lattice than the molecules of relatively more symmetrical trans-isomer

3. Dipole moment:
In general, the cis-isomer of an alkene is found to be more polar than the irans-isomer (in which there is a possibility of cancellation of moments of two oppositely oriented groups or atoms)

Organic Chemistry Techniques for Class 11 Chemistry
Equivalent And Non-Equivalent H-Atoms
If each of the two or more hydrogen atoms present in an organic compound on being replaced by any other atom or group in turn produces the same compound, then these hydrogen atoms are regarded as equivalent hydrogen atoms.
Example:
All 6 hydrogen atoms in the ethane (CH3—CH3) molecule are equivalent and this is because, if any one of these six H-atoms is replaced by an atom (or group) such as Br, the same compound ethyl bromide (CH3CH2Br) is obtained.
Again, in a propane (CH3CH2CH3) molecule, the 6 hydrogens of two methyl groups are equivalent because the replacement of any one of them by Clatom, produces the same compound, 1-chloropropane (CH3CH2CH2Cl) .
Also, the 2 hydrogens of the methylene ( —CH3—) group are equivalent because the replacement of either of them by Cl-atom produces the same compound, 2-chloropropane (CH3CHClCH3). However, one methyl hydrogen and one methylene hydrogen are non¬equivalent because each of them when displaced by Cl atom gives two different compounds.
Therefore, propane contains two types of non-equivalent H atoms. In the given compounds, equivalent H -atoms are marked by the same English letter while the non-equivalent H atoms are designated by different English letters

In following compounds, all H-atoms are equivalent:

Determination Of No. Of Covalent Bonds In An Organic Compound From Its Molecular Formula
If the total number of electrons required for the completion of If the total number of electrons required for the completion of the molecule is x and the total number of valence electrons of all the atoms present is y, then the total number of covalent bonds \(\frac{(x-y)}{2}\) (for the formation of a covalent bond, 1 electron pair is required hence, the division by 2 has been effected).
If the molecular formula of a compound is CaHbOc, then the number of electrons required for the completion of octets of several C -atoms = 8a, the number of electrons required for the completion of duplets of b number of H-atoms = 2b, and the number of electrons required for the completion of octets of c number of 0 -atoms = 8c.
Hence, the total number of electrons required for the completion of duplets of H-atoms and octets of C and O -atoms present in the molecule, x = (8a + 2b + 8c). The number of valence electrons for several C -atoms, b number of H-atoms, and c number of 0 -atoms are 4a, lb and 6c respectively. Therefore, the total number of valence electrons of C, H and O -atoms present in the molecule, y = (4a + b + 6c). So, the total number of covalent bonds present in the molecule of compound
⇒ \(\mathrm{C}_a \mathrm{H}_b \mathrm{O}_c=\frac{(8 a+2 b+8 c)-(4 a+b+6 c)}{2}\)
Examples:
1. Determine the number of covalent bonds in a compound having the molecular formula, C2H4O2.
Answer:
The total number of electrons required for the completion of octets of two C and two O -atoms and duplets of four H atoms present in the molecule = (2×8 + 2×8 + 4×2 ) = 40 and the total number of valence electrons of all these atoms =(2 × 4 + 2 ×6 + 4 × 1) =24.
⇒ \(\mathrm{C}_2 \mathrm{H}_4 \mathrm{O}_2=\frac{40-24}{2}\)
= \(\frac{16}{2}\)
= 8
2. Determine the number of covalent bonds in a compound having the molecular formula, C2H2Cl4. Write the probable structure and name of the compound.
Answer:
The total number ofelectrons required for the completion of octets of two C and four Cl -atoms and duplets of two H atoms present in the molecule =(2× 8 + 4×8 + 2×2) = 52
and the total number of valence electrons of all these atoms
=(2×4 + 4×7 + 2 × 1) = 38.
Thus, the no. of covalent bonds present in the compound having molecular formula
⇒ \(\mathrm{C}_2 \mathrm{H}_2 \mathrm{Cl}_4=\frac{52-38}{2}\)
= \(\frac{14}{2}\)
= 7
The possible structures of the compound are ClCHCHCl2 (1,1,2,2-tetrachloroethane) or ClCH2CCl (1,1,1,2- tetrachloroethane)
Double Bond Equivalent (Dbe) Or Index Of Hydrogen Deficiency (Ihd)
For determining the structure of an organic compound, it is necessary to ascertain whether unsaturation is present in that compound or not and if present, the amount of unsaturation is also needs to be known. In an organic compound, the amount of unsaturation is expressed in terms of Double Bond Equivalent (DBE).
It is also called the Index of Hydrogen Deficiency (IHD). If a hydrocarbon contains 2 hydrogen atoms less than the alkane having the same number of carbon atoms, its double bond equivalent is I, i.e., the compound may contain 1 double bond or a ring.
For example:
The compound, C4H8 contains two H -atoms less than the alkane (butane, C4H10 ) having the same number of C -atoms. So, its double bond equivalent is 1.
Thus, the compound may contain 1 double bond or it may be a cyclic one. That is, the compound may be (CH3CH2CH=CH2) but-l-ene (CH3CH=CHCH3) or cyclobutane.
SimUarly, the double bond equivalent 2 indicates the presence of 2 double or but-2-ene + 1 2 bonds or I triple bond or 1 double bond and 1 ring or 2 rings in the compound.
So, the term SODAR (Sum of Double Bonds And Rings) is also frequently used. From the molecular formula of a compound, its Double Bond Equivalent (or Index of Hydrogen Deficiency or Sum of Double bonds And Rings) can easily be calculated
The double bond equivalent (DBE) of a compound \(=\frac{\Sigma n(v-2)}{2}+1\) + j where n js the number of different types of atoms present in the molecule and v is the valency of each type of atom.
It is to be remembered that if the value of the DBE of a compound is less than 4, the compound is not a benzenoid aromatic compound.
Examples: 1. The molecular formula of a compound is C6H8. Calculate its double bond equivalent (DBE). State whether the compound is an aromatic compound or not.
Answer:
Double Bond Equivalent = \(\frac{6(4-2)+8(1-2)}{2}+1\)
= 3
Since the double bond equivalent ofthe compound is less than 4, it is not a benzenoitÿaromatic compound
2. Determine the double bond equivalent of each of the following compounds. On catalytic hydrogenation, each of the compounds consumes 2 moles of hydrogen. What is the number of rings (if present) in each of the compounds:
- C8H8Br2
- C8H10O2
- C5H6F2
- C8H9C10
Answer:
1. DBE of compound = \(\frac{8(4-2)+8(1-2)+2(1-2)}{2}\) + 1
= 4
The compound on catalytic hydrogenation consumes 2 moles of hydrogen per mole. So, there are 2 double bonds or I triple bond and (4- 2) or 2 rings present in the compound
2. DBE ofthe compound = \(\frac{8(4-2)+10(1-2)+2(2-2)}{2}\) + 1
= 4
The compound on catalytic hydrogenation consumes 2 moles of hydrogen per mole. So, there are 2 double bonds or 1 triple bond and (4- 2) or 2 rings present in the compound.
3. DBE of the compound = \(\frac{5(4-2)+6(1-2)+2(1-2)}{2}\)+ 1
= 2
Since the compound consumes 2 moles of hydrogen on catalytic hydrogenation, the compound contains 2 double bonds or 1 triple bond. There is no ring present in the compound.
4. DBE ofthe compound
= \(\frac{8(4-2)+9(1-2)+1(1-2)+1(2-2)}{2}\) + 1
= 4
Since the compound on catalytic hydrogenation consumes 2 moles of hydrogen per mole, it contains 2 double bonds or 1 triple bond and 2 rings.
3. Write the structures and the IUPAC names of all the isomeric compounds having molecular formula, C4H6 by determining its double bond equivalent.
Answer:
DBE ofthe compound = \(=\frac{4(4-2)+6(1-2)}{2}\) + 1
= 2
Therefore, the compound contains 2 double bonds or 1 triple bond or 1 double bond and 1 ring or two rings. The following 9 isomers ofthe compound are possible:
1. CH2=CH = CH=CH2 (Buta-1,3-diene)
2. CH3CH2C ≡ CH (But-l-one)
3. CH3C ≡ CCH3 (But-2-yne))
4. CH2 = C = CHCH3 (Buta-1,2-diene)

Fission Of Covalent Bond: Generation Of Reaction Intermediates
Formation of product molecule(s) from the reactant molecules involves processes like bond fission, bond formation etc. A chemical equation rarely indicates how the reaction proceeds. The mechanism of an organic reaction is a sequential account of each step of the reaction, describing details of electron movement, energetics during bond breaking and bond formation and the CH rates of conversion of reactants into products (kinetics).
During the breaking and formation of bonds, the transfer of y\H electrons is shown by the use of arrow signs. Curved arrow signs containing two bar  indicate the shifting of a pair of electrons while die transfer of one electron is indicated by curved arrow signs containing one barb
indicate the shifting of a pair of electrons while die transfer of one electron is indicated by curved arrow signs containing one barb  or ‘fish hook notation’ [it is to be noted that the symbol
or ‘fish hook notation’ [it is to be noted that the symbol  is incorrect]
is incorrect]
1. Types of the fission of covalent bonds
Cleavage of covalent bonds can take place in two ways depending upon the nature of the bond involved, the nature of the attacking agent and the conditions of the reaction.
Homolytic fission or homolysis:
If a covalent bond in a molecule undergoes fission in such a way that each bonded atom gets one electron of the shared pair, it is called homolytic fission.
This type of bond cleavage results in the formation of neutral species called free radicals. Homolytic cleavage or fission is usually favoured by conditions such as the non-polar nature of the bond, high temperature and the presence of high energy (UV) radiations.
Example:
Homolytic cleavage of a bond, A— B leads to the formation of free radicals, \(\dot{\mathrm{A}} \text { and } \dot{\mathrm{B}}\) (each containing odd electrons), may be shown as follows

This type of bond fission requires less energy than heterolytic bond fission.
Heterolytic fission or heterolysis:
If a covalent bond undergoes fission in such a way that both the bonding electrons are taken away by one of the bonded atoms, it is called heterolytic bond cleavage. This type of bond cleavage results in the formation of a cation having a sextet of electrons and an anion having an octet of electrons in the valence shells ofthe participating atoms

This type of bond cleavage resulting in the formation of charged species is favoured by the conditions such as the polar nature ofthe covalent bond and the presence of polar solvents Due to heterolytic fission of bond, ions involving charge on carbon are usually formed. According to the nature of the charge, these are of two types—carbocations and carbanions
3. Intermediates formed by the fission of bonds
Under the influence of attacking reagents, suitable bonds in most organic compounds undergo homolytic or heterolytic fission to form short-lived and highly reactive (hence cannot be isolated) chemical species called reaction intermediates.
Some common examples of reaction intermediates are :
Carbocations, carbanions, free radicals, carbenes, arynes etc.
1. Carbocations:
Chemical species having a positively charged carbon atom possessing a sextet of electrons are called carbocations.
Carbocations Formation:
Carbocations are formed by heterolytic fission in which the leaving group is removed along with its shared pair ofelectrons. These are represented by R

Example:

Carbocations Nomenclature:
In naming a carbocation, the word ‘cation’ is fission added to the name of the alkyl or aryl group.
For example: +CH3, CH3+CH2, C6H5+CH2 etc. are named methyl cation, ethyl cation, and benomyl cation respectively
Carbocations Classification:
Carbocations are classified as primary (1°), secondary (2°) and tertiary (3°) according to the positive charge is present on a primary, secondary and tertiary carbon atom respectively
Examples: Ethyl cation (CH3+CH2) is a primary, isopropyl cation [(CH3)2+CH] is a secondary and terf-butyl cation [(CH3)3+C] is a tertiary carbocation
Carbocations Structure:
The positively charged C-atom of a carbocation is sp² hybridised. Therefore the structure of a carbocation is trigonal planar and the bond angle is 120°. The vacant p-orbital is perpendicular to the plane

Carbocations Stability:
The stability of carbocations follows the order 3°>2°>1°> methyl. This stability order can be explained based on inductive effect, hyperconjugation and resonance.
Inductive effect: When an alkyl group having +1 effect (electron releasing inductive effect) is attached to a positively charged carbon atom, it reduces the positive charge on the central carbon and by doing this, the alkyl group itself becomes positively polarised, i.e., the positive charge on the central carbon atom is dispersed or delocalised.
As a result of this charge delocalisation, the carbocation is stabilised. Thus, the more the number of the alkyl groups attached to the central positively charged carbon atom greater the stability of the carbocation.
Therefore, the stability of the carbocations +CH3 ,CH3+CH2 , (CH3)2+CH and (CH3)3+C follows the order:

Hyperconjugation:
The effect of hyperconjugation which depends on the number of a —H atoms also leads to the same order of stability
Resonance:
Carbocations in which a positively charged C-atom is attached to a double bond are stabilised by charge delocalisation involving resonance. Stability due to resonance is greater than that contributed by +1 effect. For example, allyl and benzyl cations are stabilised by resonance.

It is to be rememberedResonance structure electron-releasingof benzyl cation groups, by J H their +1 or +R effect stabilise a carbocation (by dispersing the positive charge) while electron attracting groups by their -I or -R effect destabilise a carbocation (by intensifying the positive charge)
Reactivity:
Carbocations are chemically very reactive species because the positively charged carbons present in them have 6 electrons in their valence shell and hence they have strong tendency to complete their octets. The order of their reactivity is opposite to that of their stability and hence, the order of their reactivity is: methyl cation > primary (1°) > secondary (2°) > tertiary (3°). Carbocations behave as electrophiles.
2. Carbanions:
Chemical species carrying a negative charge on carbon atom possessing eight electrons in its valence shell are called carbanions.
Carbanions Formation:
Carbanions are produced by heterolytic cleavage of covalent bonds in which the bonding electron pair remains with the carbon atom. Carbanions are represented by the symbol, Re

Carbanions Classification:
Like carbocations, carbanions are also classified as primary (1°), secondary (2°) and tertiary
(3°) according as the negative charge is present on a primary, secondary and tertiary carbon atom respectively.
Examples:
Ethyl anion (CH3C–H2) is a primary, isopropyl anion [(CH3)2C–H] is a secondary, and terf-butyl anion [(CH3)3C– ] is a tertiary carbanion.
Carbanions Structure:
In alkyl carbanions, the negatively charged carbon atom is sp³ -sp³-hybridised.
Thus, the negative carbon contains 4 pairs of electrons one of which exists as a lone pair. The structure of simple carbanions is usually pyramidal just like that of ammonia. However, the central carbon in resonance stabilised carbanions are sp² – hybridised and therefore, their structures are planar. For example, the structure of allyl anion is planar

Carbanions Stability:
The stability of the carbanions follows the order: CH3 > primary (1° ) > secondary ( 2° ) > tertiary (3° ). This order of stability can be explained based on the following factors.
1. Inductive effect:
When an alkyl group having a +1 effect (electron-releasing inductive effect) is attached to a negatively charged carbon atom, it tends to release electrons towards that carbon.
As a result, the intensity of the negative charge on that carbon is increased and so, the carbanion gets destabilised. Evidently, the greater the number of alkyl groups on the carbon carrying the negative charge, the greater the intensity of the negative charge on the carbon atom and hence the carbanion will be less stable.
Hence, the stability of the carbanions \(\stackrel{\ominus}{\mathrm{C}} \mathrm{H}_3, \mathrm{CH}_3 \stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2,\left(\mathrm{CH}_3\right)_2 \stackrel{\ominus}{\mathrm{C}} \mathrm{H}\) and \(\left(\mathrm{CH}_3\right)_3 \stackrel{\ominus}{\mathrm{C}}\) follows the order as given below:

Electron-attracting groups (Example: —CN, — NO2, — Br, —Cl, — F etc.) by their -I effect stabilise a carbanion by dispersing the negative charge. Thus, the greater the number of electron-attracting groups, the more will be the stability of the carbanion.
For example:

2. Resonance:
When the negatively charged carbon of a carbanion remains attached to an unsaturated system or to a benzene ring, tire carbanion gets stabilised by resonance. For example, tire negative charge in each of the acetone anion and benzyl anion is highly delocalised by resonance and consequently, they get stabilised.

Reactivity:
Carbanions are very reactive species because the carbon-bearing negative charge is electron-rich and can easily donate its unshared electron pair to some other group or atom to form a covalent bond.
Hence, carbanions behave as nucleophiles. The order of reactivity of carbanions is reverse ofthe order of stability, i.e., tertiary (3°) > secondary (2°) > primary (1°) >CH3
3. Free radicals
An atom or a group of atoms possessing an odd (unpaired) electron is called a free radical. Homolytic cleavage of a covalent bond leads to the formation of free radicals
Examples:

Free radicals containing odd electrons on carbon are collectively called alkyl free radicals or simply alkyl radicals. For example, methyl radical (•CH3), ferf-butyl radical (Me3•C) etc.
Free radicals Classification:
Alkyl free radicals are classified as primary (1°), secondary’ (2°) and tertiary (3°) depending on the nature carbon atom bearing the unpaired electron.
Examples: CH3•CH2 (ethyl radical) is a primary (1°), (CH3)2•CH (isopropyl radical) is a secondary (2°) and (CH3)3•C (for-butyl radical) is a tertiary (3°) alkyl radical.
Free radicals Structure:
The structure of alkyl free radicals may be planar or pyramidal. The carbon atom of the planar free radical is sp² -hybridized while the carbon atom of a pyramidal free radical is sp³ -hybridised

Free radicals Stability:
The stability of radicals can be explained on the basis ofthe following factors:
Hyperconjugation:
Discussed earlier
Resonance:
Free radicals in which the carbon carrying the odd electron is attached to a double bond or a benzene ring is stabilized by resonance
Example:

Reactivity:
Free radicals are highly unstable and reactive species because they have a strong tendency to gain an additional electron, i.e., to share with some other atom or group to have a complete octet. The order of reactivity of alkyl radicals is reverse of the order of stability, Le., primary (1°) > secondary (2°) > tertiary (3°).
4. Carbenes
A neutral group of atoms which contains a carbon atom with only 6 electrons in its valence shell, out of which two electrons are unshared, are called carbenes.
For example: Methylene (: CH2), dichlorocarbene (: CCl2 etc. Because of the strong tendency to achieve an octet, carbenes are highly reactive and unstable. They behave as electrophiles.
There are two types of carbenes:
- Singlet carbene and
- Triplet carbene.
The central carbon atom of singlet carbene and most of the triplet carbene is sp² -sp²-hybridised.
In singlet carbene, the two unshared electrons occupy an sp² -hybrid orbital while in triplet carbene, these occupy one sp² -orbital and one p-orbital respectively.
The triplet carbene however may also assume a linear structure where the central carbon atom is sp -hybridised. The second one is more stable than the first

5. Arynos:
Benzenoid aromatic compounds having a carbon-carbon triple bond are known as arynes. The most simple among the arynes is benzyne or 1,2-dehydrobenzene.
Arynes are neutral, unstable and highly reactive intermediates. In an aryne molecule, the two sp² -hybrid orbitals, because of their diverging orientation, overlap to a very small extent to form the additional bond and for this weak overlapping, arynes are highly reactive.
It is to be noted that the triple bond in benzyne is not like the triple bond in acetylene because in acetylene two sp -orbitals overlap to form a cr -bond and two pairs of p -orbitals overlap to form two n -bonds

Various reactive intermediates at a glance:

Stability Order:

Organic Chemistry Techniques for Class 11 Chemistry
Classification Organic Reactionsof The Mechanisms Of
Based on carbon-carbon bond cleavage, mechanisms of organic reactions are divided into two classes—
- The free radical mechanism,
- Polar or ionic mechanism.
1. Free radical mechanism:
When a chemical reaction occurs through the formation of free radicals, then the mechanism is called the free radical mechanism. This type of mechanism, therefore, applies to heat or light-induced organic reactions in which homolytic bond fission takes place. Many substitution and addition reactions occur by this mechanism.
2. Polar or Ionic mechanism:
When a chemical reaction occurs through the formation of ions, then the mechanism is called a polar or ionic mechanism. This type of mechanism, therefore, applies to organic reactions in which heterolytic bond fission takes place. Many substitution and addition reactions occur also by this mechanism.
In reactions involving polar mechanisms, the reagents generally participate as ions. Depending on the nature of the charge on reagents, they can be divided into two classes—
Electrophiles or electrophilic reagents:
An electrophile or electrophilic reagent {Greek: electron loving) is an electron-deficient species that can accept an electron pair from an electron-rich species (molecule or anion) to form a covalent bond with it
Positively charged electrophiles:
H+,HO+, Cl,+NO,+NO, ->C+(Carbocation) etc.,
Neutralelectrophilles:
BF3,AlCl3,SbCl5,FeCl3, SO3,:CCl2 etc.
Electrophiles are Lewisacidsastheycan accept electron-pair
Nucleophiles or nucleophilic reagents:
A nucleophile or nucleophilic reagent (Greek: nucleus loving) is an electron-rich species that can donate an electron-pair to an electron-deficient species (molecule or cation) to form a covalent bond with it. Nucleophiles tend to attack electrophiles
Negatively charged electrophiles:
Cl–, Br–, OH–, RO–, RS–, CN–, CH3C≡C–etc.
Neutral nucleophiles:

Since the nucleophiles are electron-donors, they are considered as Lewis bases
Negatively charged nucleophiles:
Cl-,Br-, OH-, RO-, RS-, CN~, CH3C = C- etc.
O Neutral nucleophiles:
H20, ROH, NHg, RNH2, RgN etc.
Since the nucleophiles are electron-donors, they are considered as Lewis bases
Classification Of Organic Reactions
Since the number of organic compounds is quite large, the number of their chemical reactions is also expected to be numerous.
All organic reactions in general can be classified into the following four types:
- Substitution reactions
- Addition reactions
- Elimination reactions and
- Rearrangement reactions.
1. Substitution reactions
Substitution reactions Definition:
Reactions involving the replacement or substitution of an atom or group in organic molecules by some other atom or group without any change in the remaining part of the molecules are called substitution reactions
The products formed as a result of substitutions are called substitution products.
Depending upon the nature of the attacking species (nucleophile, electrophile or free radical),
The substitution reactions may further be classified into the following 3 types:
1. Nucleophilic substitution reactions:
Substitution reactions involving nucleophiles as the attacking agents are called nucleophilic substitution reactions.
- These reactions are usually designated as SN {Substitution Nucleophilic) reactions.
- Again, depending upon the number of species (molecule, ion or free radical) participating in the rate-determining step (r.d.s.) of the reaction, the SN reactions are further classified as SN1 and SN2 reactions.
- The number of particles taking part in the rate-determining step is called the molecularity of the reaction.
- If the molecularity of a reaction is 1, i.e., if the reaction is unimolecular, then the mechanism of the reaction is said to be SN1 {Substitution Nucleophilic Unimolecular) and if the molecularity is 2 i.e., if the reaction is bimolecular, then the mechanism of the reaction is called SN2 {Substitution Nucleophilic Bimolecular).
SN1 Reaction
The reactions which proceed through SN1 mechanism are called SN1 reactions. These are two-step processes. In the first step of such a reaction, the atom or group that is to be replaced (the leaving group) is removed to form a stable carbocation. This is the rate-determining step (i.e., the slowest step) of the reaction in which only one particle (the substrate) participates. In the second step, the nucleophile gets covalently attached to the carbocation to form the substituted compound. The rate of an SN1 reaction depends only on the concentration of the substrate
Example: The hydrolysis of tert-butyl bromide by aqueous KOH solution to form tert-butyl alcohol proceeds via KOH solution to form tert-butyl alcohol proceeds via

Reaction mechanism:
In the first step of the reaction, the C—Br bond of ferf-butyl bromide undergoes fission to form tert butyl cation (a stable carbocation) and bromide ion (the leaving group). This is the slowest or rate-determining step. The rate of the reaction depends on the concentration of the substrate, (CH3)3CBr. In the second step [fast), the nucleophile (here OH-) attacks the carbocation to form ferf-butyl alcohol.

The reactions which proceed through SN2 mechanism are called SN2 reactions. In this type of reaction, the removal of the leaving group (bond breaking) and attachment of the nucleophile with the substrate (bond formation) take place simultaneously, i.e., the reaction occurs in one step. Therefore, it is the rate-determining step of the reaction.
The rate ofan SN2 reaction depends on the molar concentration of both the substrate and the nucleophile.
Example: Hydrolysis of CH3Cl by aqueous KOH solution to produce CH3OH proceeds via SN2 mechanism

Reaction mechanism: Because of the much higher electronegativity of Cl compared to C, the C-atom of CH3CI becomes The rate of an SN1 reaction depends only on the electrophilic centre. The nucleophile, OH” attacks the C atom of CH3Cl from the opposite side of the Cl -atom i.e., at an angular distance of 180°).
Such an approach of the nucleophile requires the lowest energy as this avoids electrostatic repulsions between the negatively charged nucleophile and the leaving group. A backside attack is also more feasible sterically. In this one-step reaction, the formation of C— O and cleavage of the C —Cl bond take place simultaneously. The reaction, thus, proceeds
through a single transition state in which the carbon is partially bonded to both —OH group and Cl-atom and full-bonded to the three H-atoms. In the transition state, the —OH group possesses a diminished negative charge as it starts sharing its electrons with carbon while chlorine acquires a partial negative charge as it tends to depart with the bonding electron pair.
The C-atoms and the three Hatoms become coplanar (bond angle 120° ) and the plane is perpendicular to the line containing the grouping HOδ- ——C -—Clδ- . Once the transition state is formed, HO5– further approaches the C-atom to form a frill covalent bond while the Cl5– atom is eliminated as Cl– by taking full possession of the electron pair of C—Cl bond.

Comparison of SN1 And SN2 mechanism
Alkyl halides may undergo hydrolysis by both SN1 and SN2 mechanisms. SN1 and SN2 mechanisms of hydrolysis of RX may be compared concerning the following factors:

The terms “transition state” and “intermediate” are not synonymous. Although intermediates are (For example: Carbocations, carbanions, free radicals etc.), very unstable they have real existence, but the transition states represent hypothetical arrangements of atoms possessing a definite shape and charge distribution. These have no real existence. Each step of every reaction proceeds through a transition state but each reaction may or may not proceed through an intermediate.
For example:
There is no intermediate involved in a one-step reaction such as SN2 but the two-step reaction SN1 proceeds through the formation of an intermediate
Electrophilic substitution reactions:
The substitution reaction in which the attacking reagent is an electrophile is called an electrophilic substitution reaction. These reactions are expressed by the symbol, SE (Substitution Electrophilic)
Example:
Nitration of benzene with mixed acid (concentrated HNO3 and concentrated H2SO4) yields nitrobenzene. It is an electrophilic substitution reaction. Nitronium ion (NO2) produced by the reaction between HNO3 and H2SO4, acts as an electrophile in this reaction.
⇒ \(\mathrm{HNO}_3+2 \mathrm{H}_2 \mathrm{SO}_4 \rightleftharpoons \stackrel{\oplus}{\mathrm{N}} \mathrm{O}_2+\stackrel{\oplus}{\mathrm{H}_3} \mathrm{O}+2 \mathrm{HSO}_4^{\ominus}\)

Reaction mechanism :
The reaction occurs in two steps. In the first step, the NO2 ion is attacked by the n-electrons of a benzene ring and gets attached to any of the six carbon atoms to form a resonance-stabilised carbocation (complex). This is the rate-determining (slow) step of the reaction. In this step, benzene loses its aromaticity. In the second step, the HSO4 ion accepts a proton (H+) from the tr -complex and results in the formation ofthe stable aromatic compound i.e., nitrobenzene. In this step, the aromaticity of the ring is regained.

Free radical substitution reaction:
The substitution reactions in which the attacking reagent is a free radical are called free radical substitution reactions.
Example:
Chlorination of methane in the presence of heat or diffused sunlight to give methyl chloride and hydrogen chloride occurs by a free radical mechanism

Reaction Mechanism:

Addition reactions
Reactions Definition:
Reactions In which two reacting molecules combine to give a single product molecule arc called addition reactions.
This type of reaction is typical for compounds containing multiple (double or triple) bonds. Depending upon the nature of the attacking species (electrophiles, nucleophiles or free radicals), addition reactions may be classified into the following three types
1. Nucleophilic addition reactions:
Addition reactions In which the attacking reagent Is u nucleophile arc are called nucleophilic addition reactions. Example: Base-catalysed addition of HCN to acetaldehyde to form acetaldehyde cyanohydrin is an example of a nucleophilic addition reaction

Reaction mechanism:
In this reaction, NaOII acts as a catalyst. The reaction between HCN and NaOH gives rise to the nucleophile, CN” ion

Step 1:
Because of resonance and electromeric effect, the carbonyl carbon atom of acetaldehyde acquires a partial positive charge. The positively polarised carbonyl carbon undergoes nucleophilic attack by CN– ion to form an alkoxide ion. This Is the rate-determining (slowest) step.

Step 2:
In this step, the strongly basic alkoxide ion almost Immediately takes up a proton from HCN or from the reaction medium (i.e, H2O ) and finally gets converted to the cyanohydrin compound

2. Electrophilic addition reactions:
Addition reactions in which the attacking reagent is an electrophile are called electrophilic addition reactions.
Example: The addition of HBr to propene to form 2-bromopropane as the major product is an example of this type of reaction.

Reaction mechanism: The reaction proceeds through the following steps:
Step 1:
Hydrogen bromide provides an electrophile H+, which attacks the double bond and becomes attached to C-l of propene to form preferably the relatively stable secondary (2°) isopropyl cation. It is the rate-determining (slowest) step of the reaction

Step 2: The carbocation is attacked by the nucleophile Br© ion to form 2-bromopropane predominantly

3. Free radical addition reactions:
Addition reactions in which the attacking reagent is a free radical are called free radical addition reactions.
Example:
The addition of HBr to propene in the presence of peroxides to form 1-bromopropane as the major product is an example of a free radical addition reaction

Reaction mechanism:
The reaction follows the given steps:
1. Initiation:
The organic peroxide undergoes homolytic fission of the O — bond in the presence of heat or light to form alkoxy free radicals. These free radicals then take up H from HBr to Br

2. Propagation:
Bromine free radical gets attached to C-l of propene to form a relatively more stable secondary (2°) free radical. This alkyl radical then takes up H from HBr to from 1-bromopropane predominantly.
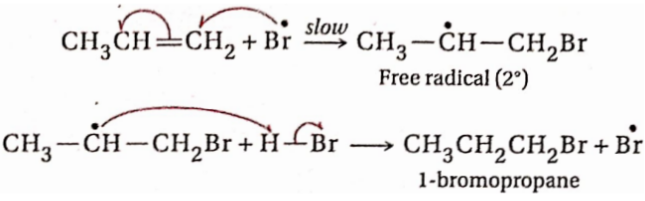
3. Termination:
Two bromine free radicals combine to form bromine molecule.

Elimination reactions
Elimination reactions Definition:
The reactions in which two atoms or groups get eliminated from the substrate molecule leading to the formation of a carbene, a multiple bond (double or triple) or a cyclopropane derivative are called elimination reactions
Depending upon the relative positions of the groups or HBr atoms eliminated, these reactions are classified as α (alpha), β(beta) and γ (gamma) elimination reactions.
α Elimination reactions:
The elimination reactions in which the loss of two atoms or groups occurs from the same atom of the substrate molecule are called α –elimination reactions. Base-catalyzed dehydrochlorination of chloroform to form dichlorocarbene is an example of α – an elimination reaction

β -Elimination reactions:
The elimination reactions in which the loss of two atoms or groups occurs from the adjacent positions (α, β) of the substrate molecule to form multiple bonds are called β-elimination or, 1,2-elimination reactions. Based on the mechanism involved, β-elimination reactions are discussed through E2, E1 and E1cB mechanisms
E2 mechanism:
The E2 mechanism is a single-step process.
- Base (BI:-) pulls a proton away from the β -carbon atom and simultaneously a leaving group (Y–) is removed from the β -β-carbon atom resulting in the formation of a carbon-carbon double bond.
- In the transition state, there are three partial bonds — one between the base and β -H atom, one between β -H atom and β-C atom and one between the a -carbon atom and the leaving group, Y.
- The carbon-carbon single bond also acquires a partial double bond character.
- Since the rate-determining step (in this case, it is the only step) involves a reaction between a substrate and a base, the mechanism is designated as E2 (Elimination bimolecular).
- Rate of the reaction is proportional to the molar concentrations of both the reactant and the base.
Example: When 2-bromopropane is heated with alcoholic KOH, propene is obtained

Reaction mechanism: The mechanism of the reaction is:

E1 Mechanism:
Elimination reactions by the El mechanism form to form dichlorocarbene is an example of takes place in two steps.
- In the first step, the substrate undergoes heterolytic fission of the cr -bond between the a -carbon atom and the leaving group to form a carbocation.
- This is the rate-determining (slowest) step of the action.
- Since the rate-determining step (in this case, it is the first step) involves only the substrate, the mechanism is designated as El (Elimination Unimolecular).
- In the second step, the β-C atom loses a proton to the base to produce an alkene.
- The rate of the reaction is proportional to the molar concentration of the substrate only
Example: When an alcoholic solution of tert-butyl chloride is heated, 2-methylpropene is formed by El mechanism.
Reaction mechanism:
The reaction proceeds via the following steps:

ElcB mechanism:
- The substrates having acidic β- hydrogen and a very poor leaving group undergo an elimination reaction by the ElcB mechanism.
- This type of reaction takes place in two steps.
- In the first step, one acidic hydrogen atom attached to β -the carbon atom of the substrate is abstracted by the base to form a stable carbanion.
- This carbanion is the conjugate base of the substrate molecule. In the second step, the leaving group becomes detached from the a -carbon atom as an anion to form an alkene.
- This step is generally the rate-determining (slow) step ofthe reaction. Since the rate of the reaction depends only on the concentration of the conjugate base, so this mechanism is known as ElcB (Elimination Unimolecular Conjugate Base).
Example:
The reaction of sodium ethoxide with 2,2-dichloro-l, 1,1-trifluoroethane gives rise to l,l-dichloro-2,2- difluoroethene. The reaction follows the ElcB mechanism because two electronegative Cl-atoms and electron-withdrawing —CF3 group causes the β —H atom to become sufficiently acidic and the F- ion behaves as a very poor leaving group

Reaction mechanism:
The mechanism is as follows:

γ -Elimination reactions:
The reactions in which the loss of two atoms or groups occurs from α and γ-positions of the molecule leading to the formation of three-membered rings are called γ-elimination reactions.

Rearrangement reaction
Rearrangement reaction Definition:
The reaction involving the shift or migration of an atom or group from a particular position in a molecule or ion to another position under suitable conditions to form a rearranged product is called rearrangement reactions
Atom or group generally shifts with bonding electron pair.
Example: When pinacol is treated with concentrated H2SO4, it undergoes dehydrative rearrangement to give pinacolone.

Reaction mechanism:
In the first step, pinacol takes a proton to form its conjugate acid. In the second step, the H2O molecule is eliminated to form a carbocation. In the third step, a methyl group migrates to the adjacent positive C with its bonding electron pair (1, 2-shift) to form a resonance-stabilized carbocation. In the fourth step, the carbocation (the conjugate acid of pinacolone) loses a proton to yield pinacolone.