Sulphonamides And Sulfones Introduction
The sulfonamide drugs were the first effective chemotherapeutic agents to be employed systemically for the prevention and cure of bacterial (pyrogenic bacterial) infections in humans. Sulfonamide can be considered as derivatives of Para-aminobenzene sulfonamide (sulfanilamide).
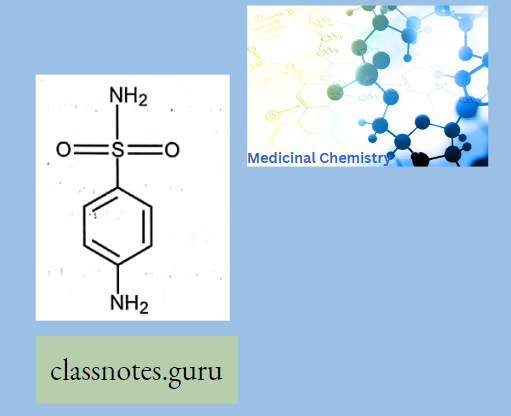
- The S2NH2 group (Nl) is not essential and governs Solubility, Potency, and Pharmacokinetic properties.
- The para-NH2 group (the N of which has been designated as N4) is essential for anti-bacterial activity. Most of them are relatively insoluble in water, but their sodium salts are readily soluble.
Antimicrobial Activity
Antimicrobial activities of the sulfonamides depend on the substituent and their position in the benzene ring. Sulphonamides are bacteriostatic.
Read and Learn More Medicinal Chemistry III Notes
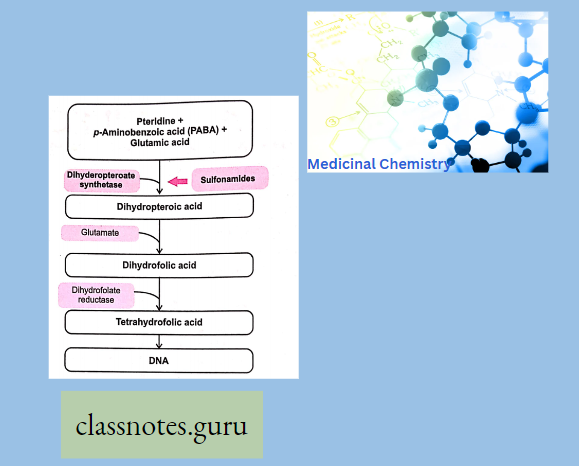
- The sulphonamide-sensitive micro-organisms require p-Amino benzoic acid (PABA) for the synthesis of folic acid which is essential for the synthesis of DNA and RNA.
- Due to the structural resemblance of sulphonamides with PABA, sulphonamides competitively inhibit PABA. This causes folic acid deficiency, resulting in arrest of bacterial growth and cell division
Classification Of Sulfonamide
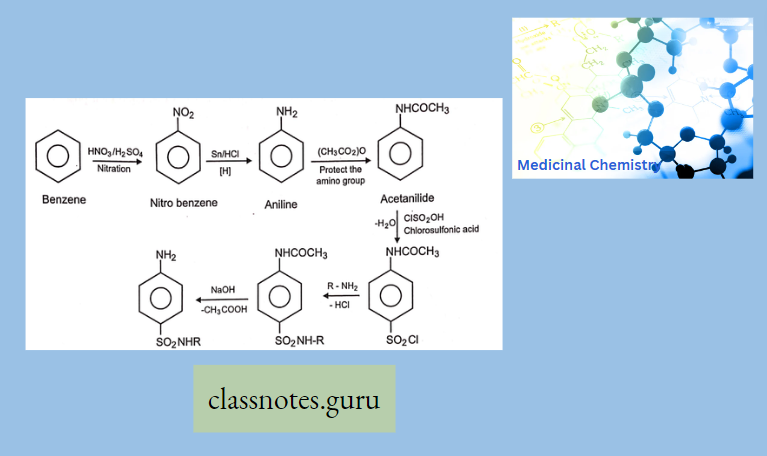
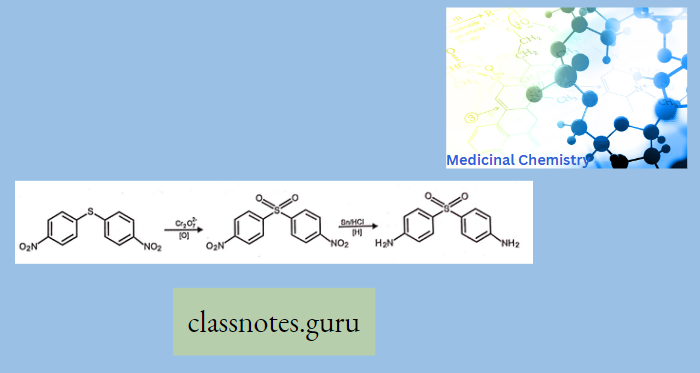
Synthesis Of Sulfonamide
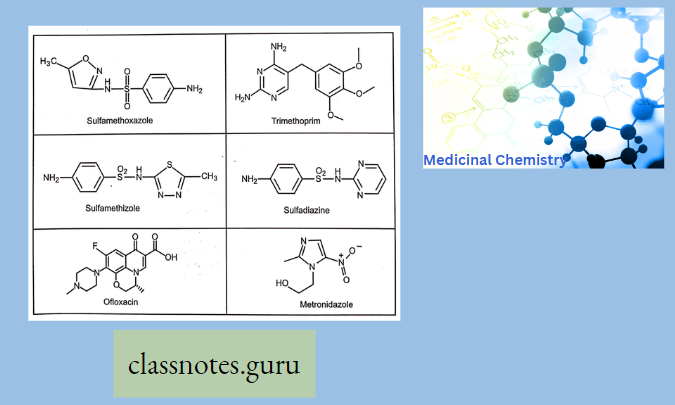
Some Examples Of Sulfonamide
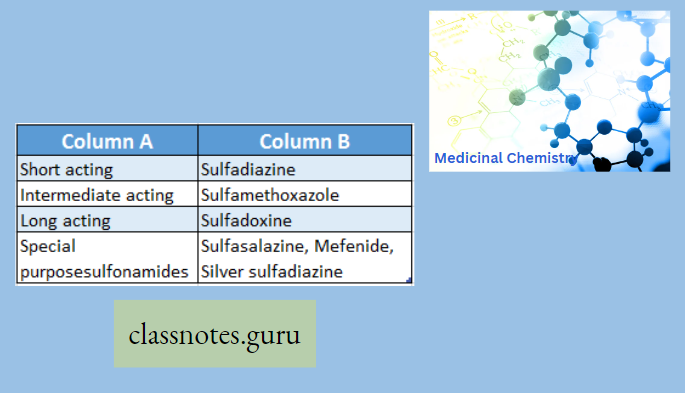
Classification According To Mode Of Action Sulphonamides For General Infections
Sulphonamides For General Infections: Sulphanilamide (Prontosil album) (Protonsil is the first identified sulphonamide)
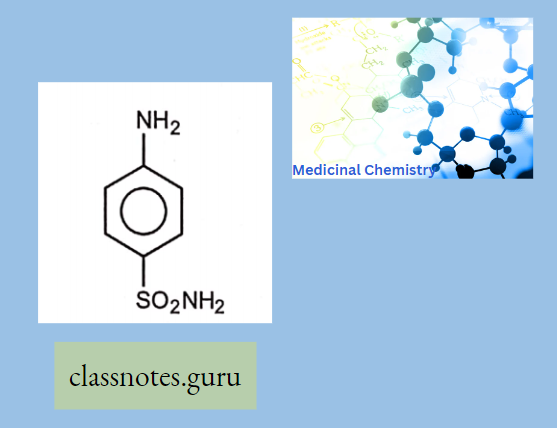
Sulphanilamide Use: Because of its high toxicity, it is not in practice now. But still used in veterinary practice as an antibacterial agent.
Sulphamethoxazole (Gantanol):
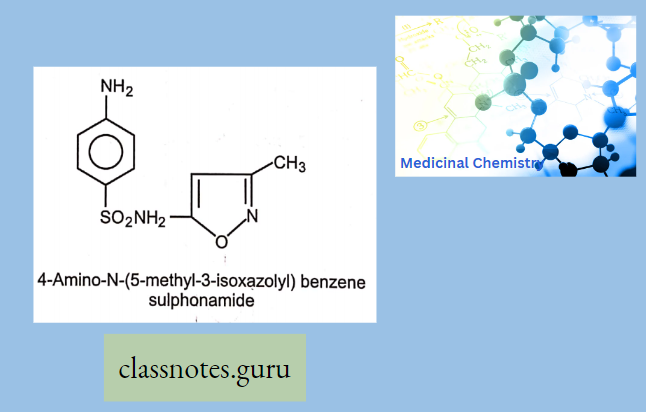
Sulphamethoxazole Use: It is used in the treatment of meningitis, and lower urinary tract infections caused by Escherichia coli and Proteus mirabilis.
Sulphonamides For Intestinal Infections: Sulphasalazine (Saaz, Salazar)
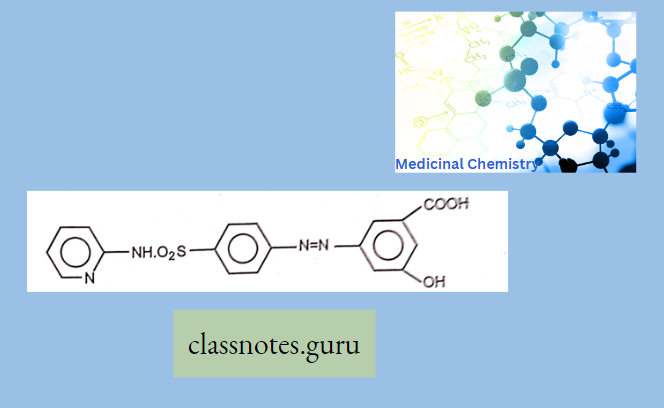
Sulphasalazine is a prodrug, which cleaved at the N4 position in the large intestine to m-Ammo salicylic acid and Sulphapyridine by azo reductase. Sulphapyridine acts as an antibacterial and aminosalicylic acid has an anti-inflammatory effect on the colon.
The advantage of this prodrug is the release of aminosalicylic acid, which prevents the absorption of the agent (prevents the systemic absorption) so that the duration of action increases (concentrates the drug in the active site). It is mainly used in intestinal infections
Sulphasalazine Use: It is used to treat ulcerative colitis and rheumatoid arthritis
Sulphonamides For Local Infections: A. Sulphacetamide (Setride, Zincoren) Sulfacetamide is a simple acetyl derivative of sulphanilamide, known as Albucid SO2NHCOCH3 N-sulphonyl acetamide
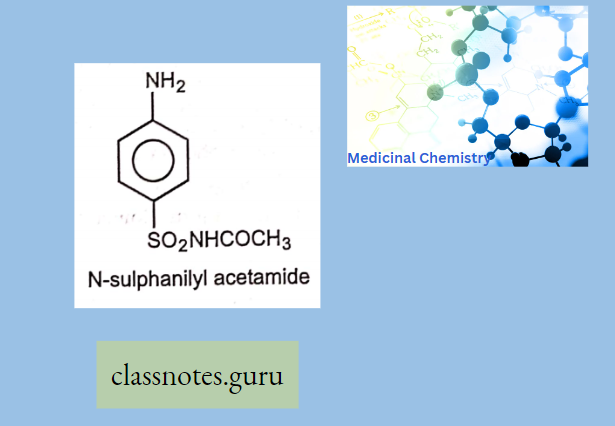
Use: Sulphacetamide sodium is used to treat infection or injuries of the eye.
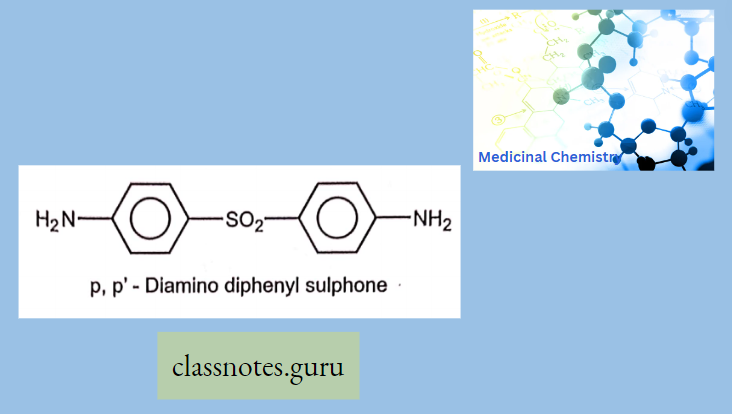
Sulphonamide For Dermatitis: Dapsone (Dds) is Used in the treatment of leprosy and nocardiosis.
Sar Of Sulfonamide
The large numbers of synthetic antibacterial sulfonamides with their various modifications have permitted to investigation of the influence of structural alteration on the antibacterial activity. Since sulfonamides are rather small molecules (as shown in the key structure) and there aren’t too many variations that can be carried out without changing the basic nucleus, these have led to the following conclusions
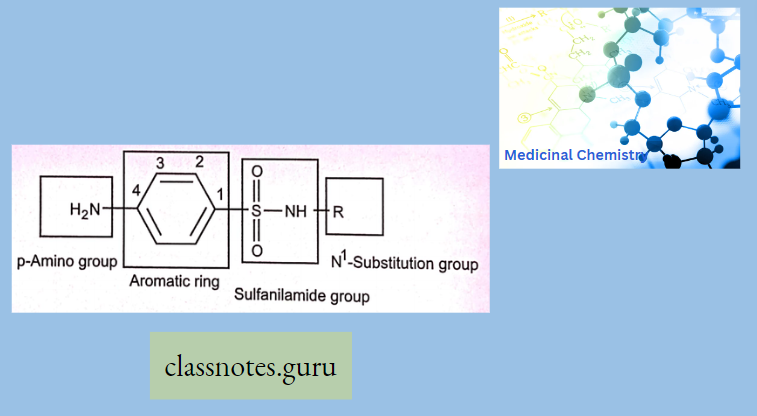
The amino & Sulphonyl groups on the benzene ring are essential and should be in 1,4-position Replacement of the Aromatic ring by other ring systems or the introduction of additional substituents on it decreases or abolishes activity.
- Exchange of the -SO2NH group by -CO-NH reduces the activity.
- Substitution of Aromatic Heterocyclic nuclei at N1- yields highly potent compounds.
- N-Di substitution in general leads to inactive.
- 1-The (N4) amine and sulfonamide groups should be on the benzene ring in the 1,4 para positions for antibacterial activity. However, the N4 should be unsubstituted or substituted to form azo or amide prodrugs or new analogs. Prodrugs are inactive unless they can be hydrolyzed in vivo to regenerate the parent drugs.
- 2-Replacement of the benzene ring with another ring system or its substitution on position other than 1,4 position will decrease or abolish the antibacterial activity.
- 3-Exchanging the sulfonamide group SO2NHR with the sulfone SO2Ph-pNH2 will retain the activity.
- The 4-Sulfonamide group is essential for biological activity and the amide should be secondary (Nl). The presence of p-aminobenzensulfonyl moiety has an important role in maintaining antibacterial activity.
- Therefore, all of the attention is focused on the Nl-substituents. These substituents seem to affect the physicochemical and pharmacokinetic characteristics of the drug. The already established substitution sites are:
- A- Nl substitution with various heteroaromatic and non-heteroaromatic (R”) influence the extent of plasma protein binding that in turn affects the drugs’ plasma concentration in addition to their onset and duration of action. Furthermore, the nature of the (R”)group influences the drug’s pka, its lipophilic-lipophobic solubility behavior, its excretion, and its toxicity profile. B- N4 azo and acyl derivatives as prodrugs
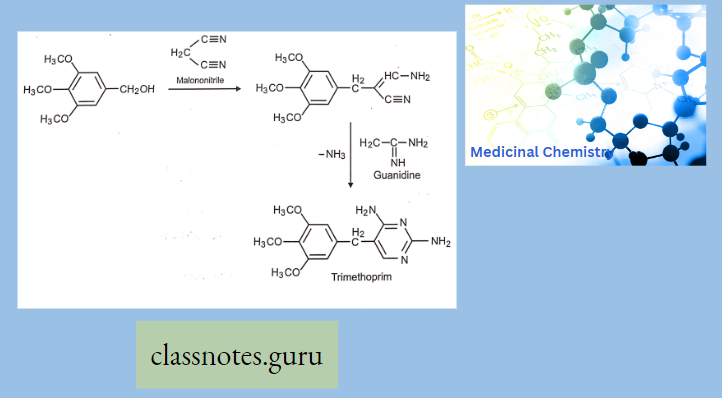
Synthesis Of Trimethoprim
Synthesis Of Dapsone
Sulphonamides And Sulfones Multiple Choice Question And Answers
Question 1. Sulphonamides are derivative of…
- Sulphanilamide
- PABA
- Sulpher
- None
Answer:1. Sulphanilamide
Question 2. Short-acting Sulfonamide is
- Sulfamethoxazole
- Sulfadiazine
- Sufapirazine
- None
Answer: 2. Sulfadiazine
Question 3. Sufapirazine is …
- Long-acting
- Short-acting
- Intermediate-acting
- Special purpose
Answer: 4. Special purpose
Question 4. The mechanism of sulfonamide is inhibition of…
- DNA Polymerase
- PABA
- Both
- None
Answer: 2. PABA
Question 5. Sulfonamide inhibits PABA incorporation
- Competitively
- Non-Competitively
- Proportionality
- None
Answer: 1. Competitively
Sulphonamides And Sulfones Short Question And Answers
Question 1. Write the mode of action of Sulfonamide.
Answer:
The Mode of Action Of Sulfonamide
Due to the structural resemblance of sulphonamides with PABA, sulphonamides competitively inhibit PABA. This causes folic acid deficiency, resulting in the arrest of bacterial growth and cell division.
Question 2. Write the SAR of sulfonamide.
Answer:
The SAR Of Sulfonamide
SAR Of Sulfonamide:
- The – SO2NH2 group (Nl) is not essential and governs Solubility, Potency, and Pharmacokinetic properties.
- The para-NH2 group (the N of which has been designated as N4) is essential for anti-bacterial activity.
- Most of them are relatively insoluble in water, but their sodium salts are readily soluble.
Question 3. Write the use of sulfonamide.
Answer:
The Use Of Sulfonamide: Use of sulfonamide Because of its high toxicity it is not in practice now. But still used in veterinary practice as an antibacterial agent.
