Fluorides Definition
Water Fluoridation
Water Fluoridation is defined as the upward adjustment of the concentration of fluoride ions in a public water supply in such a way that the concentration of fluoride ions in the water may be consistently maintained at one part per million by weight to prevent dental caries with minimum possibility of causing dental fluorosis
Topical Fluorides
Topical Fluorides are used to describe those delivery systems that provide fluoride for a local chemical reaction to exposed surfaces of the erupted dentition
Defluoridation
Defluoridation is the process of removing excess naturally occurring fluoride from drinking water in order to reduce the prevalence and severity of dental fluorosis
Fluorides Important Notes
1. Fluoride Varnishes
- They maintain fluoride ions in intimate contact with enamel for longer periods
- Commonly used are
- Duraphat – fluoride concentration is 22600 ppm
- Fluor protector – fluoride concentration is 7000 ppm
- Duraphat is the first fluoride varnish
- Fluoride varnish was first developed in Europe by Schmidt
- Varnishes increase the time of contact between enamel surface and topical fluoride agents favoring the deposition of more permanently bound fluorapatite.
2. Fluoride Concentration
- In community water fluoridation – 0.7-1.2 ppm
- In-school water fluoridation – 3.5-4.5 ppm
Read And Learn More: Percentive Communitive Dentistry Question And Answers
3. Sodium Fluoride NaF
- NaF mouthrinses commonly used are
- 0.2% NaF containing 99 ppm fluoride, for weekly use
- 0.05% NaF containing 225 ppm of fluoride, for daily use
- When NaF is applied to the tooth surface, there is the formation of CaF
- This initial rapid reaction is followed by a drastic reduction in its rate and the phenomenon is called “choking off
3. APF (Acidulated Phosphate Fluoride)
- APF solution/ Brudevold’s solution is prepared by dissolving 20 gms of NaF in 1 liter of 0.1 M phosphoric acid and to this 50% hydrofluoric acid is added to adjust pH 3 and fluoride ion concentration at 1.23%
- APF gel is prepared by adding gelling agents like methylcellulose and hydroxyl ethyl cellulose and pH is adjusted between 4-5
- APF is most commonly used in dental clinics
- Thixotropic gels are another form of APF gel with identical properties of APF.
- APF denotes a solution that sets in a gel-like state
- On application of pressure, thixotropic gels behave like a solution
- They are more easily forced into the interproximal areas
Fluoride Questions And Answers
4. Solution And Their Application
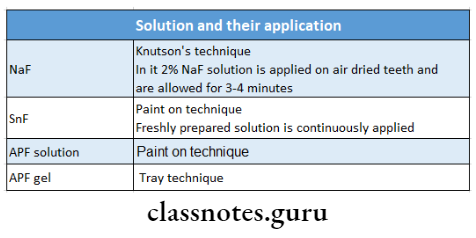
5. Snf
- So it requires fresh solutions to be prepared for each patient
- 8% SnF contains 19500 ppm of fluoride
- The pH of the freshly prepared solution is 2.5 and after that, it increases to 7.
- When SnF is applied in low concentration, tin-hydroxy-phosphate is formed, which gets dissolved in oral fluids and is responsible for the metallic taste after topical supplication of stannous fluoride
- At very high concentrations, calcium tri-fluoro-stannate gets formed along with tin-tri-fluorophosphate
- The tin-tri-fluorophosphate is responsible for making the tooth structure more stable and less susceptible to decay
- Calcium fluoride is the end product
- Snf reacts with hydroxyapatite and a small fraction of fluor hydroxyapatite also gets formed
6. Milk Fluoridation
- Introduced by Zeigler in the Swiss city of Winterthur in 1953
- In 1971, Dr. Edgar Borrow established the Borrow Foundation for the use of milk fluoridation
- The first community-based milk fluoridation scheme was introduced in 19898, in Bulgaria
7. Salt Fluoridation
- Switzerland was the first country to adopt salt fluoridation
- The recommended level of fluoride to be added is 1.5 mg/5 gm of salt
8. Fluoride Toxicity
- In acute fluoride toxicity, orally soluble calcium in the form of milk calcium gluconate, or calcium lactate solution is given
- Crippling fluorosis is seen in several forms of dental and skeletal fluorosis when the water fluoride level is more than 8 ppm or more
- In it, the spine becomes rigid and joints stiffen, virtually immobilizing the patient
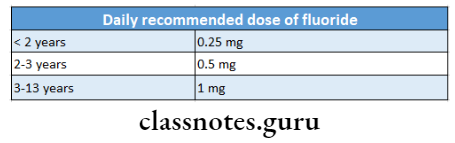
9. Fluoride Tablets
- Available in 0.55,1.1,2.2 mg tablets containing 0.25 mg, 0.5mg, 1 mg of fluoride
- Below 18 months of age, fluoride tablets are not given since the child cannot swallow
Dental Fluorides Questions
10. Nalgonda Technique
- Nalgonda Technique is a defluoridation technique
- Nalgonda Technique was developed by the National Environmental Engineering Research Institute at Nagpur in 1961.
- Useful in both domestic and community water supplies
- Involves the addition of aluminate or lime, bleaching powder, and filter alum to fluoride water
- Alumina is an important component
11. Daily recommended dose of fluoride
Fluorides Long Essays
Question 1. Describe the anticancer mechanism of fluoride on teeth, and discuss the self-applied fluoride.
(or) Acute fluoride toxicity.
Answer:
Anticaries Mechanism Of Fluoride:
1. Increased Enamel Resistance/ Reduction In Enamel Solubility
- Dental caries involves the dissolution of enamel by acid formation
- This dissolution is inhibited by fluoride as the fluoride forms fluorapatite which reduces enamel solubility
- Fluoride reduces enamel solubility also by promoting the precipitation of hydroxyapatite and phosphate mineral
- Fluoride inhibits demineralization by
- Reducing bacterial acid production
- Reducing equilibrium solubility of apatite
- By fluoridation of apatite crystal
2. Increased Rate of post-eruptive maturation
- Newly erupted teeth have hypomineralised areas and the enamel surface is also prone to dental caries
- Fluoride increases the rate of mineralization in these areas
- Organic material is also deposited over the enamel surface which increases its resistance to dental caries
3. Remineralization of incipient lesions
- Fluoride enhances remineralization by the deposition of minerals into the damaged areas
- This reduces enamel solubility through the growth of crystals which are more resistant to acid
- Fluoride enhances remineralization from calcium phosphate solution by the formation of calcium fluoride which prevents hydroxyapatite crystal growth
4. Interference With micro-organisms
- In two ways
- In high concentrations- bacteriocidal
- By reducing plaque
- In low concentrations- bacteriostatic
- Inhibits enzymes responsible for acid metabolism
5. Modification in tooth morphology
- If fluoride is ingested during tooth development it results in the formation of
- More caries-resistant tooth
- A tooth with smaller and shallow fissures
- Smaller diameter and cusp depth
- All these make them more self-cleansing
Anticaries Mechanism Of Fluoride Self-Applied Fluorides:
1. Dentrifices:
- Fluoride compounds in dentifrices
- Sodium fluoride
- Stannous fluoride
- Mono fluorophosphate
- Amine fluoride
Anticaries Mechanism Of Fluoride Indications:
- Dental caries prevention
- Caries risk patient
- Desensitization
Fluoride Chemistry Questions
Anticaries Mechanism Of Fluoride Mechanism:
- Monofluorophosphate gets deposited in the crystalline lattice and intra- crystalline transposition
- Fluoride is released
- This replaces the hydroxyl group to form fluorapatite
- Mono fluorophosphate may exchange with the phosphate group in apatite crystals
Recommendation:
Anticaries Mechanism Of Fluoride Adverse Effects:
- Detergents and flavoring agents
- Irritate stomach
- Cause vomiting
- Abrasive
- Interfere with complete intestinal absorption of fluoride
- Regular ingestion of fluoride by children < 6 years
- Dental fluorosis
2. Mouth Rinses:
As described by Bibby et al in 1946
Anticaries Mechanism Of Fluoride Contra-Indications:
- Children less than 6 years of age
- Persons with problems in oro-facial musculature due to which they cannot rinse
Sodium Fluoride Mouth Rinses:
- Formulated at
- 0.2% concentration- for weekly use
- 0.05% concentration- for daily use
- Preparation
- Prepared by dissolving 200 mg sodium fluoride tablet in 5 teaspoons of fresh clean water
- Sodium Fluoride Mouth Rinses can be used for 4 members (2 adults and 2 children)
Anticaries Mechanism Of Fluoride Mechanism:
- Fluoride forms fluorapatite from hydroxyapatite
- Fluoride inhibits bacterial metabolism and plaque acid formation
Anticaries Mechanism Of Fluoride Indications:
- If the concentration of fluoride in drinking water is 0.3 ppm or less
- Patients with increased caries risk
- School fluoride programs Advantages:
- 30-40% reduction in caries incidence
Anticaries Mechanism Of Fluoride Gels:
Anticaries Mechanism Of Fluoride Include:
- Neutral sodium fluoride and acidulated phosphate fluoride with a fluoride concentration of 5000 ppm
- Stannous fluoride with a concentration of 1000 ppm
Anticaries Mechanism Of Fluoride Method of Use:
- Brushing for 1 minute with the gel
- Placing several drops in each tray and hold in contact with the teeth for 5 minutes
Anticaries Mechanism Of Fluoride Disadvantages:
- Violate the principle of delivering low concentrations of fluoride
- Cause fluoride toxicity
- Tedious to use
Fluoride Uses In Dentistry
Question 2. Classify the various fluoride delivery methods in dentistry. Write in detail about the preparation, application and recommended age groups in Knutson’s technique.
(or) Knutson’s technique.
Answer:
Fluoride Delivery Methods:
- Topical fluoride
- Placed directly on the teeth
- Systemic fluoride
- Circulate through the bloodstream and are incorporated into developing teeth
Knutson’s Technique:
Knutson’s Technique Preparation:
- Prepared by dissolving 20 grams of sodium fluoride powder in one liter of distilled water
- Knutson’s Technique is stored in a plastic container as the fluoride may react with the silica of glass forming silicon fluoride, thus reducing the availability of free active fluoride
Knutson’s Technique Method Of Application:
- Initial appointment
- Cleaning and polishing of teeth
- Isolating with cotton rolls
- Drying with compressed air
- Using a cotton-tipped applicator stick, the 2% sodium fluoride solution is painted on dried teeth
- Allow to dry the solution for 3-4 minutes
- Repeat for each quadrant
- Instruct patient to avoid eating, drinking, or rinsing for 30 minutes
- 2nd, 3rd and 4th appointments are given at weekly appointments
Recommended Age:
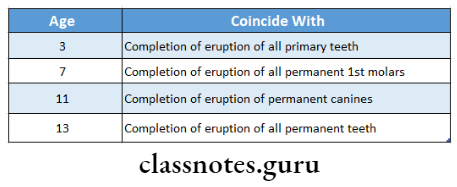
Question 3. Define water fluoridation. Discuss the feasibility of community water fluoridation in India.
Answer:
Fluoridation in India Definition:
Fluoridation in India is defined as the upward adjustment of the concentration of fluoride ions in a public water supply in such a way that the concentration of fluoride ions in the water may be consistently maintained at one part per million by weight to prevent dental caries with minimum possibility of causing dental fluorosis
Fluoridation in India Feasibility:
- The water fluoridation procedure is feasible only if
- There is a municipal water supply reaching a reasonable number of homes
- People drink this water rather than water from individual wells or tanks
- Suitable equipment is present
- Supply of fluoride is assured
- Workers are available
- Money should be available
Question 4. Describe the mechanism of action of fluoride. Add a note on the toxicity of fluoride.
Answer:
Toxicity Of Fluoride:
Toxicity Of Fluoride Acute Toxicity
- Results from rapid excessive ingestion of fluoride at one time
- Symptoms
- Nausea, vomiting, diarrhea
- Abdominal cramps
- Increased salivation
- Dehydration o Thirst
- After 2-4 hours
- Fatality
- Death due to
- Blockage of normal cellular metabolism
- Cardiac failure
- Respiratory paralysis
- If a death has not occurred after 24 hours, the prognosis is good
Toxicity Of Fluoride Pathological Changes:
- Oral corrosive changes
- Hemorrhagic stomach contents
- Changes in intestine
Toxicity Of Fluoride Management:
- Administration of milk or egg
- Lime water
- Aluminum hydroxide gels
- Inducing vomiting
Toxicity Of Fluoride Dental Fluorosis:
Due to long-term ingestion of smaller amounts
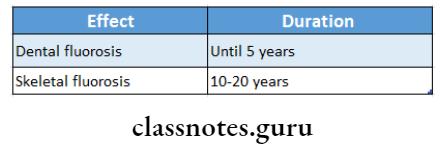
Toxicity Of Fluoride Dental Fluorosis:
Toxicity Of Fluoride Mottled Enamel
- Presence of hypoplastic areas
- Mottled areas may stain yellow/ brown
- Fluoride occurs symmetrically within dental areas, commonly affecting premolars
Toxicity Of Fluoride Skeletal Fluorosis:
- Severe pain in
- Backbones
- Joints
- Hips
- Stiffness in joints and spine
- Knock-knee syndrome
- Outward bending of legs and hands
- Damage to fetus
- Blocking and calcification of blood vessels
- Cripping fluorosis
Toxicity Of Fluoride Effect On Kidney:
Mav aggravates renal disease
Fluoride In Dentistry Mcq
Question 5. Define community water fluoridation and the requirements for community water fluoridation.
Answer:
Community Water fluoridation Requirements:
- The equipment must be adapted to local conditions and needs of the water network
- Equipment must be efficient, safe, and precise
- Equipment should have well-defined precision limits
- It should be of standard type
- Equipment should be provided with the safety mechanism
- Adjustment of the distribution must be sufficient, easy and rapid
- The apparatus should operate between 20-80% of its total capacity
- In each fluoridation system, an antisiphon mechanism should be installed in the pipes
- Maintenance and control
- The fluoridation system must be carefully maintained
- A sufficient quantity of spare parts should be available
- Control at the water treatment plant
- Regular monitoring of water supplies is essential
- A uniform concentration of fluoride ions should be maintained
- Analysis should be made several times in a day
- Control of the quality of analysis
- The corresponding authority should send 3 “blind” samples each month for analysis and returned within 48 hours
- Control of the quality of water in the network
- Samples are taken from the distribution network once a week and send for analysis
- Control of the quality of fluoride used
- Samples should be analyzed every time the delivery of fluoride is received
Community Water fluoridation Equipment Used:
- Saturation system
- Dry feeder
- Solution feeder
- Venture fluoridator system
- Saturation-suspension cone
Community Water fluoridation Fluoride Used:
- Sodium fluoride
- Fluorspar
- Silicon fluoride
- Sodium silicofluoride
- Hydrofluosilicic acid
- Ammonium silicofluoride
Question 6. Define topical fluoride. Describe in detail the method of preparation, application, mechanism of action, and advantages and disadvantages of Mahler’s solution.
(or) Stannous fluoride.
Answer:
Topical Fluoride Definition:
Topical Fluoride is used to describe those delivery systems that provide fluoride for a local chemical reaction to exposed surfaces of the erupted dentition
Topical Fluoride Definition Mahler’s Solution:
Topical Fluoride Definition Preparation:
- 0.8 grams of stannous fluoride is dissolved in 10 ml of distilled water in a plastic container
- The solution thus prepared is shaken briefly
- The solution is then applied immediately to the teeth
Topical Fluoride Definition Application:
- Cleaning and polishing of tooth surfaces
- Isolating the teeth with cotton rolls
- Drying with compressed air
- The freshly prepared solution is painted over the tooth surface
- The solution is allowed to dry for 4 minutes
- Repeat applications are made every 6 months or more
Topical Fluoride Definition Mechanism Of Action:
- At low concentration
- Hydroxyphosphate is formed
- This gets dissolved in oral fluids
- At very high concentration
- Calcium tri-fluorostannate gets formed along with tin tri-fluorophosphate
- Tin tri-fluorophosphate makes the tooth structure more stable and less susceptible to decay
- Calcium fluoride is the end product of both
- Topical Fluoride further reacts with hydroxyapatite and a small amount of fluor hydroxyapatite also gets formed
Topical Fluoride Definition Advantages:
- Application required only once per year
- Conforms to the practicing dentist’s usual patient recall system
Topical Fluoride Definition Disadvantages:
- Material is not stable in aqueous solution
- Has to be prepared freshly every time
- Metallic taste
- Causes a reversible tissue irritation
- Causes pigmentation of teeth occasionally
Fluoride Uses In Dentistry
Question 7. Define water fluoridation. Describe various methods of systemic administration of fluoride for the prevention of dental caries.
Answer:
Systemic Fluorides:
Systemic Fluorides Consist Of The:
1. Water Fluoridation:
- Water Fluoridation is the most common form of systemic fluoride administration
- Water Fluoridation constitutes of addition of fluoride to public water supplies.
- The optimal level of fluoride in water for protection against dental caries is approximately 1 ppm
Systemic Fluorides Equipment Used:
- Saturation system
- Dry feeder
- Solution feeder
- Venture fluoridator system
- Saturation-suspension cone
Systemic Fluorides Fluoride Used:
- Sodium fluoride
- Fluorspar
- Silicon fluoride
- Sodium silicofluoride
- Hydrofluosilicic acid
- Ammonium silicofluoride
2. Milk Fluoridation:
- Milk is an excellent source of calcium and phosphorous
- Milk Fluoridation contains all the essentials for the development of bone and teeth
- Milk fluoridation was first mentioned by Ziegler in 1956
Systemic Fluorides Rationale:
- The nutritional value of milk has been well-documented
- Provide a cost-effective and convenient vehicle
- All forms of milk products are suitable
- Can be targeted to those in greatest need
- The bioavailability of fluoride is not reduced by milk
- It keeps a permanently low level of ionized fluoride within the oral cavity promoting remineralization
- The confirmed dual action of fluoride: topical and systemic
- Greater preventive effect
Systemic Fluorides Advantages:
Staple food for children and infants
Systemic Fluorides Disadvantages:
- The cost would be considerably higher
- A centralized milk supply should exist
- Variation in intake and quantity of milk
3. Salt fluoridation:
Introduced in Switzerland in 1955
Systemic Fluorides Method of Preparation:
- Fluoride is added to salt by spraying concentrated solutions of sodium fluoride and potassium fluoride on salt on a conveyor belt
- Sodium fluoride and calcium fluoride are first mixed with slightly moist salt or mixed with a flow conditioner such as tri-calcium phosphate and this pre-mixed granule are added to the dry salt
Systemic Fluorides Advantages:
- Minimal fluorosis
- Safe
- Economic
- Freely available
- Easy to monitor
- Effective to supply
- Readily accepted
Systemic Fluorides Disadvantages:
- No precise control
- Sodium can cause hypertension
4. Fluoride Tablets/ Drops/ Lozenges:
Fluoride tablets/ drops/ lozenges may be prescribed to individual patients or may be part of a school or home-based public health preventive dentistry program
Systemic Fluorides Fluoride Used:
- Sodium fluoride
- APF
- Potassium fluoride
- Calcium fluoride
Systemic Fluorides Dosage:
Systemic Fluorides Age
- 6 months-3 years- 0.25 gms
- 3-6 years – 0.25-0.5 gms
- 6-16 years- 0.5-1 grams

Fluorides Short Essays
Question 1. APFgel.
Answer:
APFgel Method Of Preparation:
- APFgel is prepared by dissolving 20 gms of sodium fluoride in 1 liter of 0.1 M phosphoric acid
- Followed by the addition of 50% hydrofluoric acid
- A gelling agent methylcellulose or hydroxyethyl cellulose is added to the solution
APFgel Application:
- Oral prophylaxis
- Isolate the teeth
- Dry the teeth
- Apply APF solution by loading in a tray
- Maintain it for 4 minutes
APFgel Frequency:
Twice a year
APFgel Mechanism Of Action:
- On applying APF gel it leads to dehydration and shrinkage in the volume of hydroxyapatite crystals
- Formation of dicalcium phosphate dehydrate
- Fluoride penetrates the crystals
- This leads to the formation of fluorapatite
Fluoride Toxicity Questions
Question 2. School water fluoridation.
Answer:
School water fluoridation
- Initiated as a pilot study in 1954 at St. Thomas Virgin Islands, United States
- It is used only if the surrounding areas from which the students come have a low fluoride content
- The concentration of fluoride in the school water is 4.5 ppm in contrast to 1 ppm of the community water supply
- This is to compensate for the reduced water intake
School Water Fluoridation Advantages:
- About 40% of reduction in DMFT was observed
- No effort is required by the recipient
- Effective public health measure
- Target population-school children
- Quite economical
School Water Fluoridation Limitations:
- Need for cooperation from school authorities
- Children may not attend all school days
- There is intermittent fluoride exposure
- Limited pre-eruptive benefits to primary teeth
- Possible confrontation by antifluoridation groups
- The cost of installation, supplies and maintenance compete with other needs of the school budget
- Custodial and backup personnel are required to be trained
Question 3. Topical fluoride.
Answer:
Topical Fluoride Definition:
It is used to describe those delivery systems that provide fluoride for a local chemical reaction to exposed surfaces of the erupted dentition
Topical Fluoride Indication:
- Caries active individual
- Children shortly after tooth eruption
- Those who take medication that reduces salivary flow
- After periodontal surgery
- Patients with fixed or removable prosthesis
- Patients with eating disorders
- Mentally and physically challenged individual
Classification Of Topical Fluoride:
- Professionally applied products
- Dispensed by a dental professional
- It includes
- Sodium fluoride
- Minimum 4 applications with 2% give caries reduction of about 30%
- Stannous fluoride
- Used as 8% concentration
- Acidulated phosphate fluoride gel
- Fluoride varnishes
- Duraphat
- floor protector
- Sodium fluoride
- Composition
- It is a dichlorosilane-ethyl difluoro hydroxy silane
- Fluoride content is 22.6 mg F/ ml
- Self-administered
- Fluoride dentifrices
- Sodium fluoride
- Fluoride mouth rinses
- Dentrifices containing monofluorophosphate
Question 4. Defluoridation.
Answer:
Defluoridation
Defluoridation is the process of removing excess naturally occurring fluoride from drinking water in order to reduce the prevalence and severity of dental fluorosis
Defluoridation Methods:
Defluoridation Ion Exchange Resins:
- Carbon
- Carbon is a cation exchange resin of good durability and can be used on sodium and hydrogen cycles
- Defluoron
- A sulfonated sawdust impregnated with 2% alum solution
- Defluoron 2
- Defluoron 2 is a sulfonated coal using the aluminum solution as a regenerate
Defluoridation Nalgonda Technique:
- This technique was developed in India in 1975
- By National Environmental Engineering Research Institute
- Defluoridation Nalgonda was constructed in the district of Nalgonda in Andhra Pradesh in the town of Kathri
Defluoridation Procedure:
- Raw water is collected in a tank
- Add alum solution to it
- Next, depending on alkalinity and lime
- Stir gently for 10 minutes
- Results in the formation of floes
- Allow it to settle
Defluoridation Advantages
- Can be used at domestic and community levels
- Manually operated
- Cost-effective
- Defluoridation meets with standards laid down by the Bureau of Indian Standard
Multiple-Choice On Fluorides
Question 5. Milk fluoridation.
Answer:
Milk fluoridation
- Milk is an excellent source of calcium and phosphorous
- It contains all the essentials for the development of bone and teeth
- Milk fluoridation was first mentioned by Ziegler in 1956
Milk Fluoridation Rationale:
- The nutritional value of milk has been well-documented
- Provide a cost-effective and convenient vehicle
- All forms of milk products are suitable
- Can be targeted to those in greatest need
- The bioavailability of fluoride is not reduced by milk
- It keeps a permanently low level of ionized fluoride within the oral cavity promoting remineralization
- The confirmed dual action of fluoride: topical and systemic
- Greater preventive effect
Milk Fluoridation Advantages:
Staple food for children and infants
Milk Fluoridation Disadvantages:
- The cost would be considerably higher
- A centralized milk supply should exist
- Variation in intake and quantity of milk
Question 6. Shoe leather survey.
Answer:
Shoe leather survey
- Conducted by Dr. H Trendley Dean
- It was conducted in 97 localities
- It was done with the help of questionnaires
- During this survey, Trendley Dean visited each and every house in that particular community
- He traced the dental status of 7000 children who drank naturally fluoridated water
Shoe Leather Survey AIM:
To find the level of fluoride at which the tooth starts to blemish
Shoe Leather Survey Report:
- In 1943, he reported that the ideal amount of fluoride was 1 ppm of water
- This concentration was demonstrated to result in healthy, attractive teeth that had l/3rd as many cavities as might otherwise be expected and no staining
Question 7. Fluoride varnishes.
Answer:
Fluoride Varnishes Commonly Used:
- Duraphat
- Fluorprotector
Fluoride Varnishes Composition:
- Fluoride Varnishes Composition is a dichlorosilane-ethyl difluoro hydroxy silane
- Fluoride content is 22.6 mg F/ ml
Fluoride Varnishes Application
- Oral prophylaxis
- Dry the teeth
- Apply varnish with a single tufted small brush first on the lower arch and then on the upper
- Maintain it for 4 minutes
- Avoid rinsing, drinking, eating for 1 hour
Fluoride Varnishes Dose:
- 0.5 ml of dura phat containing 11.3 mg F fluoride
- 0.5 ml of floor protector containing 3.1 mg F fluoride
Fluoride Varnishes Mechanism Of Action:
- On application of varnish, results in a reservoir of fluoride ions around the enamel
- Results in deeper penetration of fluoride and formation of fluorapatite
Fluorides Short Answers
Question 1. Colorado Stains.
Answer:
Colorado Stains
- The history of fluoridation started with the contribution of Dr. Frederick McKay
- He arrived in Colorado Springs, Colorado, USA in 1901
- He noticed that many of his patients, particularly those who had lived in the area all their lives, had an apparently permanent stain on their teeth which was known to the local inhabitants as “Colorado Stains”
- He called the stain “mottled enamel” and said that it was characterized by minute white flecks or yellow or brown spots or areas, scattered irregularly
- Or streaked over the surface of a tooth or
- Colorado Stains may be a condition where the entire tooth surface is of a dead paper-white, like the color of a china dish
Question 2. Fluoridated Salt.
Answer:
Fluoridated Salt
Introduced in Switzerland in 1955
Fluoridated Salt Method Of Preparation:
- Fluoride is added to salt by spraying concentrated solutions of sodium fluoride and potassium fluoride on salt on a conveyor belt
- Sodium fluoride and calcium fluoride are first mixed with slightly moist salt or mixed with a flow conditioner such as tri-calcium phosphate and these pre-mixed granules are added to the dry salt
Fluoridated Salt Advantages:
- Minimal fluorosis
- Safe
- Economic
- Freely available
- Easy to monitor
- Effective to supply
- Readily accepted
Fluoridated Salt Disadvantages:
- No precise control
- Sodium can cause hypertension
Fluorides Mcq
Question 3. Nalgonda technique.
Answer:
Nalgonda technique
- Method of defluoridation
- This technique is developed in India in 1975
- By National Environmental Engineering Research Institute
- Nalgonda was constructed in the district of Nalgonda in Andhra Pradesh in the town of Kathri
Nalgonda Technique Procedure:
- Raw water is collected in a tank
- Add alum solution to it
- Next, depending on alkalinity and lime
- Stir gently for 10 minutes
- Results in the formation of floes
- Allow it to settle
Nalgonda Technique Advantages:
- Can be used at domestic and community levels
- Manually operated
- Cost-effective
- Nalgonda meets with standards laid down by the Bureau of Indian Standard
Question 4. Optimum levels of fluoride.
Answer:
Optimum levels of fluoride
- The optimum level of fluoride in water in a temperate climate is 1 ppm
- For warmer and colder climates the amount can be adjusted from approximately 0.7 ppm to 1.2 ppm
- Optimum levels of fluoride is adapted according to the water consumed
Question 5. Chemical properties of fluoride.
Answer:
Chemical properties of fluoride
- Fluoride is a compound of fluorine with a metal
- Chemical properties of fluoride is an anion, the reduced form of fluorine
- Chemical properties of fluoride is a monovalent ion
- The solution of inorganic fluoride in water contains fluorine and fluorine ion
- Few inorganic fluorines are soluble in water
- Fluorides are more strongly solvated
- Fluoride is effective in preventing tooth decay
- Fluoride tends to be dangerous only in large doses
Question 6. Requirements of community water fluoridation.
Answer:
Requirements of community water fluoridation
- The equipment must be adapted to local conditions and needs of the water network
- Equipment must be efficient, safe and precise
- Equipment should have well-defined precision limits
- It should be of standard type
- Equipment should be provided with a safety mechanism
- Adjustment of the distribution must be sufficient, easy and rapid
- The apparatus should operate between 20-80% of its total capacity
- In each fluoridation system, an antisiphon mechanism should be installed in the pipes
- Maintenance and control
- The fluoridation system must be carefully maintained
- A sufficient quantity of spare parts should be available
- Control at the water treatment plant
- Regular monitoring of water supplies is essential
- A uniform concentration of fluoride ions should be maintained
- Analysis should be made several times in a day
- Control of the quality of analysis
- The corresponding authority should send 3 “blind” samples each month for analysis and returned within 48 hours
- Control of the quality of water in the network
- Samples are taken from the distribution network once a week and send for analysis
- Control of the quality of fluoride used
- Samples should be analyzed every time the delivery of
Fluorides Mcq
Question 7. Choking off effect.
Answer:
Choking off effect
- When NaF is applied to the tooth surface, there is the formation of CaF
- This initial rapid reaction is followed by a drastic reduction in its rate and the phenomenon is called “choking off
Fluorides Viva Voce
- The relative atomic weight of fluorine is 19
- The concentration of fluoride in human milk is 15-20 mg/1
- Bone is a major route of fluoride absorption
- Dr. McKay coined the term mottled enamel for fluorosis
- Dental fluorosis was termed denti di chiaie by Dr J.M. Eager
- The element fluoride was identified as a factor responsible for mottled enamel in 1931
- 22 cities study conducted by Dr. Trendley H Dean determined the extent and severity of mottled enamel
- The inverse relationship of dental caries and mottled enamel was given by Trendley H Dean
- The world’s first artificial fluoridation plant was set up in Grand Rapids.
- Dean proposed that fluoridating water supplies by 1 ppm of fluoride reduces dental caries by 60%
- The kidney has the highest concentration of plasma fluoride level
- The renal clearance of fluoride in the adult ranges from 30-60 ml/minute
- Plasma fluoride concentration is lowest in the brain
- Anti cariogenic effect of fluoride is rendered by interference with micro-organisms
- The maximum loss of fluoride from tooth structure is due to tooth wear
- Fluoride toothpaste is the best method for topical fluoridation in school children
- Salt fluoridation is a method of choice if water fluoridation is not feasible
- Fluoride toothpaste is recommended at night time because it is a fluoride reservoir
- The maximum plasma concentration of fluoride after fluoride ingestion reaches upto 0.20 ppm
- 8 ppm of fluoride causes skeletal fluorosis
- Knutson’s technique for 3 years inhibits caries by about 35%
- Fluoride acts on enolase enzyme to render anticaries effect
- Fluoride remains on the tooth surface after dura phat application for up to 12 hours
- The application of sodium fluoride solution is called Knutson’s technique
- The aqueous solution of topical fluoride is continuously reapplied for 4 minutes
- The amount of fluoride present in fluoride varnish is 22600 ppm
- Fluoride varnishes are applied once in six months
- Knutson’s technique is recommended for the age of 3, 7,11, and 13 years
- APF solution is also known as Brudevold solution
- Fluoride rendered by APF solution is 12300 ppm
- 123% APF can reduce dental caries by about 66%
- A 0.05% concentration of sodium fluoride mouth rinses is recommended for daily use
- Children under six years of age are contraindicated for the use of fluoride rinses
- Certainly, the lethal dose of fluoride is 32-64 mg of fluoride per kg of body weight
- Safety tolerated dose of fluoride for a 70 kg adult is 8 16 mg F/kg body weight
- Abdominal pain is the first sign of acute fluoride toxicity
- The premolar is the most commonly affected tooth by fluorosis
- A combination of fluoride, calcium, and vitamin D results in increased wall thickness of osteoblasts
- Fluoride toothpaste is not safe for children of about 2-4 years of age
- Fluoride toothpaste is recommended twice a day in mixed dentition period
- Fluoride dentifrices can inhibit caries up to 35%
- Water fluoridation is the most economical method for caries prevention in urban areas
- The consumption of 1.5 ppm fluoridated water can result in fluorosis after 10-15 years
- In fluoride toxicity drug of choice is calcium chloride
- Cardiac failure is the cause of death in acute toxicity of fluoride
- 4.5 to 6.3 ppm is the recommended level for school water fluoridation
- The second stage of the Nalgonda technique is flocculation
- Ziegler introduced milk fluoridation
- Permanent teeth have higher fluoride levels than primary teeth because of the long pre-eruptive maturation period
- 30% of hydrogen peroxide is required to bleach fluorosis teeth
- The Nalgonda technique is a defluoridation technique
- The use of fluoride varnishes reduces the number of patient’s visit
- Post secretory phase and early mineralization phase are the critical periods for fluorosis.
