Fluorides Important Notes
1. APFgel
- APF solution/ Brudevold’s solution is prepared by dissolving 20 gms of NaF in 1 liter of 0.1 M phosphoric acid and to this 50% hydrofluoric acid is added to adjust pH 3 and fluoride ion concentration at 1.23%
- APF gel is prepared by adding gelling agents like methylcellulose and hydroxyl ethyl cellulose and pH is adjusted between 4-5
2. Sodium fluoride
- It is chemically stable
- Has pleasant taste
- No irritation to the tissues
- Cheap
- The method of application is known as the Knutson technique
- Knutson and Feldman recommended this technique
- In this 4 applications of 2% NaF at weekly intervals in a year 3,7,11 and 13 years is done
Read And Learn More: Pedodontics Short Essays Question And Answers
3. Stannous fluoride
- It is an unstable solution due to the formation of Sn(OH)2
- So it requires fresh solutions to be prepared for each patient
4. Nalgonda technique of defluoridation
- It was developed by National Environmental Engineering Research Institute at Nagpur in 1961.
- Involves the addition of aluminate or lime, bleaching powder, and filter alum to fluoride water
5. Fluoride varnishes
- Commonly used are
- Duraphat – fluoride concentration is 22600 ppm
- Fluor protector – fluoride concentration is 7000 ppm
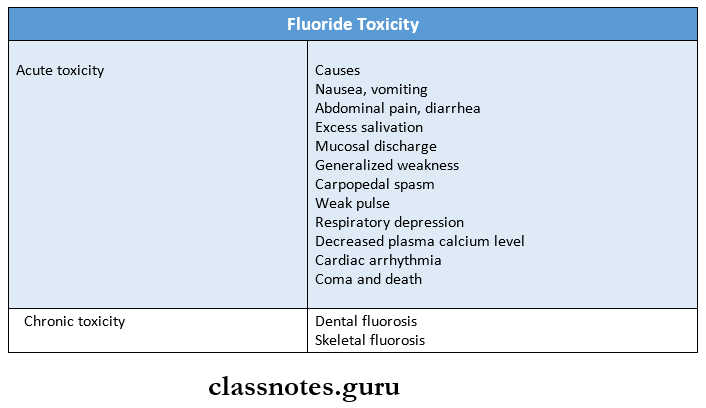
6. Fluoride toxicity
Fluorides Long Essays
Question 1. What is topical fluoride? Explain about APF gel and Sodium fluoride.
Answer:
Topical Fluoride:
- It refers to the use of systems containing relatively large, concentrations of fluoride that are applied locally, or topi¬cally, to erupted tooth surfaces to prevent the formation of dental caries
Sodium Fluoride- 2%:
- Preparation: It is prepared by dissolving 20 gms. of Sodium fluoride powder in 1 liter of distilled water.
Sodium Fluoride Application (Knutson Technique):
- Clean and polish the teeth
- Isolate both the arches
- Dry the teeth thoroughly
- Apply 2% NaF with cotton applicators
- Maintain it for 4 minutes
- Repeat it for the remaining quadrant
- Avoid eating, drinking/rinsing for 30 minutes
- Repeat applications at weekly intervals
- Recommended ages – 3,7,11,13
Sodium Fluopide Mechanism Of Action:

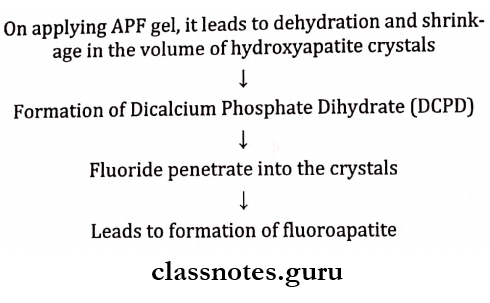
Apf (Acidulated Phosphate Fluoride) – 1.23%:
Method Of Preparation (Brudevold’s Solution):
- It is prepared by dissolving 20 gms. of sodium fluoride in 1 liter of 0.1M phosphoric acid
- Followed by the addition of 50% hydrofluoric acid
- A gelling agent methylcellulose or hydroxyethyl cellulose is added to the solution
Brudevold’s Solution Application:
- Oral prophylaxis
- Isolate the teeth
- Dry the teeth
- Apply APF solution by loading in a tray
- Maintain it for 4 minutes
Brudevold’s Solution Frequency: – Twice in a year
Brudevold’s Solution Mechanism Of Action:
Question 2. Write in detail about the mechanism of action of fluoride in preventing dental caries. Add a note on topical fluorides for home use.
Answer:
Mechanism Of Action Of Fluoride
1. Fluoride incorporation in enamel
- Pre-eruptive incorporation:
- Fluoride gets incorporated in the fluid-filled sac surrounding the developing tooth
- Then it enters the developing tooth
- Post-eruptive incorporation:
- Fluoride enters the enamel surfaces
- Conversion of carbonated apatite and hydroxyapatite to fluorapatite and fluoro- hydroxyapatite takes place
2. Remineralization of acid
- Equilibrium exists between minerals of tooth enamel and minerals of saliva
- This is disturbed by the organic acid produced by carbohydrates
- There is a drop in pH
- Minerals leach out
- This is prevented by the remineralization by fluoride
- Fluoride penetrates into enamel rods
- It forms larger crystals that are more acid resistant
Topical Fluorides
1. Dentrifices:
- Fluoride compounds in dentifrices
- Sodium fluoride
- Stannous fluoride
- Mono fluorophosphate
- Amine fluoride
Topical Fluorides Indications:
- Dental caries prevention
- Caries risk patient
- Desensitization
Topical Fluorides Mechanism:
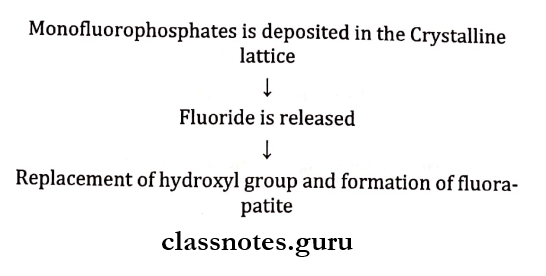
- Monofluorophosphate gets deposited in the crystalline lattice and intra- crystalline transposition
- Fluoride is released
- This replaces the hydroxyl group to form fluorapatite
- Mono fluorophosphate may exchange with the phosphate group in apatite crystals
Topical Fluorides Adverse Effects
- Detergents and flavoring agents
- Irritate stomach
- Cause vomiting
- Abrasive
- Interfere with complete intestinal absorption of fluoride
- Regular ingestion of fluoride by children <6 years
- Dental fluorosis
2. Mouth Rinses:
- Described by Bibby et al in 1946
Contra-Indications:
- Children less than 6 years of age
- Persons with problems in oro-facial musculature due to which they cannot rinse
Sodium Fluoride Mouth Rinses:
- Formulated at
- 0.2% concentration- for weekly use
- 0.05% concentration- for daily use
- Preparation
- Prepared by dissolving 200 mg sodium fluoride tablet in 5 teaspoons of fresh clean water
- It can be used for 4 members (2 adults and 2 children)
Mechanism:
- Fluoride forms fluorapatite from hydroxyapatite
- Fluoride inhibits bacterial metabolism and plaque acid formation
Mouth Rinses Indications:
- If the concentration of fluoride in drinking water is
- 0. 3 ppm or less
- Patients with increased caries risk
- School fluoride programs
Mouth Rinses Advantages:
- 30-40% reduction in caries incidence
3. GELS:
- Include
- Neutral sodium fluoride and acidulated phosphate fluoride with a fluoride concentration of 5000 ppm
- Stannous fluoride with a concentration of 1000 ppm
GELS Method of Use:
- Brushing for 1 minute with the gel
- Placing several drops in each tray and held in contact with the teeth for 5 minutes
GELS Disadvantages:
- Violate the principle of delivering low concentration of fluoride
- Cause fluoride toxicity
- Tedious to use
Fluorides Short Essays
Question 1. School water fluoridation.
Answer:
- Initiated as a pilot study in 1954 at St. Thomas Virgin Islands, United States
- It is used only if the surrounding areas from which the students come have a low fluoride content
- The concentration of fluoride in the school water is 4.5 ppm in contrast to 1 ppm of the community water supply
- This is to compensate for the reduced water intake
School water fluoridation Advantages:
- About 40% of reduction in DMFT was observed
- No effort is required by the recipient
- Effective public health measure
- Target population-school children
- Quite economical
School water fluoridation Limitations:
- Need for cooperation from school authorities
- Children may not attend all school days
- There is intermittent fluoride exposure
- Limited pre-eruptive benefits to primary teeth
- Possible confrontation by antifluoridation groups
- The cost of installation, supplies, and maintenance compete with other needs of the school budget
- Custodial and backup personnel are required to be trained
Question 2. Defluoridation.
Answer:
- Defluoridation is the process of removing excess naturally occurring fluoride from drinking water in order to reduce the prevalence and severity of dental fluorosis
Defluoridation Methods
1. Ion Exchange Resins:
- Carbion
- It is a cation exchange resin of good durability and can be used on sodium and hydrogen cycles
- Defluoron 1:
- A sulfonated sawdust impregnated with 2% alum solution
- Defluoron 2:
- It is a sulfonated coal using aluminum solution as regenerate
2. Nalgonda Technique:
- This technique is developed in India in 1975
- By National Environmental Engineering Research Institute
- It was constructed in the district of Nalgonda in Andhra Pradesh in the town of Kathri
Nalgonda Technique Procedure:
- Raw water is collected in a tank
- Add alum solution to it
- Next, depending on alkalinity add lime
- Stir gently for 10 minutes
- Results in the formation of floes
- Allow it to settle
Nalgonda Technique Advantages:
- Can be used at domestic and community levels
- Manually operated
- Cost-effective
- It meets with standards laid down by the Bureau of Indian Standard
Question 3. Fluoride Toxicity.
Answer:
- It refers to the symptoms manifested as a result of overdosage or excessive administration.
Fluoride Toxicity Types:
- Acute: Due to single ingestion of large amounts of fluoride
- Chronic: Due to long-term ingestion of smaller amounts
Fluoride Toxicity Symptoms:
- GIT disturbances
- Nausea
- Vomiting
- Diarrhea
Pain:
- Abdomen
- Extremities
- Difficulty in speech
- Thirst
- Perspiration
- Weak pulse
- Coma
- Convulsion
- Cardiac arrhythmia
Pathological Changes:
- Oral corrosive changes
- Hemorrhagic stomach contents
- Changes in intestine
Fluoride Toxicity Management:
- Administration of Milk or egg
- Lime water
- Aluminum hydroxide gels
- Inducing vomiting
Question 4. Fluoride Varnish.
Answer:
- Commonly used
- Duraphat
- Fluorprotector
Fluoride Varnish Composition:
- It is a dichlorosilane-ethyl diflurohydroxysilane
- The Fluoride content is 22.6 mg F/ml.
Fluoride Varnish Application:
- Oral prophylaxis
- Dry the teeth
- Apply varnish with a single tufted small brush first on the lower arch, then on upper
- Maintain it for 4 minutes
- Avoid rinsing, drinking, and eating for 1 hour.
Fluoride Varnish Dose:
- 0.5 ml of dura phat containing 11.3 mg F fluoride
- 0.5 ml of floor protector containing 3.1 mg F fluoride
Fluoride Varnish Mechanism Of Action:
- On application of varnish, results in a reservoir of fluoride ions around the enamel
- Results in deeper penetration of fluoride and formation of fluorapatite
Question 5. Fluoride Dentrifices.
Answer:
Commonly Used Agents:
- Sodium mono fluoro phosphates
- Sodium fluoride
Fluoride Dentrifices Indication:
- Prevention of caries
- Caries – risk patients
- Desensitization
Fluoride Dentrifices Mechanism Of Actions:
Fluoride Dentrifices Procedure:
- Selection of proper dentifrices
- Place a pea size amount of dentifrice on the toothbrush tips
- Proceed with correct brushing.
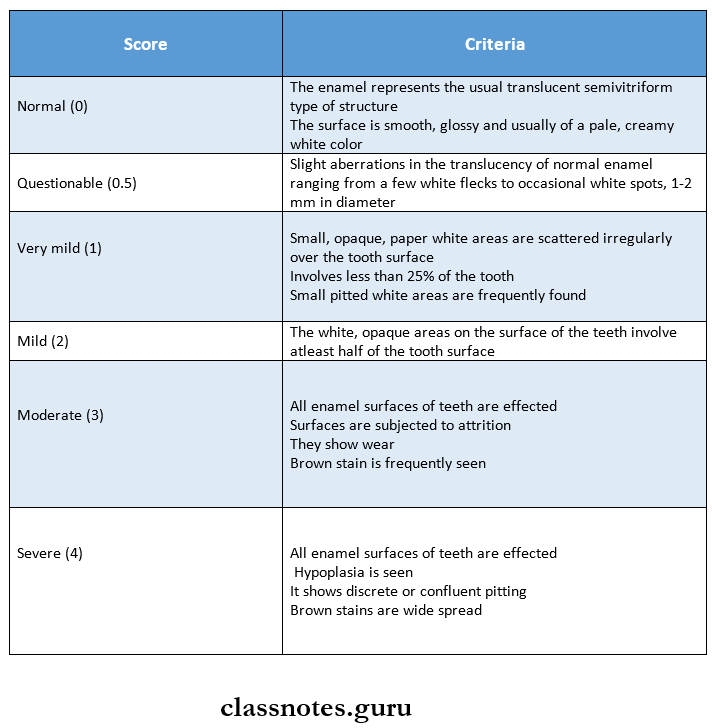
Question 6. Dean’s Index.
Answer:
Question 7. Topical Fluorides.
Answer:
- Definition: It is used to describe those delivery systems which provide fluoride for a local chemical reaction to exposed surfaces of the erupted dentition
Topical Fluorides Indications:
- Caries active individual
- Children shortly after tooth eruption
- Those who take medication that reduces salivary flow
- After periodontal surgery
- Patients with fixed or removable prosthesis
- Patients with eating disorders
- Mentally and physically challenged individual
Topical Fluorides Classification:
1. Professionally applied products:
- Dispensed by a dental professional
- It includes
- Sodium fluoride
- Minimum 4 applications with 2% gives caries reduction of about 30%
- Stannous fluoride
- Used as 8% concentration
- Acidulated phosphate fluoride gel
- Fluoride varnishes
- Duraphat
- fluoroprotector
Composition:
- It is a dichlorosilane-ethyl difluoro hydroxy silane
- Fluoride content is 22.6 mg F/ ml
2. Self-administered:
- Fluoride dentifrices
- Sodium fluoride
- Fluoride mouth rinses
- Dentrifices containing monofluorophosphate
Question 8. Miller’s acidogenic theory
Answer:
- Postulated by WD Miller in 1889.
- It states that
- Acids formed due to the fermentation of dietary carbohydrates by oral bacteria lead to progressive decalcification of the tooth structures with subsequent disintegration of organic matrix
- It states that the process of dental caries involves two stages
- Initial stage
- Acid production due to fermentation of carbohydrates by plaque bacteria
- Late stage
- Decalcification of enamel followed by dentin by acids
- This causes total destruction of enamel and den-tin
- Initial stage
- According to Miller, the process of caries involves four factors
- Dietary carbohydrates
- Micro-organisms
- Acids
- Dental plaque
Fluorides Short Answers
Question 1. Duraphat.
Answer:
- It is a fluoride varnish
Duraphat Composition:
- It is a dichlorosilane-ethyl diflurohydroxysilane
- The Fluoride content is 22.6 mg F/ml.
Duraphat Application:
- Oral prophylaxis
- Dry the teeth
- Apply varnish with a single tufted small brush first on the lower arch, then on upper
- Maintain it for 4 minutes
- Avoid rinsing, drinking, and eating for 1 hour.
Duraphat Dose:
- 0.5 ml of dura phat containing 11.3 mg F fluoride
Question 2. Mechanism of fluoride varnish.
Answer:
- On application of varnish, results in a reservoir of fluoride ions around the enamel
- Results in deeper penetration of fluoride and formation of fluorapatite
Question 3. Brudevold’s technique.
Answer:
- It is a method of preparing APF gel
- It is prepared by dissolving 20 gms. of sodium fluoride in 1 liter of 0.1M phosphoric acid
- Followed by the addition of 50% hydro fluoride acid
- A gelling agent methylcellulose or hydroxyethyl cellulose is added to the solution
Question 4. Choking off phenomenon.
Answer:
- It is seen on the application of 2% of sodium fluoride
- When sodium fluoride is applied a layer of calcium fluo¬ride gets formed
- This interferes with the further diffusion of F to react with hydroxyapatite
- This is called choking-off phenomenon
Question 5. Sodium Fluoride Mouth washes.
Answer:
Sodium Fluoride Mouthwashes Preparation:
- Dissolving 200 mg NaF tablet in 5 teaspoons of fresh clean water
Sodium Fluoride Mouthwashes Method Of Use:
- Rinse daily with 1 teaspoon after brushing
- Rinse for 60 seconds
- Then expectorant
Sodium Fluoride Mouthwashes Advantages:
- 30-40% reduction in DMFT
Question 6. Shoe Leather survey.
Answer:
- Conducted by Dr HTrendley Dean
- It was conducted in 97 localities
- It was done with the help of a questionnaire
AIM:
- To find the level of fluoride at which the tooth starts to blemish
Question 7. Dental Fluorosis.
Answer:
Etiology:
- Excessive intake of fluoride during tooth development
Features:
- Lustreless enamel
- Opaque white patches
- The mottled, striated, and pitted surface
- Yellow/brown stains
- Enamel hypoplasia
Fluorides Viva Voce
- Safely tolerated dose – 8-16 mg/kg body weight
- Toxic dose – 16-32 mg/kg body weight
- Lethal dose – 32-64 mg/kg body weight
- Fluoride varnishes are applied once in six months
- Knutson’s technique is recommended at the age of 3, 7,11, and 13 years
- APF solution is also known as Brudevold solution
- The premolar is most commonly affected tooth by fluorosis
- Duraphat is the most effective varnish in caries reduction
- Chronic toxicity includes dental fluorosis and skeletal fluorosis
