Drugs Acting On Autonomic Nervous System Introduction
The central nervous system (CNS) is composed of the brain and spinal cord. These neurons cannot be regenerated if damaged. The peripheral nervous system (PNS) is made up of peripheral nerves that connect the CNS to the rest of the body. These neurons can be regenerated if damaged. Nerves that exit from the cranium are called cranial nerves while those exiting from the spinal cord are called spinal nerves. There are 31 pairs of spinal nerves and 12 pairs of cranial nerves.
Functional Importance:
- Sensory: Gathers information about changes occurring within and around the body; sensory receptors, at the ends of peripheral nerves, send signals to CNS.
- Integrative: Information is “brought together,” interpreted, to create sensations, create thoughts, add, to memory, make decisions, etc.
- Motor: Sending of signals from CNS to muscles and/or glands to elicit a response.
Peripheral Nervous System
Nerves that transmit signals from the brain are called motor or efferent nerves, while those nerves that transmit information from the body to the CNS are called sensory or afferent. Spinal nerves serve both functions and are called mixed nerves.
The motor (efferent) portion of the nervous system is divided into three separate subsystems, the somatic, autonomic, and enteric nervous systems. Both autonomic and enteric nervous systems function involuntarily.
- The somatic nervous system (mediate voluntary activities).
- The autonomic nervous system (mediate involuntary activities).
- The enteric nervous system (functions to control the gastrointestinal system).
The autonomic nervous system is largely independent (activities are not under direct conscious control). The cranial and spinal nerves connect CNS to heart, stomach, intestines and glands. It controls unconscious activities.
Read and Learn More Medicinal Chemistry III Notes
It is concerned primarily with visceral functions such as cardiac output, blood flow to various organs and digestion. The ANS is further subdivided into the sympathetic and the parasympathetic nervous systems. The sympathetic nervous system is activated in cases of emergencies to mobilize energy, while the parasympathetic nervous system is activated when organisms are in a relaxed state.
The somatic division is largely concerned with consciously controlled functions such as movement, respiration, and posture. The cranial and spinal nerves connect CNS to skin and skeletal muscles. It controls conscious activities.
Autonomic Nervous System (ANS):
The ANS has two major portions: The sympathetic (thoracolumbar) division and the parasympathetic (craniosacral) division. Both divisions originate in nuclei within the CNS and give rise to preganglionic efferent fibers that exit from the brain stem or spinal cord and terminate in motor ganglia.
- The sympathetic preganglionic fibers leave the CNS through the thoracic and lumbar spinal nerves.
- The parasympathetic preganglionic fibers leave the CNS through the cranial nerves (especially the 3rd, 7th, 9th and 10th) and the 3rd and 4th sacral spinal roots.
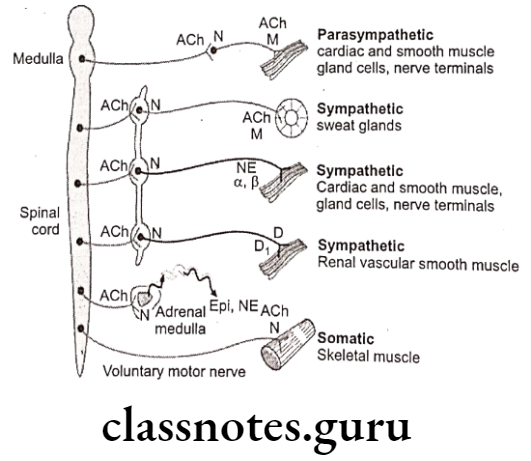
The sympathetic system leads to decrease in digestion, pupil size, urinary output and at the same time increases heart rate, bronchiole dilation, blood glucose, blood to skeletal muscle more of “fight or flight” response.
The parasympathetic system leads to decrease in heart rate, bronchiole dilation, blood glucose, blood to skeletal muscle and whilst, increase in digestion, pupil size, urinary output; more of “rest and digest” response.
Cholinergic Drugs
Nerve impulses elicit responses in smooth, cardiac, and skeletal muscles, exocrine glands, and postsynaptic neurons by liberating specific chemical neurotransmitters. By using drugs that mimic or block the actions of chemical transmitters, we can selectively modify many autonomic functions. These functions involve a variety of effector tissues, including cardiac muscle, smooth muscle, vascular endothelium, exocrine glands and presynaptic nerve terminals. Autonomic drugs are useful in many clinical conditions.
Cholinergic Neurotransmitters:
Cholinergic nerves (Parasympathetic nerves) are found in the peripheral nervous system and central nervous system (CNS) of humans. Parasympathetic nerves regulate processes connected with energy assimilation (food intake, digestion, absorption) and storage. These processes operate when the body is at rest, allowing increased bronchomotor tone and decreased cardiac activity.
Secretion of saliva and intestinal fluids promotes the digestion of foodstuffs, transport of intestinal contents is speeded up because of enhanced peristaltic activity and lowered tone of sphincter muscles.
To empty the urinary bladder (micturition), wall tension is increased by detrusor activation with a concurrent relaxation of sphincter tonus. Activation of ocular parasympathetic fibers results in narrowing of the pupil and increased curvature of the lens, enabling near objects to be brought into focus (accommodation).
Cholinergic neurons release acetylcholine (Ach) as the primary neurotransmitter, serves as mediator at terminals of all postganglionic parasympathetic fibers, in addition to fulfilling its transmitter role at ganglionic synapses within both the sympathetic and parasympathetic divisions and the motor endplates on striated muscle. However, different types of receptors are present at these synaptic junctions.
Synthesis, Storage and Release of Acetylcholine Biosynthesis:
Acetylcholine (Ach) is biosynthesized in cholinergic neuron, in which active transport mechanism is involved in picking up choline molecule from extra synaptic fluid into axoplasm. This transport depends on concentration of Na* and K+ ions. Choline is acetylated in cytoplasm by acetyl coenzyme-A (acetyl-CoA) which is biosynthesized in mitochondria present in nerve terminal. The acetylation is catalyzed by the enzyme choline acetyltransferase (biosynthesized itself in the cholinergic neurons).
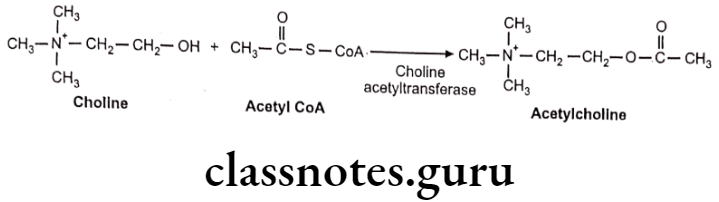
Some of the choline is biosynthesized from amino acid serine, but most of choline used to form Ach is recycled following the enzymatic hydrolysis of Ach in synaptic space.
Storage:
As soon as acetylcholine is synthesized, it is actively transported into cystolic storage vesicle (5000-10000 Ach) located in presynaptic nerve ending, where it is maintained until it is released. Some Ach remains in cystol and is eventually hydrolyzed to acetate and choline. Only the stored form of Ach serves as the functional neurotransmitter.
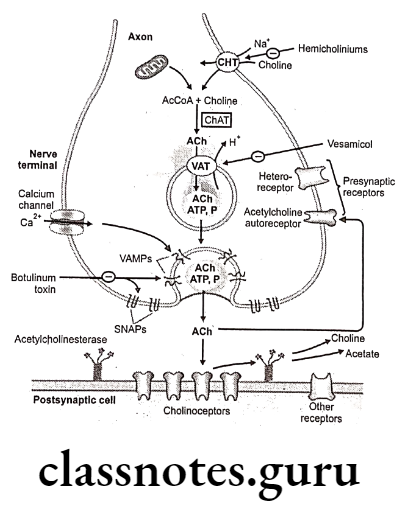
Release:
When impulse reaches to the nerve terminal, it results in release of Ach due to depolarization of the nerve terminal by the action potential. It alters membrane permeability to Ca++, which results into opening of voltage dependant Ca** channel affording an influx of Ca** causes exocytotic release of Ach into the synaptic cleft. (4 Ca** are taken up for each molecule of ACh release).
The ruptures cystolic (synaptic) vesicle is again re-shaped to store the fresh neurotransmitter. Each synaptic vesicle contains a quantum of Ach (one Quanta is equal to 12,000-60,000 molecules of ACh). A single action potential causes the release of several hundred quanta of ACh into the synapse.
Metabolism:
At the postsynaptic effector cell membrane, ACh interacts with its receptors. Because these receptors can also be activated by the alkaloid muscarine, they are referred to as muscarinic (M) cholinoceptors. In contrast, at ganglionic and motor endplate cholinoceptors, the action of ACh is mimicked by nicotine, therefore they are also said to be nicotinic (N) cholinoceptors.
Released ACh is rapidly hydrolyzed and inactivated by a specific acetylcholine esterase (AChE), present on pre- and post-junctional membranes, or by a less specific serum choline esterase (butyryl choline esterase), a soluble enzyme present in serum and interstitial fluid. Enzymatic hydrolysis, results into formation of choline which poorly binds to acetylcholine receptors.
There are enough AChE present in synapse to hydrolyse approximately 3 x 108 molecules of Ach in 1 millisecond. Thus there is adequate enzyme activity to hydrolyse all of ACh released by one action potential.
The empty synaptic vesicles, which are returned to the axonal terminal bulb by endocytosis, are filled with ACh.

The vascular release of ACh is reported to be inhibited by excess of Mg**. The release of ACh along with the extracelluler Ca** then mobilize the intracellular Ca** from sac present on sarcoplasmic reticulum. The increase in concentration of free intracellular Ca** activates calmodulin-dependent myosin light kinase and phosphorylation of myosin takes place which leads to muscle contraction.
Cholinergic Receptors
There are two different types of cholinergic receptors i.e. nicotinic receptor and muscarinic receptor. These receptors differ in composition, location and pharmacological functioning. They have specific agonists and antagonists activity. They have been characterised as nicotinic and muscarinic on the basis of their ability to be bound by the natural alkaloids nicotine and muscarine respectively. Both the receptors have subtypes that differ in location and specificity.
Nicotinic Receptors
Nicotinic receptors are coupled directly to ion channels and when activated by Ach, mediate very rapid responses. Ion channels are responsible for the electrical excitability of nerve and muscle cells and for the sensitivity of sensory cells. The channels are pores that open or close in an all or nothing fashion on time scales ranging from 0.1 to 10 milliseconds to provide aqueous pathways through the plasma membrane that ions can transverse.
The nicotinic ACh receptor is a glycoprotein embedded into the polysynaptic membrane and consists of 5 subunits of polypeptide chains. Subunits are arranged like a rosette surrounding the Na channel. The two alpha subunits carry two ACh binding sites with negatively charged groups which combine with the cationic group of ACh and open Na* channel.
When the neurotransmitter ACh binds to the nicotinic receptor, it causes a change in the permeability of the membrane to allow passage of small cations Ca** and Na* and K+. The. physiological action is to temporarily depolarize the nerve end plate to muscular contraction at a neuromuscular junction.
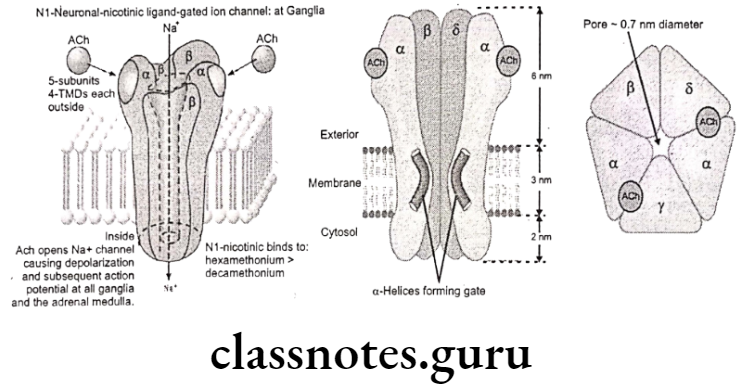
Nicotinic Receptor Subtypes:
Nu-cholinoceptors (N):
- Location: Neuromuscular junction.
- Function: Depolarization of muscle end plate and contraction of skeletal muscle.
- They are blocked by succinylcholine, d-tubocurarine and decamethonium, and are stimulated by phenyl trimethyl ammonium.
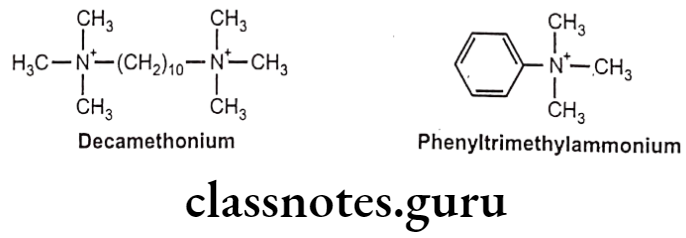
NN-cholinoceptors (N2):
- Location: Autonomic ganglia.
- Function: Depolarization of post ganglonic membrane (in adrenal medulla- catecholamine release).
- They are blocked by hexamethonium and trimethaphan, but are stimulated by tetramethyl ammonium and dimethyl-4-phenylpiperazinium (DMPP).
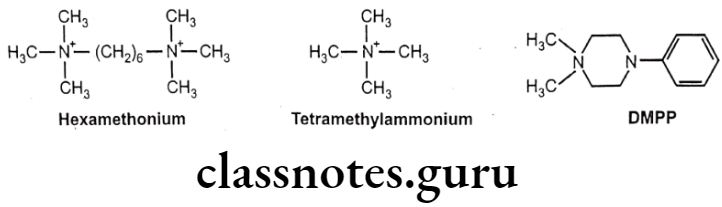
Muscarinic Receptors
Muscarinic receptors play an important role in regulating the functions of organs innervated by the autonomic nervous system. The action of ACh on muscarinic receptors is to stimulate secretions from salivary and sweat glands, secretions and contraction of the gut and constriction of the respiratory tract. It inhibits contraction of the heart and relaxes smooth muscle of blood vessels.
Muscarinic receptors mediate their effects by activating guanosine triphosphate (GTP)- binding proteins (G-proteins). These receptors have seven protein helixes that passed through the plasma membrane, creating four extracellular domains and four intracellular domains. The extracellular domain of the receptor contains the binding site for Ach. The intracellular domain couples with G-proteins to initiate the biochemical changes that results in pharmacological action from receptor activation.
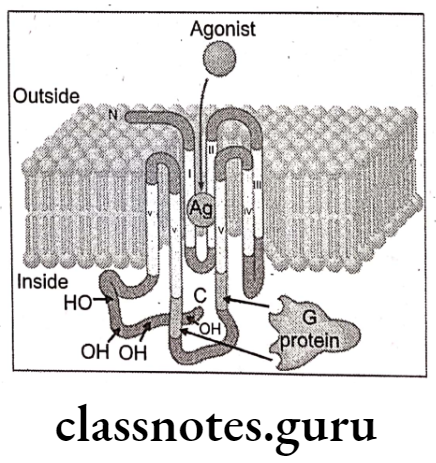
Muscarinic Receptor Subtypes:
Subtypes of muscarinic receptors are located at the CNS and peripheral nervous system. Muscarinic receptor subtypes have been defined on the basis of their affinity for agonists, antagonists and the pharmacological effects cause.
They are classified into subtypes according to their molecular structure, signal transduction, and ligand affinity in the M1, M2, M3, M4 and Ms.
- M1-receptors are present on nerve cells, exocrine glands and autonomic ganglia, where they mediate a facilitation of impulse transmission from preganglionic axon terminals to ganglion cells. In humans, these receptors seem to affect the rapid eye movement (REM), sleep, emotional responses, affective disorders including depression and modulation of stress. They are also located in intramural ganglia of stomach wall which on stimulation cause gastric secretion.
- M2-receptors are also called cardiac muscarinic receptors because they are located in the atria and conducting tissue of the heart. Their stimulation causes a decrease in the strength and rate of cardiac muscle contraction. These effects may be produced by affecting intracellular K+ and Ca** levels in heart tissue. Opening of K+ channels leads to slowing of diastolic depolarization in sinoatrial pacemaker cells and a decrease in heart rate. These receptors may also act through an inhibitory G protein (G1) to reduce adenylate cyclase activity and lower cyclic 3′,5′-adenosine monophosphate (CAMP) levels in cardiac cells. M2-receptors can also serve as autoreceptors and inhibit release of ACh’ on presynaptic terminals of postganglionic cholinergic nerves.
- M3-receptors are referred to as glandular muscarinic receptors. They are located in exocrine glands and smooth muscles and increase secretory activity such as secretions from lacrimal, salivary, bronchial, pancreatic and mucosal cells in the GIT. Stimulation of M3-receptors also results into contraction of visceral smooth muscles.
- M4-receptors, like M2-receptors, they act through G1 protein to inhibit adenylate cyclase. They also function by a direct regulatory action on K* and Ca++ ion channels. Stimulation of M4-receptors in tracheal smooth, inhibit the release of Ach in same manner as M2-receptors.
- Ms-receptors, messenger RNA (mRNA) is found in the substantia nigra. It may regulate dopamine release at terminals within the striatum.
Classification Of Para-Sympathomimetic Agents
Acetylcholine receptor stimulants and cholinesterase inhibitors:
Acetylcholine receptor stimulants comprise a large group of drugs that mimic ACh (cholinomimetic agents). Cholinoceptor stimulants are classified by their spectrum of action depending on the type of receptor-muscarinic (M) or nicotinic (N) that is activated. They are also classified by their mechanism of action, as some cholinomimetic drugs bind directly to (and activate) cholinoceptors, while others act indirectly by inhibiting the hydrolysis of endogenous ACh.
Direct-Acting Cholinergic Drugs:
- Choline ester (stimulants of M- and N-receptors):
e.g. Acetylcholine, Carbachol. - Cholinomimetic Alkaloids:
- Stimulants of M-receptors
e.g. Pilocarpine, Cevimeline, Bethanechol, Musacarine, Phalloidin
- Stimulants of M-receptors
- Stimulants of N-receptors
e.g. Nicotine, Cytisine, Lobeline
- Stimulants of N-receptors
Indirect-Acting Cholinergic Drugs (anticholinesterase (Anti-ChEs) drugs):
- Reversible drugs (carbamates class)
- With N3+ (cross BBB)
e.g. Alkaloids: Galantamine, Physostigmine
e.g. Synthetic drugs: Donepezil, Rivastigmine, Tacrin
- With N3+ (cross BBB)
- With N4+ (do not cross BBB)
e.g. Demecarium, Edrophonium, Neostigmine, Pyridostigmine
- With N4+ (do not cross BBB)
- Irreversible anticholinesterase agents (organophosphates class)
- Thiophosphate insecticides:
e.g. Parathion, Malathion
- Thiophosphate insecticides:
- Nerve paralytic gases (chemical warfare):
e.g. Tabun, Sarin, Soman.
- Nerve paralytic gases (chemical warfare):
Structure-Activity Relationship: Choline ester (stimulants of M- and N- receptors):
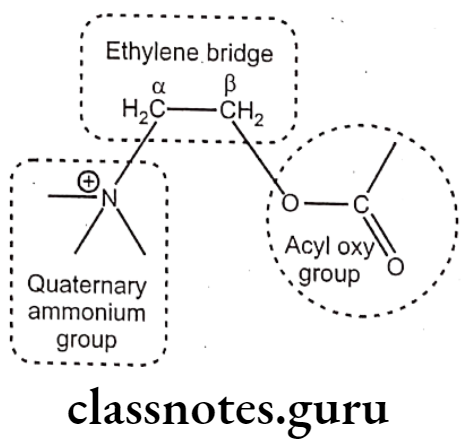
Quaternary Ammonium Group (N4*):
- The quaternary ammonium group is essential for activity; at the cationic head is the major point of receptor interaction.
- The replacement of nitrogen (N) by sulfur (S), arsenic (As) or selenium (Se) leads to decrease in activity.
- The conversion of the quaternary ammonium group (N) to primary, secondary or tertiary leads to less active derivatives.
- The replacement of the methyl (CH3) substitution with higher carbon skeleton (more ‘C’-atoms) functional groups like Propyl (C3H7) or higher alkyl groups leads to inactive derivatives.
- The quaternary ammonium group should be followed by a chain of five atoms for maximal muscarinic activity; it is referred as “five-atom rule”.
Ethylene Bridge (-CH2-CH2-):
- With the increase or decrease in the carbon chain length, the activity of the derivatives is rapidly reduced.
- Substitution on the a or ẞ position with methyl (CH3) group results in decreased activity, but increased duration of action, this phenomenon is due to the reduced metabolism due to steric hindrance.
- B-substituted choline derivative exhibits longer duration of action than that of its a-substituted choline analog.
- The substitution of methyl (CH3) group on a or ẞ position leads to selectivity towards receptors.
- a-Methylcholine (methyl group substituted at a-position derivative) is less potent than Ach, but has more nicotinic activity than muscarinic activity.
- B-Methylcholine; Methacholine (methyl group substituted at B-position derivative) is less potent than ACh, but more selective to Muscarinic receptor.
- Substitution on the aor B.position with alkyl function larger than methyl group like propyl or butyl leads to derivatives with no activity.
Acyl oxy group:
- The conversion of acetyl (CH3CO) group to higher group like propionyl (C2H, CO) or butyryl (C3H,CO) functions results in decrease in activity, indicated by the five-atom rule.
- The ester derivatives of higher molecular carboxylic acids or of aromatic acids results in antagonist property.
- The acetyl (CH3CO) group/methyl ester in Ach is rapidly hydrolyzed by AchE, replacement of the same with carbomate (NH2CO) ester results in resistance to hydrolysis leading to potent derivative with increased duration of activity.
- Carbachol (carbamate ester of choline) is more stable to hydrolysis and can be administered orally.
- The replacement of the ester function with ether or ketonic function while sticking by the five-atom rule will also result in stable and potent derivatives.
- Choline ethyl ether derivative is as potent as ACh.
Direct-Acting Cholinergic Drugs
Choline Ester
Acetylcholine:
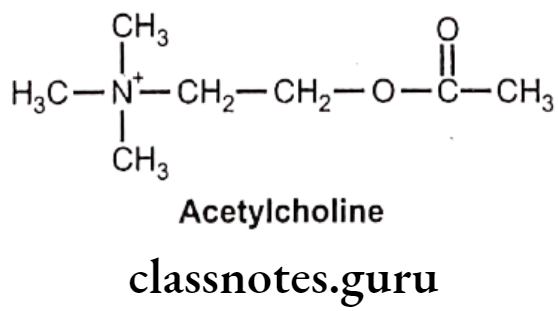
- Acetylcholine is chemically, 2-acetyloxyethyl (trimethyl)azanium.
- Acetylcholine chloride exerts a powerful stimulant effect on the parasympathetic nervous system.
- It does not have any therapeutic importance as it is rapidly hydrolyzed by cholinesterase.
- Stimulation of the vagus and the parasympathetic nervous system produces a tonic action on smooth muscle and induces a flow from the salivary and lacrimal glands. Its cardiac-depressant results in negative chronotropic effect (decrease in heart rate) and negative inotropic effect (decrease in the force of myocardial contractions).
- It also has vasodilatory action on the peripheral vascular system.
- When it gives systemically, it produces bronchial constriction as a characteristic side effect.
- A non-selective muscarinic receptor antagonist (i.e. atropine) is the most effective antagonist to the action of Ach.
Uses:
- Acetylchloline is a cardiac depressant and an effective vasodilator.
- It is used during ophthalmic surgery (cataract)..
- It is used as ex-vivo agent to study pharmacological effect of parasympathomimetic.
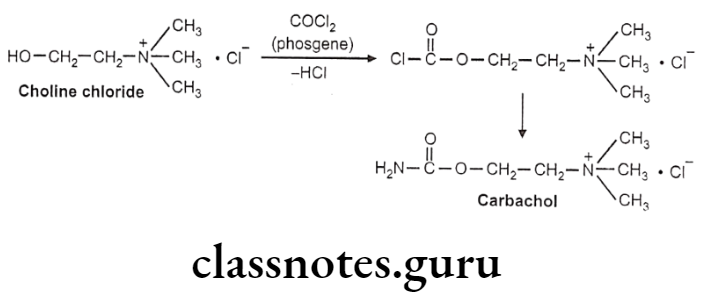
Carbachol:
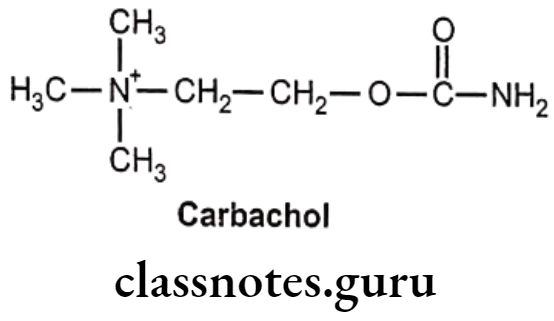
- Carbachol is chemically, 2-carbamoyloxyethyl(trimethyl)azanium.
- It is a choline carbamate, a parasympathomimetic that stimulates both muscarinic and nicotinic receptors.
- The pharmacological activity of carbachol is similar to that of ACh.
- It is not well absorbed in the gastro-intestinal tract and does not cross the blood- brain barrier.
- It is usually administered topical ocular or through intraocular injection.
- It is not easily metabolized by cholinesterase therefore, more stable in aqueous solutions.
- It can also act indirectly by promoting release of ACh and by its weak anticho- linesterase activity.
- It has a 2 to 5 minute onset of action and its duration of action is 4 to 8 hours with topical administration and 24 hours for intraocular administration.
- Since carbachol is poorly absorbed through topical administration, benzalkonium chloride is mixed in it to promote absorption.
Uses:
- Carbachol is primarily used for treating glaucoma, or during ophthalmic surgery (cataract).
- It is administered as an ophthalmic solution, its principal effects are miosis and increased aqueous humour outflow.
Bethanechol:
- Bethanechol is chemically, 2-carbamoyloxypropyl(trimethyl)azanium.
- It is B-methylcholine carbamate and non-specific, that selectively stimulates muscarinic receptors without any effect on nicotinic receptors.
- It is not readily hydrolyzed by cholinesterase and therefore has a long duration of action.
- It has pharmacological properties similar to that of methacholine.
Uses:
- Bethanechol is given orally or subcutaneously to treat urinary retention and abdominal distention resulting from general anaesthetic, diabetic neuropathy of the bladder, or a side effect of antidepressants; or to treat gastrointestinal lack of muscular tone.
- It has potential benefit in the treatment of cerebral palsy.
- It is contraindicated in patients with asthma, coronary insufficiency, peptic ulcers, intestinal obstruction and hyperthyroidism.
Methacholine:
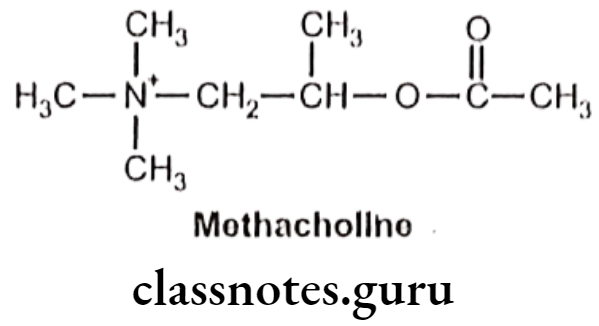
- Methacholine is chemically, 2-acetyloxypropyl (trimethyl)azanium.
- It is ẞ-methylcholine acetate and is a synthetic choline ester that acts as a non- selective muscarinic receptor agonist but has little effect on the nicotinic receptors.
- It is not readily hydrolyzed by cholinesterase and therefore has a long duration of action.
Uses:
- Methacholine is primarily used to diagnose bronchial hyper-reactivity (asthma and chronic obstructive pulmonary disease), known as the bronchial challenge test or methacholine challenge.
- It also leads to adverse cardiovascular effects, bradycardia and hypotension.
Cholinomimetic Alkaloids
Pilocarpine:
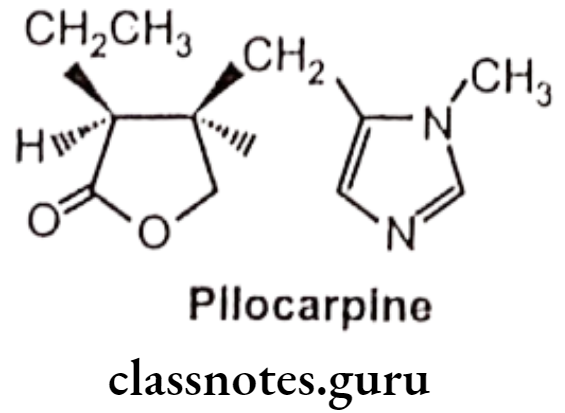
- Pilocarpine is an alkaloid obtained from the dried leaflets of Pilocarpus jaborandi or Pilocarpus microphyllus.
- It is chemically, (3S,4R)-3-ethyl-4-[(3-methylimidazol-4-yl)methyl]oxolan-2-one.
- It is a non-selective agonist on the muscarinic receptors.
- It works by activating cholinergic receptors of the muscarinic type which cause the trabecular meshwork to open and the aqueous humor to drain from the eye.
Uses:
- Pilocarpine is used to treat increased pressure inside the eye (glaucoma).
- As ophthalmic solution, it is used for angle closure glaucoma, ocular hypertension, and open angle glaucoma.
- By oral medication, it stimulates the secretion of saliva and sweat and use to treat dry mouth.
- Common side effects include irritation of the eye, increased tearing, headache, and blurry vision.
- Systemic injection of pilocarpine can cross blood-brain barrier, which can lead to chronic epilepsy and can be used as epilepsy inducing agent in rodents in order to study human epilepsy.
Indirect-Acting Cholinergic Drugs/Cholinesterase Inhibitors
The acetylcholine action at the synaptic junction is terminated by metabolism catalyzed by the action of AchE. The AchE enzyme hydrolyses Ach to choline and acetate. Inhibition of the enzyme leads to increased concentration of Ach and prolongs the life of the available Ach at the synaptic junction, thus producing a cholinergic agonist effect. The enzyme inhibitors are classified based on the affinity towards the enzyme and the type of interaction.
Reversible Cholinesterase Inhibitors
Physostigmine:
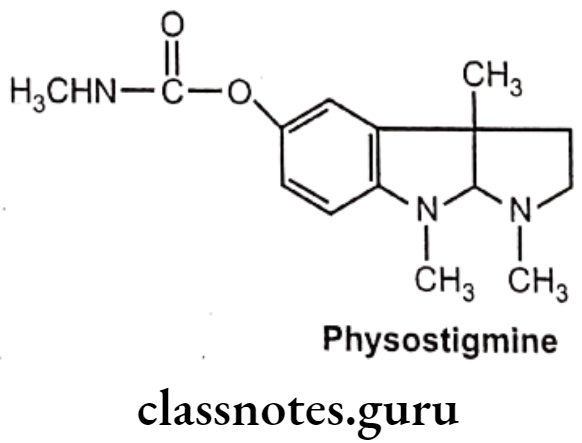
- Physostigmine is an alkaloid obtained from the dried ripe seed of Physostigma venenosum.
- It occurs naturally in the Calabar bean and the Manchineel tree.
- It is chemically, [(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-methylcarbamate.
- It is a highly toxic parasympathomimetic alkaloid, a reversible cholinesterase. inhibitor.
- It acts by interfering with the metabolism of acetylcholine.
- In solution it undergoes hydrolysis to form methyl carbamic acid and eseroline, neither of which inhibits Ach.
Uses:
- Physostigmine is used to treat glaucoma and delayed gastric emptying.
- As it enhances the transmission of acetylcholine signals in the brain and can cross the blood-brain barrier, physostigmine is used as an antidote to treat anticholinergic poisoning (Datura stramonium, Atropa belladonna, atropine, scopolamine, and other anticholinergic drug overdoses poisoning).
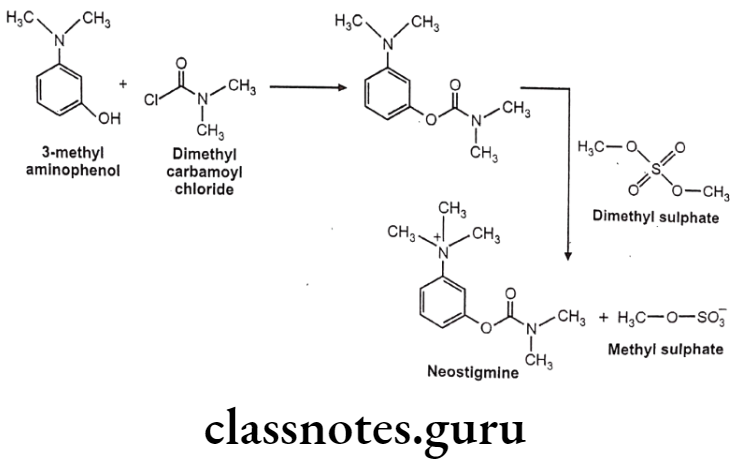
Neostigmine:
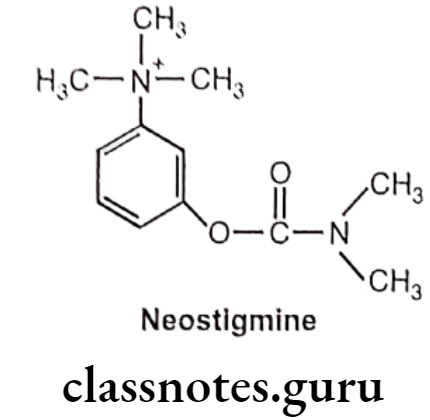
- Neostigmine is chemically, [3-(dimethylcarbamoyloxy)phenyl]-trimethylazanium.
- It has quaternary nitrogen; hence, it is polar and does not cross the blood-brain barrier, but it does cross the placenta.
- It works by blocking the action of acetylcholinesterase and therefore increases the levels of acetylcholine.
Uses:
- Neostigmine is used to treat myasthenia gravis, ogilvie syndrome (acute dilation of the colon), and urinary retention. Common side effects include nausea, increased saliva and abdominal pain.
- In myasthenia gravis there are too few acetylcholine receptors so with the acetyl cholinesterase blocked, acetylcholine can bind to the few receptors and trigger a muscular contraction.
- It is also used as an antidote to non-depolarizing neuromuscular blocking drug.
Pyridostigmine:
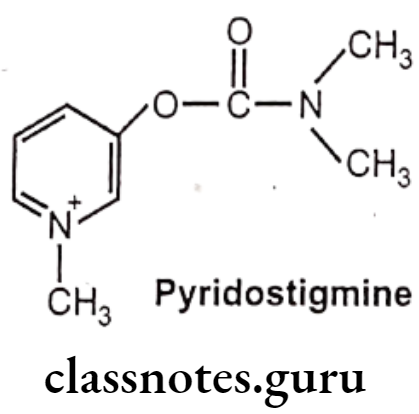
- Pyridostigmine is chemically, (1-methylpyridin-1-ium-3-yl) N,N-dimethylcarbamate.
- It is a quaternary carbamate inhibitor of cholinesterase that does not cross the blood-brain barrier.
- Common side effects include nausea, diarrhea, frequent urination, and abdominal pain.
Uses:
- Pyridostigmine is used to treat myasthenia gravis. (Long term neuromuscular disease that leads to varying degrees of skeletal muscle weekness).
- It is used to treat muscle weakness in people with myasthenia gravis or forms of congenital myasthenic syndrome.
- It is also used together with atropine to end the effects of neuromuscular blocking medication of the non-depolarizing type.
Edrophonium Chloride:
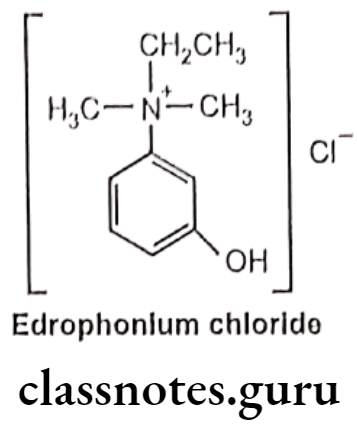
- Edrophonium is chemically, ethyl-(3-hydroxyphenyl)-dimethylazanium, chloride.
- It is a readily reversible acetylcholinesterase inhibitor and acts by competitively inhibiting the enzyme.
- It has a direct cholinomimetic effect on skeletal muscle, which is greater than that of most other anticholinesterase drugs.
Uses:
- Edrophonium chloride is used as a diagnostic agent to differentiate myasthenia gravis from cholinergic crisis and Lambert-Eaton. (Lambert-Eaton myasthenic syndrome is an autoimmune disease where the neuron is unable to release enough ACh for normal muscle function).
- In myasthenia gravis, the body produces autoantibodies which block, inhibit or destroy nicotinic acetylcholine receptors in the neuromuscular junction. Edrophonium reduces the muscle weakness in myasthenia gravis.
- In a cholinergic crisis, resulting from too much neuromuscular stimulation, edrophonium will make the muscle weakness worse by inducing a depolarizing block.
Tacrine Hydrochloride:
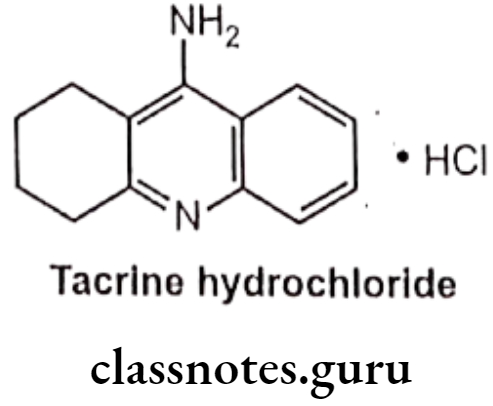
- Tacrine hydrochloride is chemically, 1,2,3,4-tetrahydro-9-aminoacridine, hydro- chloride.
- It is a centrally acting reversible anticholinesterase and indirect cholinergic agonist.
Uses:
- It was the first centrally acting cholinesterase inhibitor approved for the treatment of Alzheimer’s disease (progressive loss of brain cells that leads to memory loss and decline of other thinking skills).
Ambenonium Chloride:
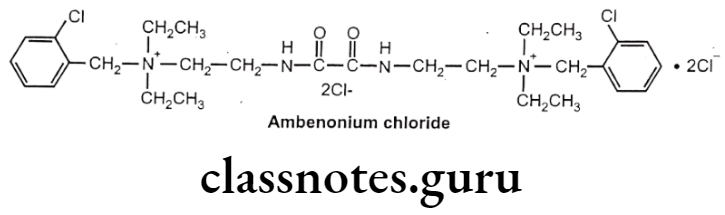
- Ambenonium dichloride is chemically, (2-chlorophenyl)methyl-[2-[[2-[2-[(2-chloro- phenyl) methyl-diethylazaniumyl)ethylamino]-2-oxoacetyl]amino]ethyl]-diethylazanium; dichloride.
- It acts by suppressing the activity of AChE.
- It possesses a relatively prolonged duration of action and causes fewer side effects in the GI tract than the other anticholinesterase agents.
- Because of its quaternary ammonium structure, it is absorbed poorly from the GI tract.
- In moderate doses, the drug does not cross the blood brain barrier.
- Ambenonium chloride is not hydrolyzed by cholinesterases.
Uses:
- Ambenonium is a reversible cholinesterase inhibitor used in the management of myasthenia gravis in patients who do not respond satisfactorily to neostigmine or pyridostigmine.
- It is used to treat muscle weakness due to myasthenia gravis disease or defect of the neuromuscular junction.
Irreversible Cholinesterase Inhibitors
They covalently bond with the AchE enzyme to irreversibly deactivate them, they are referred as nervé poisons and are used as agricultural insecticides. Due to the irreversible inhibition of the enzyme, Ach accumulates at the synaptic junction leading to a high concentration of the Ach and thus to produce agonist effect. These compounds belong to the class of Organophosphorus esters. A general formula for such compounds is as follows:
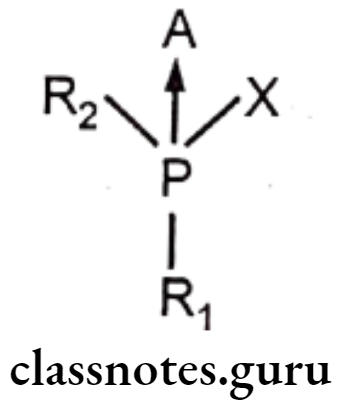
- R1 = Alkoxyl group
- R2 = Alkyl / Alkoxyl / aryl/aryloxy/Tertiary Amine.
- X = A good leaving group like F, CN, thiomalate, p-nitrophenyl
- A Oxygen / Sulphur
- ‘A’ is usually oxygen or sulphur, but may also be selenium.
- When ‘A’ is other than oxygen, biological activation is required before the compound becomes effective as an inhibitor cholinesterase.
- Phosphorothionates (when ‘A’ is ‘S’) have much poorer electrophilic character and 105 folds weaker anticholinesterase activity than their oxygen analogues.
- ‘X’ is the leaving group when the molecule reacts with the enzyme.
- The ‘R’ moiety imparts lipophilicity to the molecule and contributes to its absorption through the skin.
- Inhibition of AChE by organo-phosphorous compound takes place in two steps, association of enzyme and inhibitor and the phosphorylation step.
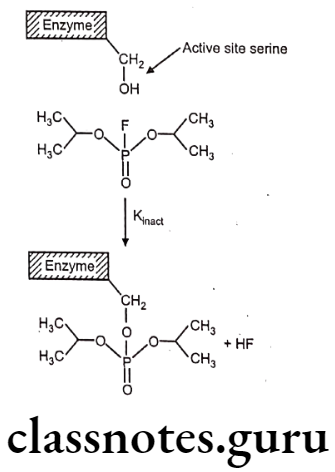
Isofluorophate (Diisopropyl fluorophosphate):
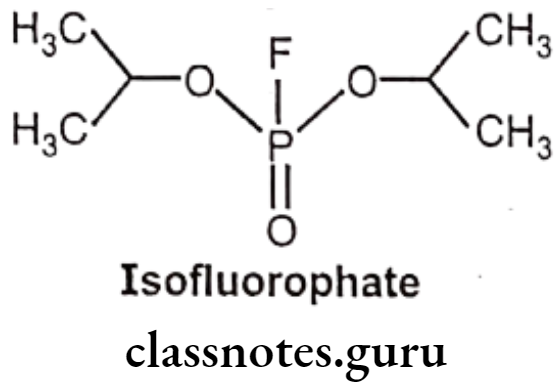
- Diisopropyl fluorophosphates is chemically, 2-[fluoro(propan-2-yloxy) phosphoryl]- oxypropane.
- It is an organophosphorus insecticide, a parasympathomimetic drug irreversible anti- cholinesterase.
- Since it irreversibly inhibits cholinesterase, its activity lasts for days or even weeks.
- It must be handled with extreme caution, as it can be absorbed readily through intact epidermis and more so through mucous tissues.
Uses:
- Isofluorophate has been used in ophthalmology as a miotic agent in treatment of chronic glaucoma.
- It is also used as a miotic in veterinary medicine.
- It is used as an experimental agent in neuroscience because of its acetyl- cholinesterase inhibitory properties and ability to induce delayed peripheral neuropathy.
Echothiophate Iodide:
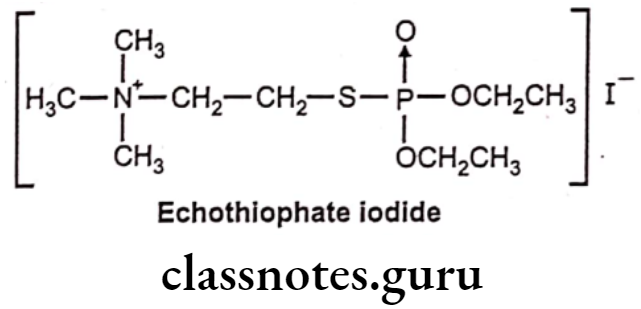
- Echothiophate iodide is chemically, 2-diethoxyphosphorylsulfanylethyl (trimethyl)- azanium; iodide.
- It is also known as Phospholine. It is a parasympathomimetic drug, an irreversible acetylcholinesterase inhibitor.
- It has long duration of action and its effects can last a week or more.
Uses:
- Echothiophate iodide is used as an ocular antihypertensive in the treatment of chronic glaucoma and in accommodative esotropia.
Parathion:
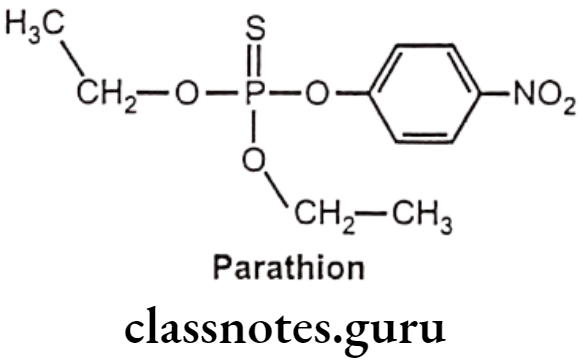
- Parathion is chemically, O,O-Diethyl O-(4-nitrophenyl) phosphorothioate.
- It is a relatively weak cholinesterase inhibitor.
- It is highly toxic to non-target organisms, including humans, so its use has been restricted.
- Absorbed parathion is rapidly metabolized to paraoxon by enzymes present in liver microsomes and insect tissue.
- Parathion is also metabolized by liver microsomes to yield p-nitrophenol and diethylphosphate; the later is inactive as an irreversible cholinesterase inhibitor.
Uses:
- Parathion is an organophosphate insecticide and acaricide.
- It is used as an agricultural insecticide.
Malathion:
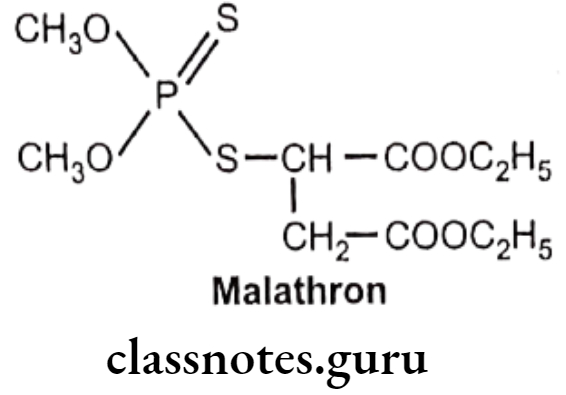
- Malathion is chemically, 2-[(dimethoxyphosphinothioyl)-thio]butanedionic acid diethyl ester, or diethyl-2-dimethoxyphosphinothioyl sulfanyl butane dioate.
- It is a poor inhibitor of cholinesterases.
- It is an organophosphate insecticide which acts as an acetylcholinesterase inhibitor.
Uses:
- Malathion is used as an agricultural insecticide.
Acetylcholine Sterase Reactivators
Oxime class of compounds can provide a nucleophilic attack on the phosphorylated AchE (deactivated) and can regenerate the free enzyme. Choline-reactivating oximes are effective antidotes in organophosphorus insecticide or sulfonate poisoning, a state of acetylcholine excess because of cholinesterase inhibition.
Pralidoxime chloride:
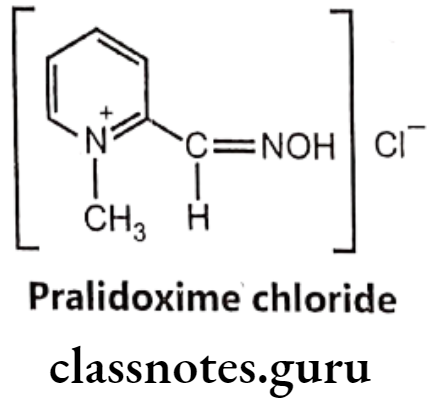
- Pralidoxime chloride is chemically, 2-formyl-1-methylpyridinium chloride oxime.
- It is also said to be 2-pyridine aldoxime methyl chloride or 2-PAM, usually as the chloride or iodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase.
- These drugs antagonize the effects of accumulated acetylcholine at the cholinergic synapses by reactivating the inhibited cholinesterase.
- Reactivation of cholinesterases is importance for the survival due to organo- phosphorus insecticide poisoning.
- It is a quaternary ammonium compound and most effective by intramuscular, subcutaneous, or intravenous administration.
Uses:
- Pralidoxime chloride or 2-PAM is used to combat poisoning by organophosphates or acetyl cholinesterase inhibitors (nerve agents) in conjunction with atropine.
- 2-PAM is the most popular oxime for this purpose.
- It may be effective against some phosphates that have a quaternary nitrogen.
- It is also an effective antagonist for some carbamates, such as neostigmine methylsulfate and pyridostigmine bromide.
Cholinergic Blocking Agents
Agents that block or antagonize the action of acetylcholine, at post ganglionic nerve ending are called as cholinergic blockers or parasympatholytic agents. They act by inhibiting muscarinic action of acetylcholine on smooth muscle contraction and including exocrine gland secretion.
Cholinergic blockers are classified into two classes, based on mechanism of action:
- Agents that inhibit, synthesis or release of Ach: These agents are not been effective clinically and hence not used.
- Agents that block Muscarinic receptor at nerve ending: These agents are also said to be parasympathetic post ganglionic muscarinic receptor blockers. These competitive reversible antagonists are large molecules, derived from muscarinic antagonist with one or more bulky groups, thus capable of binding to the receptor.
Structure-activity relationship of cholinergic blockers:
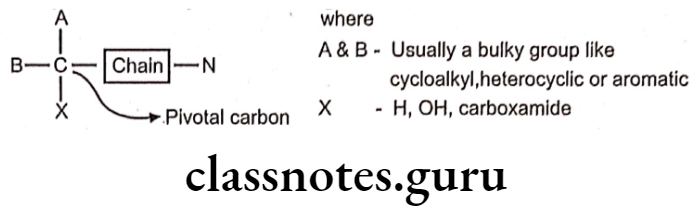
- The Pivotal carbon is of crucial importance in relation to the development of cholinergic antagonists.
- Generally, the amine function need to be a quaternary ammonium group (N4*) or it can be tertiary (N3*) that is protonated at physiological pH to form the cationic head. The quaternary ammonium group is essential for activity, as the cationic head is the major point of receptor interaction.
- The amine function is separated from the pivotal carbon by a chain / bridge, the chain may be a ester, ether or a hydrocarbon function bridge.
- The substitution ‘A’ and ‘B’ contains at least one aromatic moiety that can exhibit Van der Waals’ interaction with the receptor and the other one as cycloalkyl, aliphatic hydrocarbon or alkyl group for hydrophobic interaction with the receptor.
- The ‘X’ function can be hydroxy function or a carboxamide group or just hydrogen to exhibit hydrogen bond interaction with the receptor.
The Amine Function:
- The amine group should be cationic in nature, which is the primary point for interaction with the receptor core.
- It may be a quaternary ammonium group (N4) or it can be tertiary (N3+) that is protonated at physiological pH to form the cationic head.
The ‘X’ Functions as Hydrogen (H) / Hydroxyl group (OH):
- The hydroxyl group (OH) is not prerequisite for activity, but suitably placed alcoholic hydroxyl function enhances the activity.
- The ‘OH’ group contributes in hydrogen bonding with the amino acid residues present in the receptor.
The Chain Function:
- The chain function can be an ester/ether / amino alcohol / hydrocarbon moiety.
- The ester moiety is found to be more effective, this can be indicated as the agonist, also poses a ester function, contributing for a better binding affinity at the receptor site.
- The ether amino alcohol/ hydrocarbon moieties are also clinically effective derivatives.
The Cyclic Substituents – ‘A’ and ‘B’:
- At least one cyclic moiety as a substituent at position ‘A’ / ‘B’ is prerequisite for activity.
- The cyclic substituent may be phenyl, cyclohexyl etc., the phenyl moiety predominant as an important substitution.
- Aromatic acid ester derivatives produce decrease in anticholinergic activity, but results in compounds with potential local anaesthetic activity.
Classification Of Muscarinic Receptor Blockers
- Natural and semi-synthetic derivatives:
- Solanaceous alkaloids and their synthetic analogs
- Synthetic derivatives:
- Amino alcohol ester derivatives
- Amino alcohol ether derivatives
- Amino alcohol derivatives
- Amino amide derivatives
- Miscellaneous derivatives
Natural and Semi-Synthetic Derivatives
Solanaceous Alkaloids and their Synthetic Analogs:
The solanaceous alkaloids, represented by hyoscyamine, atropine and scopolamine (hyoscine) are the major class of antimuscarinic drugs. These alkaloids are found principally in Henbane (Hyoscymus niger), deadly nightshade (Atropa belladonna) and Jimson weed (Datura stramonium).
Crude drugs containing these alkaloids have been used since early times for their marked medicinal properties which depend largely on inhibition of the parasympathetic nervous system and stimulation of the higher nervous centers.
- Belladonna alkaloid (Atropine) possesses a weak local anaesthetic activity and has been used topically for its analgesic effect on hemorrhoids, certain skin infections and various itching dermatoses.
- It is also used as mydriasis.
- It increases the heart rate (by depression of the vagus nerve).
- It depresses the motility of the GIT, and acts as an antispasmodic on various smooth muscles (ureter, bladder, and biliary tract).
- It also directly stimulates the respiratory center.
- The action of scopolamine containing drugs differs from those containing hyoscyamine and atropine in having no CNS stimulation; they have significant narcotic or sedative effect.
- The use of this group of drugs is accompanied by a fairly high incidence of reactions because of individual idiosyncrasies.
- Death from over dosage usually results from respiratory failure.
Atropine Sulphate:
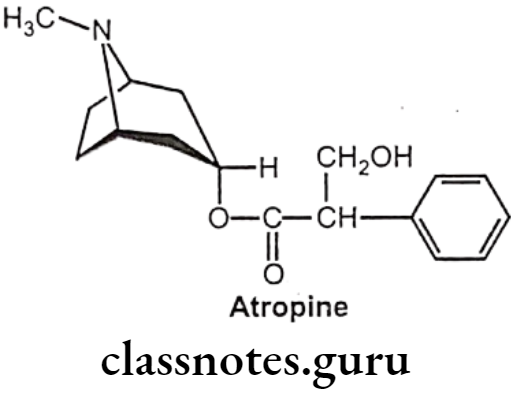
- Atropine occurs naturally in a number of plants of the nightshade family including deadly nightshade, Jimson weed, and mandrake.
- It is chemically, [(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 3-hydroxy-2-phenyl- propanoate.
- It is the tropine ester of racemic tropic acid and is optically inactive.
- It is an enantiomeric / racemic mixture of d-hyoscyamine and l-hyoscyamine, with most of its physiological effects due to l-hyoscyamine.
- Atropine sulfate is prepared by neutralizing atropine in acetone or ether with an alcoholic solution of sulfuric acid, with care used to prevent hydrolysis.
- It is an antimuscarinic drug that works by inhibiting the parasympathetic nervous system.
- Atropine is a competitive reversible antagonist of the muscarinic acetylcholine receptor types M1, M2, M3, M4 and Ms.
- In the eye, atropine induces mydriasis by blocking contraction of the circular pupillary sphincter muscle, thereby allowing the radial iris dilator muscle to contract and dilate the pupil.
Uses:
- Atropine is used to treat pesticide poisonings and to decrease saliva production during surgery.
- As an ophthalmic preparation it is used to treat uveitis (swelling of the middle layer of the eye) and early amblyopia (vision development disorder).
- Atropine induces cycloplegia by paralyzing the ciliary muscles and helps to relieve pain associated with iridocyclitis (an inflammation of the iris), and treats ciliary block (malignant) glaucoma.
- Common side effects include a dry mouth, large pupils, urinary retention and constipation.
Hyoscyamine Sulphate:
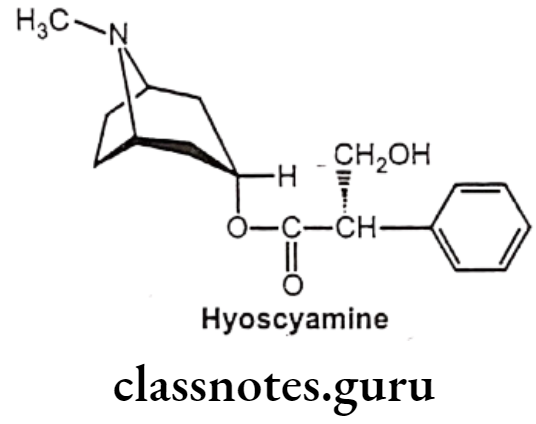
- Hyoscyamine (daturine) is a tropane alkaloid.
- It is a secondary metabolite found in certain plants of the family Solanaceae like Atropa belladonna.
- It is chemically, [(1S,5R)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] (2S)-3-hydroxy-2- phenylpropanoate.
- It is the levorotatory isomer of atropine.
Uses:
- Hyoscyamine is used to provide symptomatic relief of spasms caused by various lower abdominal and bladder disorders including peptic ulcers, irritable bowel syndrome, diverticulitis, pancreatitis, colic, and interstitial cystitis.
- It has also been used to relieve some heart problems, control some of the symptoms of Parkinson’s disease, as well as for control of abnormal respiratory symptoms and “hyper-mucus secretions” in patients with lung disease.
- It is also useful in pain control for neuropathic pain, chronic pain and palliative care; and for those with intractable pain from treatment resistant, untreatable, and incurable diseases.
- It is used to treat disorders of the urinary tract.
- It is used to treat spasms of the bladder and serves as a urinary stimulant.
- When combined with opioids, it increases the level of analgesia obtained.
Scopolamine:
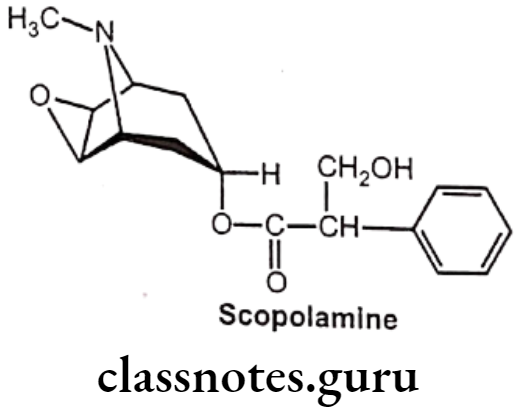
- Hyoscine is produced from plants of the family Solanaceae like Atropa belladonna.
- The name “scopolamine” is derived from one type of nightshade known as Scopolia, while the name “hyoscine” is derived from another type known as Hyoscyamusniger.
- Hyoscine is in the antimuscarinic family of medications and works by blocking some of the effects of acetylcholine within the nervous system.
Uses:
- Scopolamine (Hyoscine) is a medication used to treat motion sickness and postoperative nausea and vomiting.
- It is also used before surgery to decrease saliva.
- It is not recommended in people with glaucoma or bowel obstruction.
Homatropine:
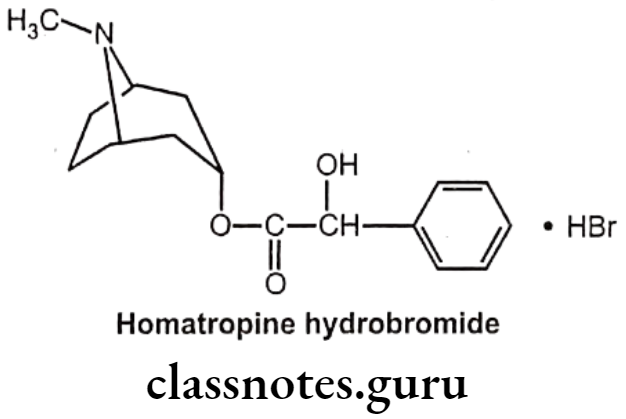
- Homatropine is chemically, [(15,5R)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl]-2-hydroxy-2-phenylacetate.
- It is available as the hydrobromide salt.
- It is an anticholinergic medication that is an antagonist at muscarinic acetylcholine receptors.
- It is less potent than atropine and has a shorter duration of action:-
Uses:
- It is used as an ophthalmic preparation as a cycloplegic (to temporarily paralyze accommodation), and as a mydriatic (to dilate the pupil).
- Homatropine is also given as an atropine substitute given to reverse the muscarinic and CNS effects associated with indirect cholinomimetic (Anti AChE) poisoning.
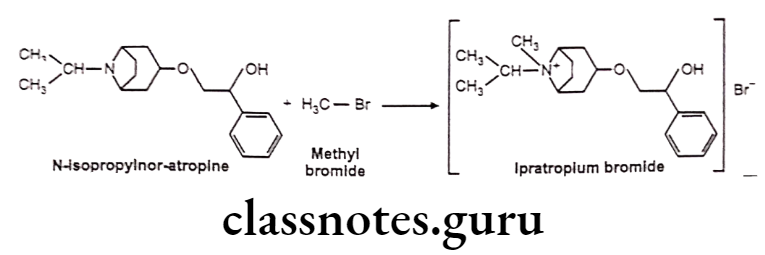
Ipratropium Bromide:
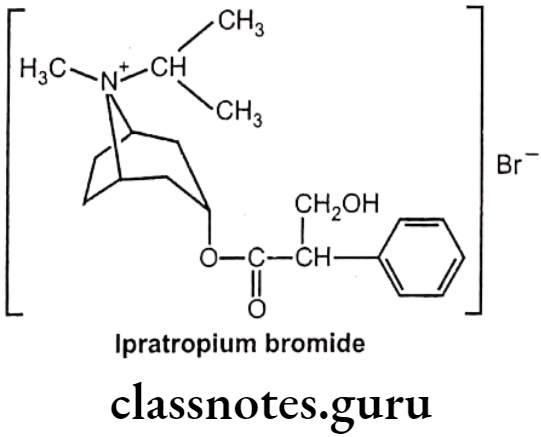
- Ipratropium bromide is chemically, (8-methyl-8-propan-2-yl-8-azoniabicyclo[3.2.1]- octan-3-yl) 3-hydroxy-2-phenylpropanoate;bromide.
- It is a muscarinic antagonist, a type of anticholinergic, which works by causing smooth muscles to relax.
Uses:
- Ipratropium bromide is used to treat the symptoms of chronic obstructive pulmonary disease and asthma.
- Ipratropium, sprayed into the nostrils as a nasal solution, can reduce rhinorrhea, but will not help nasal congestion.
- Combination with beta-adrenergic agonists increases the dilating effect on the bronchi.
- Common side effects include dry mouth, cough, and inflammation of the airways.
Synthetic Derivatives
The solanaceous alkaloids are found to be potent parasympatholytics, but they have the undesirable wide range of effects because of their non-specific action. For example, antispasmodic effects of the alkaloids most often result in side effects such as dryness of the mouth and fluctuations in pulse rate. To minimize such non-specific undesirable effects, the synthetic compounds are derived which possess specific cholinolytic actions.
The synthetic derivatives have tertiary amino function that is protonated in physiological pH or a quaternary ammonium group to form the cationic head, a crucial point of interaction. at the receptor cites. The tropic acid moiety is replaced by a large aromatic moiety like phenyl to provide hydrophobic interaction at the receptor cites.
Amino Alcohol Ester Derivatives:
Clidinium Bromide:
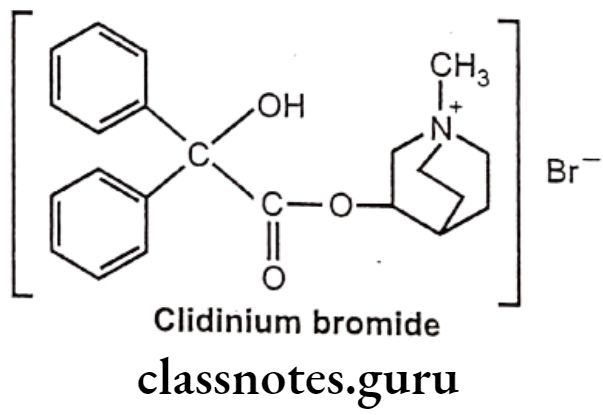
- Clidinium bromide is chemically, (1-methyl-1-azoniabicyclo[2.2.2]octan-3-yl)- 2-hydroxy-2,2-diphenylacetate;bromide.
- It is a muscarinic antagonist and finds importance in management of the symptoms of cramping and abdominal/stomach pain by decreasing stomach acid, and slowing the intestines.
Uses:
- Clidinium bromide is used in combination for the management of Peptic ulcer disease, GI motility disturbances (irritable bowel syndrome) and in acute enterocolitis.
- It is contraindicated in glaucoma.
Cyclopentolate Hydrochloride:
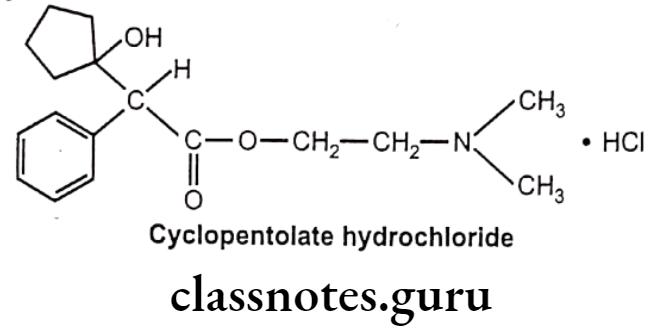
- Cyclopentolate hydrochloride is chemically, 2-(dimethylamino)ethyl 2-(1-hydroxy-cyclopentyl)-2-phenylacetate;hydrochloride.
- It blocks the acetylcholine receptor in the sphincter muscle of the iris and the ciliary muscle, thereby preventing contraction. This dilates the pupil, producing mydriasis, and prevents accommodation of the eye to different distances (cycloplegic).
Uses:
- Cyclopentolate hydrochloride is used as a mydriatic medication where it acts as parasympatholytic.
- It is commonly used during pediatric eye examinations.
- It is also administered as an atropine substitute to reverse muscarinic and central nervous system effects of indirect cholinomimetic (anti AchE) administration.
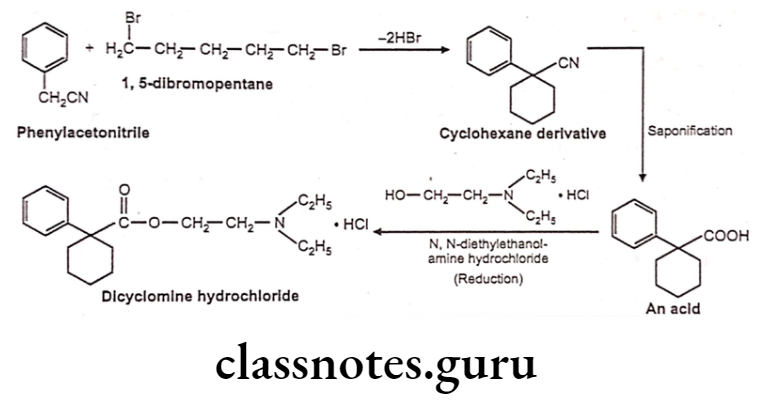
Dicyclomine Hydrochloride:
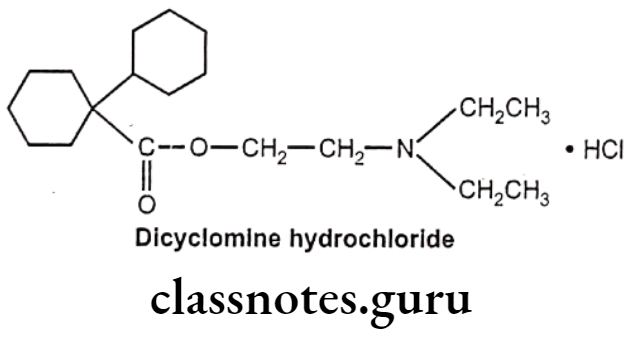
- Dicyclomine hydrochloride is chemically, 2-(diethylamino)ethyl. 1-cyclohexyl- cyclohexane-1-carboxylate;hydrochloride.
- It has some muscarinic receptor subtype selectivity. It binds more firmly to M1 and M3 than M2 and M4 receptors.
- It antagonizes muscarinic receptors on smooth muscle in the gastrointestinal (GI) tract, thereby preventing the actions of acetylcholine and reducing GI smooth muscle spasms.
Uses:
- Dicyclomine hydrochloride is used as an antispasmodic and in urinary incontinence.
- It has local anesthetic properties and is used in gastrointestinal, biliary, and urinary tract spasms.
- It is used to treat the symptoms of irritable bowel syndrome, specifically hypermotility.
- It is also useful in dysmenorrhea (pain during menstruation), pylorospasm (spasm of the pyloric sphincter) and biliary dysfunction.
Glycopyrrolate:
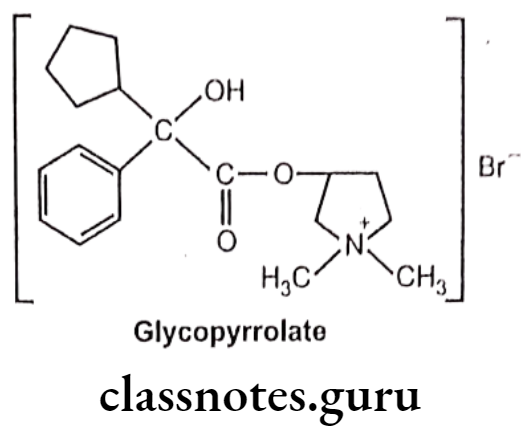
- Glycopyrrolate is chemically, (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2- hydroxy-2-phenylacetate;bromide.
- It is a synthetic quaternary ammonium amine anticholinergic agent with antispasmodic activity.
- It competitively binds to peripheral muscarinic receptors in the autonomic effector cells.
- It does not cross the blood-brain barrier and consequently has no CNS effects.
Uses:
- Glycopyrrolate is a preanesthetic medication to reduce salivary, tracheobronchial, and pharyngeal secretions as well as decreasing the acidity of gastric secretion.
- It is also used to reduce excessive saliva.
- It decreases acid secretion in the stomach and so may be used for treating stomach ulcers in combination with other medications.
Methantheline Bromide:
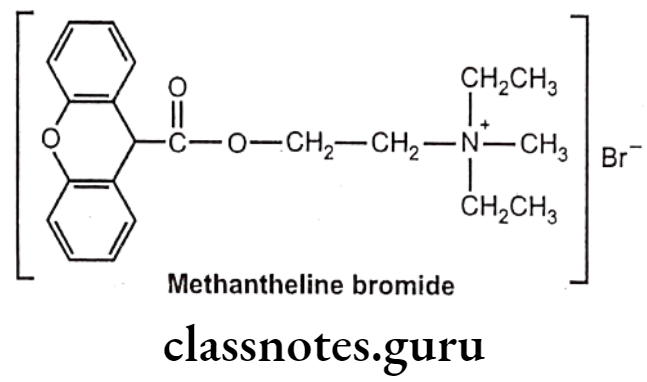
- Methantheline bromide is chemically, diethyl-methyl-[2-(9H-xanthene-9-carbonyloxy)ethyl]azanium;bromide.
- It is a muscarinic antagonist and finds importance in management of the symptoms of cramping and abdominal/stomach pain by decreasing stomach acid and slowing the intestines.
- It is an anticholinergic agent that also acts at the nicotinic cholinergic receptors of the sympathetic and parasympathetic systems as well as at the myoneural junction of the postganglionic cholinergic fibers.
Uses:
- Methantheline bromide is used in combination for the managment of peptic ulcer and irritable bowel syndrome.
- It is also used in treatment of gastritis, intestinal hypermotility, bladder irritability, cholinergic spasm and pancreatitis.
- It is contraindicated in glaucoma.
Propantheline Bromide:
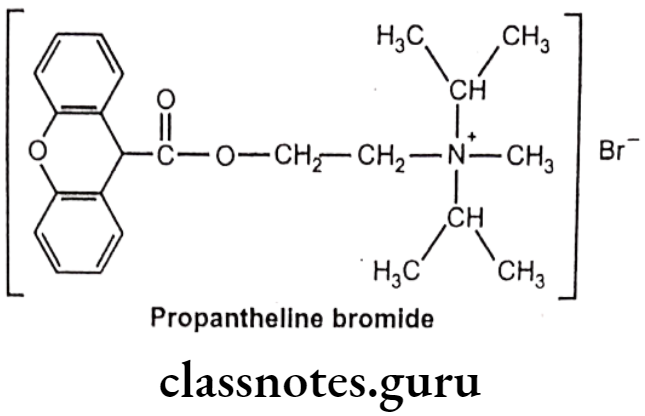
- Propantheline bromide is chemically, methyl-di(propan-2-yl)-[2-(9H-xanthene-9- carbonyloxy)ethyl]azanium;bromide.
- It competitively antagonizes acetylcholine activity mediated by muscarinic receptors at neuroeffector sites on smooth muscle and exocrine gland cells.
- It is five times more potent than methantheline and also finds importance to control the symptoms of irritable bowel syndrome.
Uses:
- Propantheline bromide is an antimuscarinic agent used for the treatment of excessive sweating (hyperhidrosis), cramps or spasms of the stomach, intestines or bladder, and involuntary urination (enuresis).
- Propantheline relaxes the gut muscle and can relieve pain in conditions caused by spasm of the muscle in the gut.
- It relaxes the smooth muscle in the bladder and prevents the involuntary spasms that can allow leakage of urine from the bladder in the condition known as enuresis (involuntary urination in adults).
- Propantheline can also be used to treat excessive sweating because acetylcholine block also reduces secretions such as sweat and tears.
Amino Alcohol Ether Derivatives:
These cholinergic blockers were introduced as antiparkinsonian drugs due to their beneficial effect in the management of parkinsonism. These derivatives also posess antihistaminic properties since they are structurally related to H1 class of a antihistaminic drugs.
Benztropine Mesylate:
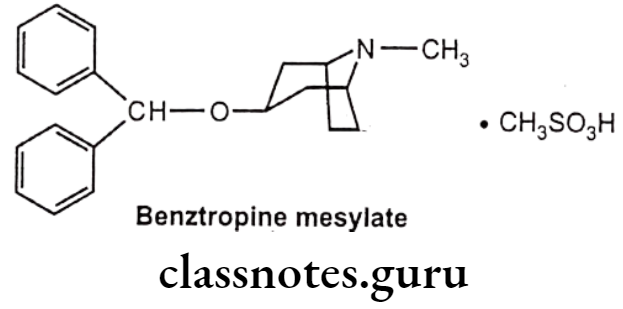
- Benztropine mesylate is chemically, 3-benzhydryloxy-8-methyl-8-azabicyclo[3.2.1] octane; methanesulfonic acid.
- It is a centrally acting anticholinergic/antihistamine agent.
- It is a selective M1 muscarinic acetylcholine receptor antagonist.
- It partially blocks cholinergic activity in the basal ganglia and has also been shown to increase the availability of dopamine by blocking its reuptake and storage in central sites, and as a result, increasing dopaminergic activity.
- It antagonizes the effect of acetylcholine, decreasing the imbalance between the neurotransmitters acetylcholine and dopamine, which may improve the symptoms of early parkinson’s disease.
Uses:
- Benztropine mesylate is a centrally active muscarinic antagonist that has been used in the symptomatic treatment of Parkinson’s disease.
- It is used to reduce extrapyramidal side effects of antipsychotic treatment.
- It improves tremor and may alleviate rigidity and bradykinesia.
- Benzatropine is also used for the treatment of dystonia, a rare disorder that causes abnormal muscle contraction, resulting in twisting postures of limbs, trunk, or face.
Orphenadrine Citrate:
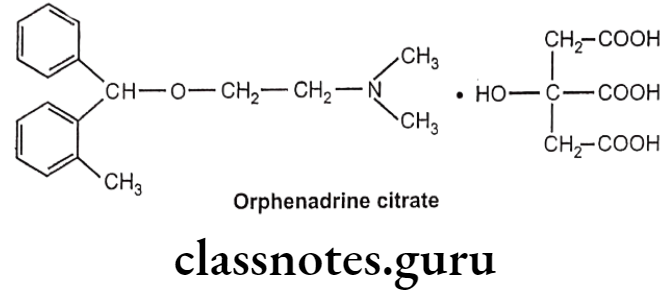
- Orphenadrine citrate is chemically, phenylmethoxy]ethanamine; 2-hydroxypropane-1,2,3-tricarboxylic acid.
- It is an anticholinergic drug of the ethanolamine antihistamine class (it is closely related to diphenhydramine).
- It blocks cholinergic receptors, thereby interfering with the transmission of nerve impulses from the spinal cord to the muscles.
Uses:
- Orphenadrine citrate is used to treat muscle pain and to help with motor control in parkinson’s disease.
- It is used to relieve pain caused by muscle injuries like strains and sprains.
- Orphenadrine and other muscle relaxants are sometimes used to treat pain arising from rheumatoid arthritis.
- It also possess antihistamine property due to H1 receptor antagonist action.
Amino Alcohol Derivatives:
These cholinergic blockers were introduced in 1940s, and were established for their antispasmodic importance. They are also used as antiparkinsonian drugs due to their beneficial effect in the management of parkinsonism.
These derivatives have structural similarity with atropine derivatives possessing a bulky group and a cyclic amino function, amino propanol arrangement with three carbon chains between the hydroxy function and the amino group is the common structural features of these amino alcohol derivatives.
Biperidin Hydrochloride:
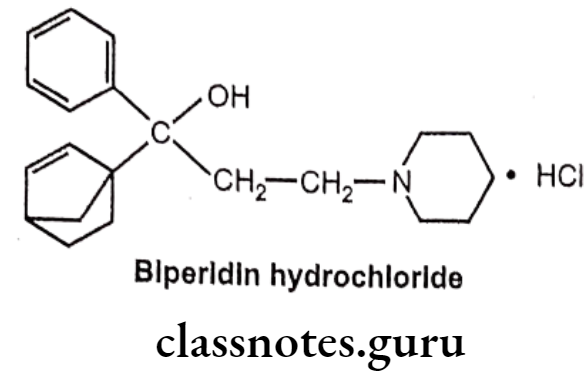
- Biperiden hydrochloride is chemically, 1-(5-bicyclo[2.2.1]hept-2-enyl)-1-phenyl-3- piperidin-1-ylpropan-1-ol;hydrochloride.
- It is a muscarinic antagonist that has effects in both the central and peripheral nervous systems.
Uses:
- Biperiden hydrochloride is used to treat Parkinson disease.
- Common side effects include blurred vision, dry mouth, sleepiness, constipation, and confusion.
- It should not be used in people with a bowel obstruction or glaucoma.
- It is used to improve acute extrapyramidal side effects, it relieves muscle rigidity, reduces abnormal sweating and salivation, improves abnormal gait (particular way of walking) and to lesser extent tremor.
- It has a strong nicotine receptor blocking effect and helpful in nicotine induced convulsions hence, biperiden has been analyzed as an alternative anticonvulsant for usage.
- It is contraindicated in all forms of epilepsy.
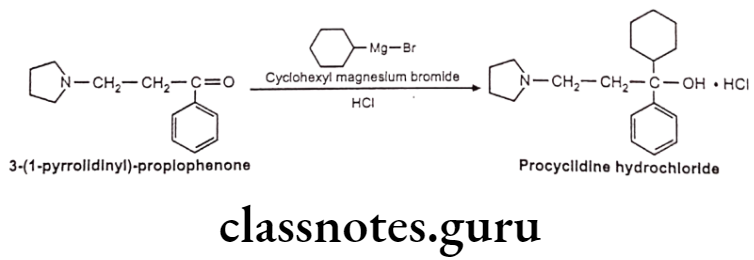
Procyclidine Hydrochloride:
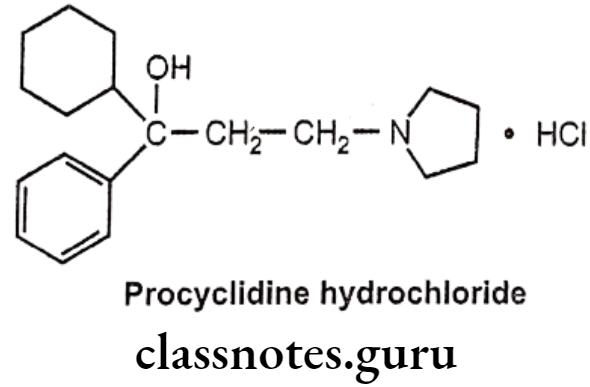
- Procyclidine hydrochloride is chemically, (1R)-1-cyclohexyl-1-phenyl-3-pyrrolidin- 1-ylpropan-1-ol;hydrochloride.
- It is a muscarinic antagonist that crosses the blood-brain barrier.
- It acts by blocking central cholinergic receptors, and thus balancing cholinergic and dopaminergic activity in the basal ganglia.
Uses:
- Procyclidine is principally used for the treatment of drug-induced extra pyramidal disorders and in parkinsonism, akathisia (feeling of inner restlessness) and acute dystonia (sustained muscle contractions) or secondary dystonia.
- It is a second-line drug for the treatment of parkinson’s disease. It improves tremor, but not rigidity or bradykinesia.
- It also has antispasmodic effect on smooth muscle and may produce mydriasis and reduction in salivation.
Tridihexethyl Chloride:
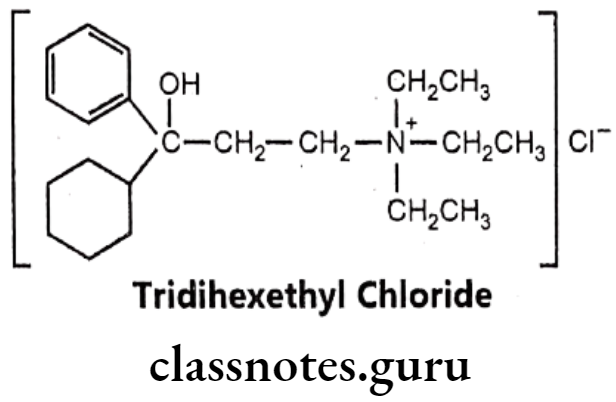
- Tridihexethyl chloride is chemically, (3-cyclohexyl-3-hydroxy-3-phenylpropyl)- triethylazanium; chloride.
- It is an anticholinergic, antimuscarinic and antispasmodic drug.
- It may block all the three (M1, M2 and M3) types of muscarinic receptors.
- It has a pronounced antispasmodic and antisecretory effect on the gastrointestinal tract.
Uses:
- It may be used, usually in combination with other drugs, to treat acquired nystagmus (involuntary eye movement) or peptic ulcer disease.
Amino Amide Derivatives:
The amino amide derivatives are structurally similar to the amino alcohol derivatives, where the polar hydroxyl function is replaced by a polar amide function resulting in reduced metabolism, hence these derivatives have increased duration of action in comparison to the amino alcohol derivatives.
They also have structural features similarity with atropin derivatives. They possess a bulky aromatic group usually a phenyl moiety with respect to the both ‘A’ and ‘B’ substitutents on the pivotal carbon and amide function.
Isopropamide Iodide:
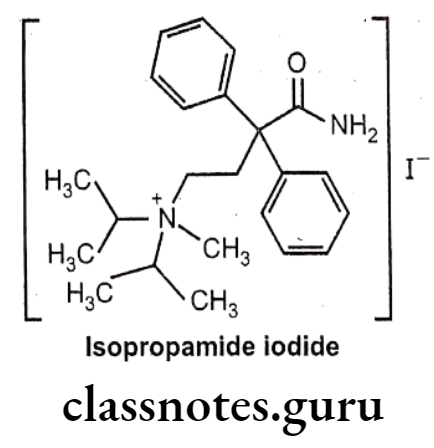
- Isopropamide iodide is chemically, (4-amino-4-oxo-3,3-diphenylbutyl)-methyl- di(propan-2-yl)azanium;iodide.
- It is most often provided as an iodide salt, but is also available as a bromide or chloride salt.
- It is a long-acting quaternary anticholinergic drug.
Uses:
- Isopropamide iodide is used in the treatment of peptic ulcers and other gastrointestinal disorders involving hyperacidity (gastrointestinal acidosis) and hypermotility.
Tropicamide:
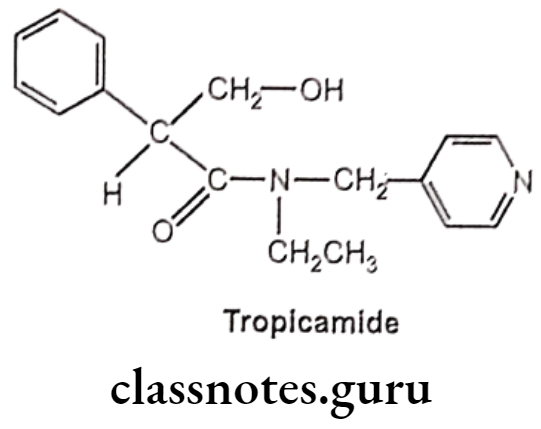
- Tropicamide is chemically, N-ethyl-3-hydroxy-2-phenyl-N-(pyridin-4-ylmethyl)- propanamide.
- It is a synthetic muscarinic antagonist with having similar action to that of atropine and with an anticholinergic property.
- It binds to and blocks the muscarinic receptors (M4) in the sphincter and ciliary muscle in the eye and thus works as a mydriatic and relaxes the sphincter muscle of the Iris and paralysis of the ciliary muscle.
Uses:
- Tropicamide is an anticholinergic drug and is used to dilate the pupil and helps in examination of the eye.
- It is a diagnostic agent and is used to produce short-duration mydriasis and cycloplegia.
Miscellaneous Derivatives:
Drugs discussed in the miscellaneous derivatives are synthetic molecules without any structural resemblance with the other synthetic anticholinergic agents.
Ethopropazine Hydrochloride:
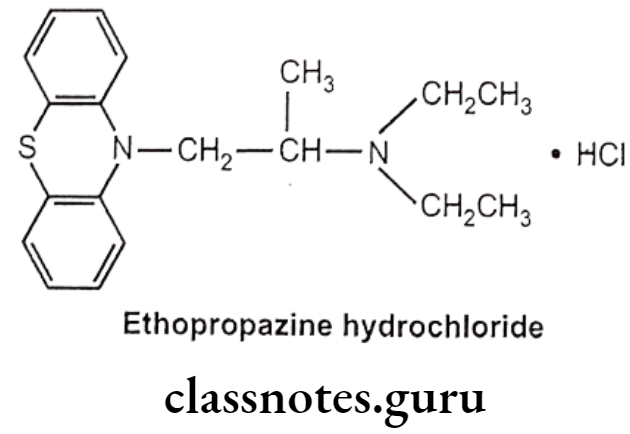
- Ethopropazine hydrochloride is chemically, N,N-diethyl-1-phenothiazin-10-ylpropan- 2-amine;hydrochloride.
- It is a phenothiazine derivative with anticholinergic activity.
- Drowsiness and dizziness are the most common side effects.
- It is contraindicated in conditions such as glaucoma because of its mydriatic effect.
Uses:
- Ethopropazine hydrochloride is used as an antiparkinsonian agent that also has antihistamine, and antiadrenergic actions.
- It is also used in the alleviation of the extrapyramidal syndrome induced by drugs.
- It is also effective on tremor, sialorrhea, (excessive salivation) and oculogyric crises (prolonged involuntary upward deviation of the eyes).
Synthesis
Carbachol
Neostigmine
Ipratropium Bromide
Dicyclomine Hydrochloride
Procyclidine Hydrochloride
Multiple Choice Questions
Question 1. Acetylcholine is not a specific neurotransmitter at:
- Sympathetic ganglia
- Sympathetic postganglionic nerve endings
- Parasympathetic ganglia
- Parasympathetic postganglionic nerve endings
Answer. 2. Sympathetic postganglionic nerve endings
Question 2. Muscarinic receptors are located in:
- Autonomic ganglia
- Skeletal muscle neuromuscular junctions
- Autonomic effector cells
- Sensory carotid sinus baroreceptor zone
Answer. 3. Autonomic effector cells
Question 3. Substitution of acetylcholine at ẞ-position with respect to the nitrogen atom:
- Decreases the nicotinic activity
- Increases the nicotinic activity
- Increases the muscarinic activity
- Decreases both nicotinic and muscarinic activity
Answer. 3. Increases the muscarinic activity
Question 4. Indicate the location of M2 cholinoreceptor type:
- Heart
- Glands
- Smooth muscle
- Endothelium
Answer. 1. Heart
Question 5. Which of the following cholinomimetics activates both muscarinic and nicotinic receptors?
- Lobeline
- Pilocarpine
- Nicotine
- Bethanechol
Answer. 4. Bethanechol
Question 6. Atropine contains
- One asymmetric carbon
- Three asymmetric carbons
- Two asymmetric carbons
- Four asymmetric carbons
Answer. 2. Three asymmetric carbons
Question 7. Indicate a cholinomimetic agent, which is related to direct-acting drugs:
- Edrophonium
- Physostigmine
- Carbachol
- Isofluorophate
Answer. 3. Carbachol
Question 8. Acetylcholine is not used in clinical practice because:
- It is very toxic.
- The doses required are very high.
- It is very rapidly hydrolyzed.
- It is very costly.
Answer. 3. It is very rapidly hydrolyzed.
Question 9. Parasympathomimetic drugs cause:
- Bronchodilation
- Mydriasis
- Bradycardia
- Constipation
Answer. 3. Bradycardia
Question 10. Which of the following direct-acting cholinomimetics is mainly muscarinic in action?
- Bethanechol
- Carbachol
- Acetylcholine
- None of the above
Answer. 1. Bethanechol
Question 11. Which of the following direct-acting cholinomimetics has the shortest duration of action?
- Acetylcholine
- Methacholine
- Carbachol
- Bethanechol
Answer. 1. Acetylcholine
Question 12. Which of the following cholinomimetics is indirect-acting?
- Lobeline
- Edrophonium
- Pilocarpine
- Carbachol
Answer. 2. Edrophonium
Question 13. Indicate a reversible cholinesterase inhibitor:
- Isofluorophate
- Carbachol
- Physostigmine
- Parathion
Answer. 3. Physostigmine
Question 14. Which of the following cholinesterase inhibitors is irreversible?
- Physostigmine
- Edrophonium
- Neostigmine
- Isofluorophate
Answer. 4. Isofluorophate
Question 15. Indicate cholinesterase activator:
- Pralidoxime
- Edrophonium
- Pilocarpine
- Isofluorophate
Answer. 1. Pralidoxime
Question 16. Which of the following cholinomimetics is commonly used in the treatment of glaucoma?
- Pilocarpine
- Lobeline
- Acetylcholine
- Neostigmine
Answer. 1. Pilocarpine
Question 17. Chronic long-term therapy of myasthenia is usually accomplished with:
- Edrophonium
- Neostigmine
- Echothiophate
- Carbachol
Answer. 2. Neostigmine
Question 18. Indicate the reversible cholinesterase inhibitor, which penetrates the blood-brain barrier:
- Physostigmine
- Edrophonium
- Neostigmine
- Piridostigmine
Answer. 1. Physostigmine
Question 19. Indicate a muscarinic receptor-blocking drug:
- Scopolamine
- Pipecuronium
- Trimethaphan
- Pilocarpine
Answer. 1. Scopolamine
Question 20. The mechanism of atropine action is:
- Competitive ganglion blockade
- Competitive muscarinic blockade
- Competitive neuromuscular blockade
- Non-competitive neuromuscular blockade
Answer. 2. Competitive muscarinic blockade
Question 21. Atropine is highly selective for:
- M1-receptor subtype
- M2-receptor subtype
- M3-receptor subtype
- All of the above
Answer. 4. All of the above
Question 22. Atropine causes:
- Miosis, a reduction in intraocular pressure and cyclospasm
- Mydriasis, a rise in intraocular pressure and cycloplegia
- Miosis, a rise in intraocular pressure and cycloplegia
- Mydriasis, a rise in intraocular pressure and cyclospasm
Answer. 2. Mydriasis, a rise in intraocular pressure and cycloplegia
Question 23. Atropine causes:
- Bradycardia, hypotension and bronchoconstriction
- Tachycardia, little effect on blood pressure and bronchodilation
- Decrease in contractile strength,conduction velocity through the AV node
- Tachycardia, hypertensive crisis and bronchodilation
Answer. 2. Tachycardia, little effect on blood pressure and bronchodilation
Question 24. Atropine is frequently used prior to administration of inhalant anesthetics to reduce:
- Muscle tone
- Secretions
- Nausea and vomiting
- All of the above
Answer. 2. Secretions
Question 25. Which of the following drugs is useful in the treatment of uterine spasms?
- Carbachol
- Vecuronium
- Atropine
- Edrophonium
Answer. 3. Atropine
Question 26. Indicate the antimuscarinic drug, which is used as a mydriatic:
- Pilocarpine
- Neostigmine
- Homatropine
- Ipratropium
Answer. 3. Homatropine
Question 27. Which of the following agents is used as an inhalation drug in asthma?
- Atropine
- Ipratropium
- Lobeline
- Homatropine
Answer. 2. Ipratropium
Question 28. Indicate an antimuscarinic drug, which is effective in the treatment of mushroom poisoning:
- Pralidoxime
- Homatropine
- Pilocarpine
- Atropine
Answer. 4. Atropine
