Gypsum Products Important Notes
1. Gypsum Types
- Type 1- dental plaster, impression
- Type 2 – Dental plaster, model
- Type 3 – Dental stone/ hydro cal
- Type 4 – dental stone, high strength/ die stone
- Type 5 – dental stone, high strength, and high Expansion
2. Gypsum Terms
- Cast – a replica of the entire arch
- Die – a replica of a single-tooth
- Investment – when plaster is mixed with silica
- Divestment – it is an investment containing die stone
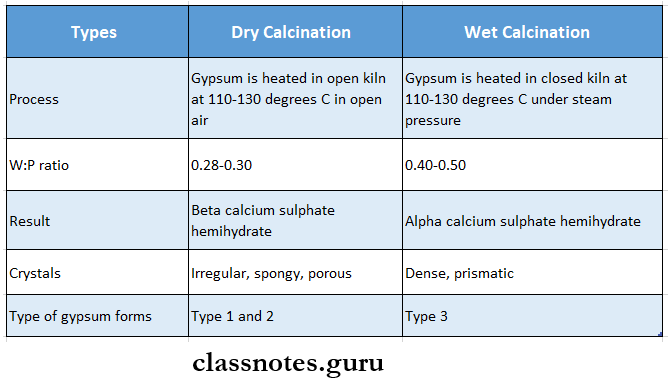
3. Gypsum Manufacture
- Calcination is the process of heating gypsum for the manufacture of plaster
- Types of calcinations
4. Gypsum Setting Reaction
- When the plaster is mixed with water calcium sulfate hemihydrate reacts with the water
- The solution gets supersaturated to form dihydrate
- Reaction is Exothermic
- (CaSO4)2. H2O + 3H2O → 2CaSO4.2H2O + unreacted (CaSO4)2 .H2O + heat
- Hemihydrate + water -> dehydrate + unreacted hemihydrate
- Hemihydrate is four times more soluble than dihydrate
Read And Learn More: Dental Materials Question and Answers
5. Gypsum Setting Time – Factors Affecting It
- Accelerators:
- It decreases setting time
- Example: potassium sulfate, NaCl in low concentration, adding a small amount of slurry water in mixing water
- Retarders
- It increases setting time
- Examples: acetates, borates, citrate, NaCl in high concentration, colloids like gelatin, glue, agar
- Other factors
- Calcination process
- Fineness – Finer particles faster the setting
- W:P ratio – more water increases setting time
- Mixing – rapid mixing shorter the setting time
- Temperature- increase in temperature shorter the setting time
Dental Gypsum Products
6. Gypsum Measurement Of Setting Time
- Initial set measurement
- Loss of gloss test
- Initial Gillimore needle test
- A small needle of 1/4 lb weight and diameter of 1/12” is used
- Final set measurement
- Vicat needle test – The needle used is 300 gm and 1 mm diameter
- Final Gillimore needle test – needle used is of 1 lb weight and 1/24” diameter
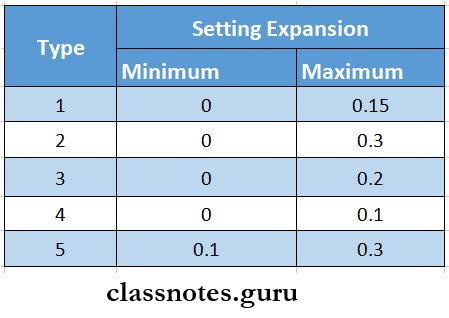
7. Gypsum Setting Expansion
- Normal setting Expansion
- Setting Expansion without water immersion
- Hygroscopic setting Expansion
- It is Expansion that occurs in water due to additional crystal growth potential
8. Factors Affecting Setting Expansion
- Factors that increase setting Expansion
- Less W:P ratio
- Setting underwater
- Alpha hemihydrate
- Finer particles
- Factors that decrease setting Expansion
- More W:P ratio
- Beta hemihydrate
- Use of modifiers
9. Gypsum Wet and dry strength
- Wet/ Green strength
- Strength occurs due to the presence of Excess water than required is called wet strength
- Diy strength
- The strength that occurs when Examplecess of water is dried
- It is 2-4 times more than that of wet strength
Gypsum Products Long Essays
Question 1. What are the various gypsum products used in dentistry? Describe the composition, manipulation, and setting reaction of dental stone.
Answer:
Types of Gypsum Products Used in Dentistry:
- Type 1- Dental plaster, impression
- Type 2- Dental plaster, model
- Type 3- Dental stone
- Type 4- Dental stone, high strength
- Type 5- Dental stone, high strength, high Expansion
Dental Stone:
Dental Stone Composition:
- Alpha hemihydrate
- Coloring agent- 2-3%
- Potassium sulfate
- Borax-retarder
Dental Stone Manipulation:
- The proper water-powder ratio is determined
- Water is measured by graduated cylinder and powder by weighing balance
- Place a measured volume of water in a parabolic, smooth bowl first
- Add preweighed powder gradually to it
- Allow settling for 30 seconds to minimize air entrapment
- The mix is stirred vigorously
- Periodically wiping inside of the bowl with the spatula is done to ensure the wetting of all of the powder and breaking up of the lumps
- Continue mixing until a uniform smooth mix is obtained
- Vibrate the mix using a mechanical vibrator or by repeated tapping against the bench
- Pour it into the impression
Dental Stone Setting Reaction:
- Calcium sulfate hemihydrates react with water
- Dissolution of calcium sulfate hemihydrates
- Formation of a saturated solution of calcium sulfate
- Aggregation of less soluble calcium sulfate dehydrate
- Precipitation of crystals
Types Of Gypsum In Dentistry
Question 2. Write in detail the setting reaction of dental plaster and indicate various uses in dentistry.
Answer:
Dental Plaster Setting Reaction:
- When the plaster is mixed with water it takes up one and a half molecules of water
- A suspension is formed that is fluid and workable
- Hemihydrates dissolve until it forms a saturated solution
- This solution gets supersaturated to form dehydrate which gets precipitated
- As dehydrate precipitates the solution is no longer saturated with the hemihydrates, so it continues to dissolve
- Dissolution of hemihydrates and precipitation of dehydrate proceeds until no further dehydrate precipitates out of the solution
- The reaction is Exothermic
(CaSO4)2. H2O + 3H2O → 2CaSO4.2H2O + unreacted (CaSO4). H2O + heat - Hemihydrates + water-> dehydrate + unreacted hemihydrates + heat
Dental plaster Theories:
- Colloidal theory
- It proposes that when mixed with water, plaster enters into the colloidal state through a sol-gel mechanism
- In a sol state, hemihydrates particles are hydrated to form dehydrate, thereby entering into an active state
- As water is consumed, the mass converts to a solid gel
- Hydration theory
- Rehydrated plaster particles join together through hydrogen bonding to the sulfate groups to form a set material
- Dissolution precipitation theory- crystallization theory
- The most widely accepted theory
- According to it, the plaster dissolves and reacts to form gypsum crystals which interlock to form a set solid
Dentistry Uses:
- For making impressions in complete denture and maxillofacial prosthesis
- Bite registration material
Question 3. Classify gypsum products and write in detail about the chemistry and properties of type 2 gypsum products.
Answer:
Gypsum Products Classification:
- Type 1- Dental plaster, impression
- Type 2- Dental plaster, model
- Type 3- Dental stone
- Type 4- Dental stone, high-strength
- Type 4- Dental stone, high strength, high Expansion
Type 2 Gypsum Product- Dental Plaster, Model
Type 2 Gypsum Product Chemistry:
- When the plaster is mixed with water it takes up one and a half molecules of water
- A suspension is formed that is fluid and workable
- Hemihydrates dissolve until it forms a saturated solution
- This solution gets supersaturated to form dehydrate which gets precipitated
- As dehydrate precipitates the solution is no longer saturated with the hemihydrates, so it continues to dissolve
- Dissolution of hemihydrates and precipitation of dehydrate proceeds until no further dehydrate precipitates out of the solution
- The reaction is Exothermic
(CaS04)2. H20 + 3H20 → 2CaS04.2H20 + unreacted (CaS04)2. H20 + heat - Hemihydrates + water → dehydrate + unreacted hemihydrates + heat
Type 2 Gypsum Product Properties:
- Natural white
- Weak
- Compressive strength is low- 9 MPa
- Tensile strength- 0.6 MPa
Properties Of Dental Gypsum
Question 4. Describe in detail the setting reaction and the various factors that affect the dimensional stability of the dental stone.
Answer:
Factors Affecting Dimensional Stability:
- Mixing method
- Mechanical mixing decreases setting Expansion
- Water powder ratio

- Modifiers- accelerators and retarders
- Reduces setting Expansion
Question 5. What are the different methods of manufacturing of gypsum products? Write the setting reaction of gypsum. Mention the uses of gypsum products.
Answer:
Methods of Manufacturing Gypsum Products
- Dry calculations
- Gypsum is ground and heated in an open kettle or kiln at a temperature of 110-130 degrees C
- The process is called dry calcination
- Beta calcium sulfate hemihydrates are formed, which have spongy, irregular large orthorhombic crystal particles
- CaSO4.2H2O → CaSO4 1/2 H2O
- Type 1 and type 2 are formed by this method
- Wet Calculations
- when gypsum is ground and heated under steam pressure at a temperature of 110-130 degrees C in a closed kettle or kiln or an autoclave the process is called wet calculations
- Alpha calcium sulfate hemihydrates are formed, which consist of smaller, regularly shaped crystalline particles in the form of rods or prisms
- Type 3 is formed by this method
- Microscopically crystals of type 3 are in the form of rods and prisms
- Dehydration By Boiling With Chemicals
- The gypsum is calcined by boiling it in a 30% calcium chloride solution
- The chlorides are then washed away or autoclaved in the presence of sodium succinate 0.5%
- Improved stone is manufactured by this method
- These particles are denser among all three types
- After controlled grinding, these powders have an even higher apparent density, and microscopically the particles that are cuboidal in shape yield an even stronger set of material
Gypsum Products Setting Reaction
- When the plaster is mixed with water, it takes up 1 1/2 molecules of water, i.e. it regains its water of crystallization and becomes calcium sulfate dehydrate
- A suspension is formed that is fluid and workable
- Hemihydrates dissolve until it forms a saturated solution
- This solution gets supersaturated to form dehydrate which gets precipitated
- As dehydrate precipitates the solution is no longer saturated with the hemihydrates, so it continues to dissolve
- Dissolution of hemihydrates and precipitation of dehydrate proceeds until no further dehydrate precipitates out of the solution
- The reaction is Exothermic
(CaSO4)2. H2O + 3H2O → 2CaSO4.2H2O + unreacted (CaSO4)2. H2O + heat
Hemihydrates + water-> dehydrate + unreacted hemihydrates + heat
Gypsum Products In Dentistry MCQs
Gypsum Products Uses:
- General use
- For preparing statues and in construction work
- Medical use
- For splinting and making plaster casts
- In dentistry
- For making an impression of the mouth and face
- To make molds, casts, and dyes over which dental prostheses and restorations are made
- To mount the casts on articulators
- For bite registration
- As dental investment materials
Question 6. Discuss the plaster of Paris in detail.
Answer:
The Plaster Of Paris
- Type 1 or impression plaster is also known as plaster of Paris
- It was the earliest impression material in dentistry
- Because of its rigidity and nonelastic nature, it often had to be fractured for its removal from undercut areas in the mouth
- The fractured pieces were then reassembled outside and a cast was poured
Plaster Of Paris Uses:
- For making an impression in complete denture and maxillofacial prosthesis
- Bite registration material
Plaster of Paris Properties
- The setting time should be under accurate control
- The dentist must have sufficient time to mix, load the impression tray, carry the loaded tray to the patient’s mouth, and place it in position
- The plaster should harden promptly once in position so that there is minimum discomfort to the patient
- The desirable setting time is 3-5 min
- The setting Expansion should be low for better accuracy
- Both setting time and Expansion are controlled by modifiers
- The plaster should have enough strength to fracture cleanly without crumbling to facilitate its removal from undercut areas
Plaster of Paris Composition:
- Dental plaster
- Potassium sulfate
- Borax- retarder
- Coloring agents- to help the dentist and technician distinguish between the cast material and the impression
- Flavoring agents- to make it more acceptable to the patient
- It may sometimes contain potato starch to make it soluble
- After the cast hardens, the impression and cast are immersed in hot water
- The starch swells and the impression disintegrates, making it easy to separate the cast from the impression
- This is called soluble plaster
Uses Of Gypsum In Dentistry
Question 7. Define calculations. What is the process of calcination?
Answer:
Calculations Definition:
The process of heating gypsum for the manufacture of plaster is known as calcination
Process of Calcination:
- Dry calcinations
- Gypsum is ground and heated in an open kettle or kiln at a temperature of 110-130 degrees C
- The process is called dry calcination
- Beta calcium sulfate hemihydrates are formed, which have spongy, irregular large orthorhombic crystal particles
- CaSO4.2H2O → CaSO4 1/2 H2O
- Type 1 and type 2 are formed by this method
- Wet calculations
- When gypsum is ground and heated under steam pressure at a temperature of 110-130 degrees C in a closed kettle or kiln or an autoclave the process is called wet calculations
- Alpha calcium sulfate hemihydrates are formed, which consist of smaller, regularly shaped crystalline particles in the form of rods or prisms
- Type 3 is formed by this method
- Microscopically crystals of type 3 are in the form of rods and prisms
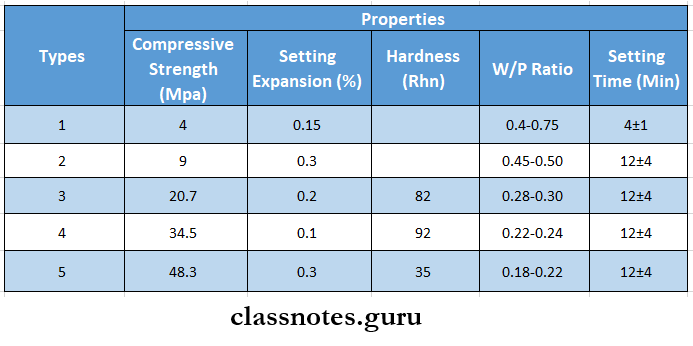
Question 8. Identify the different gypsum products with reference.
Answer:
The different gypsum products with reference
Question 9. Normal and hygroscopic Expansion
Answer:
Normal And Hygroscopic Expansion
All gypsum products show a linear Expansion during setting, due to the outward thrust of the growing crystals during setting
Normal Setting Expansion:
Setting Expansion without water immersion is called normal setting Expansion
Normal Setting Expansion Importance
- Undesirable in the construction of cast
- Causes change in occlusal relations
- Desirable in case of investment materials
Hygroscopic Expansion:
- Expansion that occurs in water is called hygroscopic Expansion
- It is a physical Expansion
- When Expansion begins, Externally available water is drawn into pores forming a setting mass
- It maintains a continuous aqueous phase in which crystal growth takes place freely
Hygroscopic Expansion Importance:
Use to Expand some gypsum bonded investments
Setting Time Of Gypsum Products
Gypsum Products Short Essays
Question 1. High strength, high Expansion dental stone
Answer:
High Strength, High Expansion Dental Stone
- It is a Type 5 gypsum product
- It is the most recent gypsum product
- It has a high compressive strength
- Improved strength is due to a lower water-powder ratio
- Setting Expansion is increased from 0.1% to 0.3%
- It is to compensate for the shrinkage of base metal alloys
High Strength, High Expansion Dental Stone Uses:
Fabrication of cast crown due to inadequate Expansion
High Strength, High Expansion Dental Stone Contraindication:
In the production of dies of inlays due to unacceptably tight fits.

Question 2. Calcium sulfate hemihydrates
Answer:
Calcium Sulfate Hemihydrates
- The principal constituent of gypsum-based products such
- as dental plasters and stones are calcium sulfate hemihydrate
- The chemical formula is (CaSO4)2 .H2O → CaSO4 1/2 H2O
- Depending on the method of calcination, different forms of hemihydrate can be obtained.
- They are:
- Alpha hemihydrate.
- It is called dental stone
- It consists of smaller, regularly shaped crystalline particles in the form of rods or prisms
- Alpha-modified hemihydrate o Beta hemihydrate
- It is known as dental plaster
- It consists of large, irregularly shaped orthorhombic crystal particles with capillary pores
- Alpha and beta forms differ in crystal size, surface area, and degree of lattice perfection
- Alpha hemihydrate requires much less water when it is mixed than beta hemihydrate
- Beta hemihydrate particles absorb more water because the crystals are more irregular in shape and porous
Question 3. Die stone
Answer:
Die Stone
- Type 4 and Type 5 gypsum products are used as die materials
- Type 4
- Have cuboidal shaped particles g Has reduced surface area
- Minimal setting Expansion
- Have a setting Expansion of 0.1% or less
- Type 5
- Have improved compressive strength e Setting Expansion is 0.3%
- Increased Expansion is used in compensation for the base metal alloy solidification shrinkage
Die Stone Advantages:
- In Expensive
- Easy to use
- Compatible with other impression materials
- Good strength
- Good working time
- Minimal shrinkage
- Have color contrast
- Has smooth surface
- Sets quickly
Die Stone Disadvantages:
- Brittle
- Susceptible to abrasion during the carving of the wax pattern
Classification Of Dental Gypsum
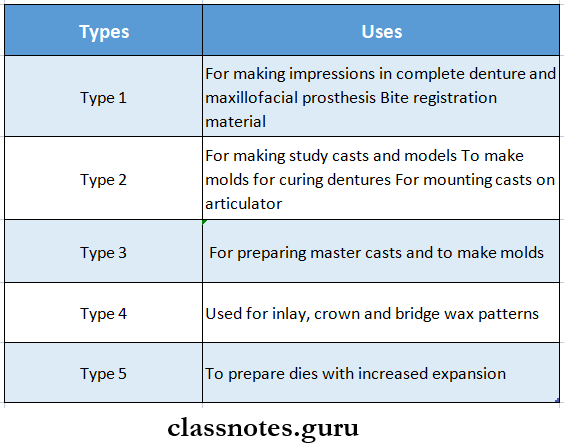
Question 4. Types of gypsum products
Answer:
Types of Gypsum Products Uses:
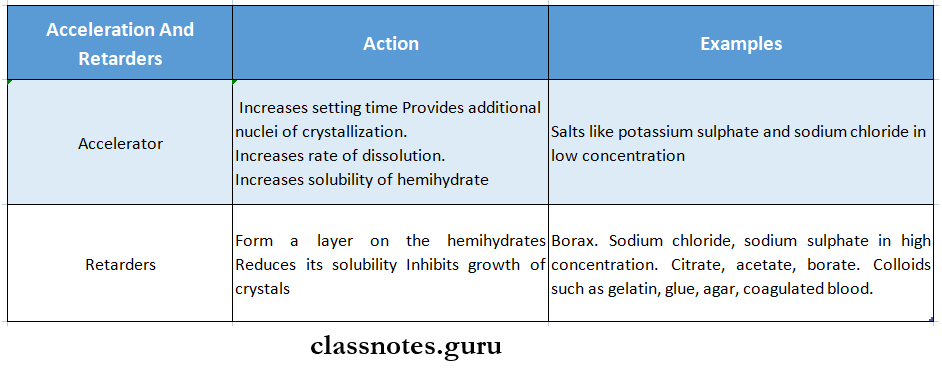
Question 5. Accelerators and retarders
Answer:
Accelerators And Retarders
Chemicals that decrease setting time are called accelerators while increase setting time are called retarders.
Question 6. Die materials
Answer:
Die Materials
A positive replica of a prepared tooth or teeth in a suitable hard substance on which inlays, crowns, and other restoration are made is called a die
Types of Die Materials:
- Gypsum products
- Type 4 dental stone
- Type 5 dental stone, high strength, high Expansion
- Type 5, dental stone, lignosulfonates
- Metal and metal-coated dies
- Electroformed
- Sprayed metals
- Amalgam
- Polymers
- Metal or inorganic-filled resins
- Epoxy
- Cement
- Silicophosphate
- Polyacrylic acid-bonded cement
- Refractory materials
Die Materials Ideal Requirements:
- Dimensionally stable
- Have good abrasion resistance
- Have smooth surface
- Reproduce surface details accurately
- Biocompatible
- Noninjurious
- Color should contrast with wax, porcelain, and alloys
- Easy and quick to fabricate
- In Expensive
Dental Gypsum Setting Reaction
Question 7. Factors controlling the setting time of plaster
Answer:
Factors Controlling The Setting Time Of Plaster
- Manufacturing Process
- If calculations are not complete, gypsum particles remain and the resulting product will set fast
- If soluble anhydrite is in Examplecess, the plaster will set fast
- If natural anhydrite is in Examplecess, plaster sets slow
- Fineness
- The finer the particle size of the hemihydrates, the faster the mix hardens because
- Hemihydrates dissolve fast
- Gypsum nuclei are more
- Crystallization is fast
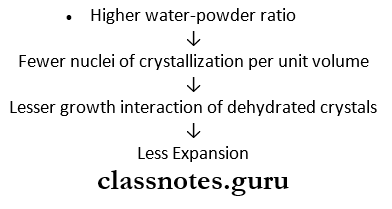
- Water-powder ratio
- More water, fewer nuclei present per unit volume
- Thus setting time is prolonged
- Mixing
- The longer and more rapidly the plaster is mixed, the shorter the setting time
- Temperature
- On increasing the temperature the rate of the reaction increases slightly and the setting time is shortened
- Modifiers
- Chemical decreasing setting time is called accelerators while that which increases setting time is called retarders
Question 8. Dental stone
Answer:
Dental Stone
Dental Stone is a type 3 gypsum product
Dental Stone Composition:
- Alpha hemihydrate
- Coloring agent – 2-3%
- Potassium sulfate – accelerator
- Borax – retarder
Dental Stone Manipulation:
- The proper water-powder ratio is determined
- Water is measured by graduated cylinder and powder by weighing balance
- Place a measured volume of water in the parabolic, smooth bowl
- Add preweighed powder gradually to it
- Allow settling for 30 seconds to minimize air entrapment
- The mix is stirred vigorously
- Periodically wiping inside the bowl with the spatula is done to ensure the wetting of all of the powder and breaking up of the lumps
- Continue mixing until a smooth uniform mix is obtained
- Vibrate the mix using a mechanical vibrator or by repeated tapping against the bench
Dental Stone Properties:
- Compressive strength – 3000-5000 psi
- Setting Expansion – 0.06-0.12%
- Hardness – 82 RHN
Dental Stone Uses:
- For preparing master casts
- To make molds
Gypsum Products Short Question And Answers
Question 1. Dental stone
Answer:
Dental Stone Composition:
- Alpha hemihydrate
- Coloring agent- 2-3%
- Potassium sulfate
- Borax-retarder
Dental Stone Manipulation:
- The proper water-powder ratio is determined
- Water is measured by graduated cylinder and powder by weighing balance
- Place a measured volume of water in a parabolic, smooth bowl first
- Add preweighed powder gradually to it
- Allow settling for 30 seconds to minimize air entrapment
- The mix is stirred vigorously
- Periodically wiping inside of the bowl with the spatula is done to ensure the wetting of all of the powder and breaking up of the lumps
- Continue mixing until a uniform smooth mix is obtained
- Vibrate the mix using a mechanical vibrator or by repeated tapping against the bench
- Pour it into the impression
Advantages And Disadvantages Of Gypsum
Question 2. Measurement of setting time of dental plaster
Answer:
Dental Plaster Setting Time:
The time that elapses from the beginning of mixing until the material
Dental Plaster Measurement:
- Loss of gloss test
- As the reaction proceeds, some of the Examplecess water is used to form dehydrate
- As a result, the mix loses its gloss
- Exothermic reaction
- As the setting reaction is Exothermic, the temperature rise of the mass is used to measure the setting time
- Initial Gillmore test
- A small Gillmore needle having % lb weight and a diameter of 1/12″ is used
- The mixture is spread out and the needle is lowered onto the surface
- The time at which it no longer leaves an impression is called the initial set
- Vicattest
- A Vicat needle weighing 300 gm and a diameter of 1 mm is used
- The needle with a weighed plunger rod is supported and held just in contact with a mix
- Soon after the gloss is lost, the plunger is released
- The time elapsed until the needle no longer penetrates the bottom of the mix is known as the setting time
- Gillmore test for the final set
- A larger Gillmore having a 1 lb weight and diameter of 1/24″ is used
- The elapsed time at which this needle leaves only a barely perceptible mark on the surface is called the final setting time
Question 3. Hygroscopic Expansion
Answer:
Hygroscopic Expansion
- Expansion that occurs in water is called hygroscopic Expansion
- It is a physical Expansion
- When Expansion begins, Externally available water is drawn into pores forming a setting mass
- It maintains a continuous aqueous phase in which crystal growth takes place freely
Hygroscopic Expansion Importance:
Use to Expand some gypsum bonded investments
Question 4. Setting Expansion
Answer:
Setting Expansion
All gypsum products show a linear Expansion during setting, due to the outward thrust of the growing crystals during setting
1. Normal Setting Expansion:
Setting Expansion without water immersion is called normal setting Expansion
Normal Setting Expansion Importance:
- Undesirable in the construction of cast
- Causes change in occlusal relations
- Desirable in case of investment materials
2. Hygroscopic Expansion:
- Expansion that occurs in water is called hygroscopic Expansion
- It is a physical Expansion
- When Expansion begins, Externally available water is drawn into pores forming a setting mass
- It maintains a continuous aqueous phase in which crystal growth takes place freely
Hygroscopic Expansion Importance:
Use to Expand some gypsum bonded investments
Dental Plaster Vs Dental Stone
Question 5. Impression plaster Or plaster of Paris
Answer:
Impression Plaster Or Plaster Of Paris
- Type 1 or impression plaster is also known as plaster of Paris
- It was the earliest impression material in dentistry
- Because of its rigidity and nonelastic nature, it often had to be fractured for its removal from undercut areas in the mouth
- The fractured pieces were then reassembled outside and a cast was poured
Plaster of Paris Uses:
- For making an impression in complete denture and maxillofacial prosthesis
- Bite registration material
Plaster of Paris Properties:
- The setting time should be under accurate control
- The dentist must have sufficient time to mix, load the impression tray, carry the loaded tray to the patient’s mouth
- and place it in the position
- The plaster should harden promptly once in position so that there is minimum discomfort to the patient
- The desirable setting time is 3-5 min
- The setting Expansion should be low for better accuracy
- Both setting time and Expansion are controlled by modifiers
- The plaster should have enough strength to fracture cleanly without crumbling to facilitate its removal from undercut areas
Question 6. Bite registration paste
Answer:
Bite Registration Paste
- Materials used for recording the occlusal relationship between natural and artificial teeth are
- Impression plaster o Impression compound
- Wax
- Resin
- Metal oxide
- Zinc oxide eugenol paste
- ZOE paste is used often because
- It has a shorter setting time to prevent distortion
- Has more plasticizers to prevent it from sticking to teeth and rims
- Has almost no resistance to closing the mandible
- The record obtained is more rigid, more stable, and more accurate
Question 7. List various gypsum products and write about methods of testing setting time.
Answer:
Types of Gypsum Products Used In Dentistry:
- Type 1- Dental plaster, impression
- Type 2- Dental plaster, model
- Type 3- Dental stone
- Type 4- Dental stone, high strength
- Type 5- Dental stone, high strength, high Expansion
Methods for Measuring Setting Time:
- Loss of gloss test
- Exothermic reaction
- Initial Gillmore test
- Vicattest
- Gillmore test for the final test
Dental Plaster Vs Dental Stone
Question 8. Wet and dry strength
Answer:
Wet And Dry Strength
Wet strength is the strength obtained when the water is Excess of that required for hydration of the hemihydrates
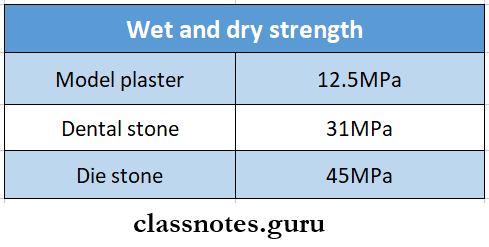
- Dry strength – When excess water in the specimen is driven off by drying, the strength obtained is dry
- It may be two or more times as high as wet strength
Wet And Dry Strength Factors Affecting it:
- Water-powder ration- the greater the ratio- the less the dry strength
- Saturation- increase in mixing time, increases strength
- Modifiers- lowers the strength
