Combinatorial Chemistry Definition
The synthesis of chemical compounds as ensembles (libraries)and the screening of those libraries for compounds with desirable properties “Potentially speedy route to new drugs, catalysts, and other compounds and materials.
Concept Of Combinatorial Chemistry
The use of combinatorial chemistry techniques has been explored as an alternative to conventional approaches for the synthesis of compounds in the drug discovery process.
- This technique is the starting point for the development of synthesis concepts that were intended to cover and explore the chemical space without having to prepare every individual compound.
- Combinatorial Chemistry technologies were developed in response to the increased screening capacities that are available.
- when drug discovery changed its screening paradigm from a pharmacology-based approach to target-oriented lead finding.
Solid Phase Synthesis
Most solid-state combinatorial chemistry is conducted by using polymer beads ranging from 10 to 750 pm in diameter.
The Solid Support Must Have The Following Characteristics For An Efficient Solid-Phase Synthesis:
- Physical stability and the right dimensions to allow for liquid handling and filtration;
- Chemical inertness to all reagents involved in the synthesis;
- An ability to swell while under reaction conditions to allow permeation of solvents and reagents to the reactive sites within the resin;
- Derivatization with functional groups to allow for the covalent attachment of an appropriate linker or first monomeric unit.
Read and Learn More Medicinal Chemistry III Notes
Types Of Solids That Are Used:
- Polystyrene Resins: In this Polystyrene is cross-linked with divinyl benzene (about 1% crosslinking) .polystyrene resins are suitable for nonpolar solvents.
- Tenta Gel Resins: Polystyrene in which some of the phenyl groups have polyethylene glycol (PEG) groups attached in the tire para position. The free OH groups of the PEG allow the attachment of compounds to be synthesized. PEG-containing resins are suitable for use in polar solvents.
- Polyacrylamide Resins: Like super blue, these resins swell better in polar solvents since they contain amide bonds, and more closely resemble biological materials.
- Glass And Ceramic Beads: These types of solid supports are used when high-temperature and high-pressure reactions are carried out.
- Linkers Used In Solid-Phase Synthesis: To support the attachment of a synthetic target, the polymer is usually modified by equipping it with a linker.
- Linkers must be stable under the reaction conditions, but they must be susceptible to cleavage.
- Some specialized linkers have been developed to meet particular reactions or product conditions this type of linker is known as a traceless linker, it can be cleaved from the resin with no residual functionality left.
- This type of linker allows the attachment of aryl and alkyl products that do not have OH or NH functionality Examples of this linker include the silyl group (-Si(CH3)2) that is sensitive to acid and can be cleaved to give unsubstituted phenyl or alkyl product.
- Protecting groups these are important for blocking and regenerating certain functional groups in a reaction sequence. Example FMOC, TBOC
- Combinatorial synthesis on solid support is usually carried out using either parallel synthesis or mixed procedures
- Parallel synthesis’In this method, the compounds are prepared in separate vessels but at the same time that is in parallel.
- Mix and split technique: ‘May be used to make both large and small combinatorial libraries using relatively few reaction steps. The history of the bead is traced by using a suitable encoding method or deconvulsion.
Combinatorial Chemistry Application
1. Synthesis Of Bis(2-picolyl)Amine (BPA) Molybdenum Conjugate: In case when the attachment of a metal complex to the peptide on the solid support is not desirable
- For example with radioactive metal isotopes, an innocent anchoring group can be attached to the peptide during solid-phase synthesis.
- The ligand-peptide conjugate is then cleaved from the resin, purified and the metal label is only added to the solution immediately before use of the bioconjugate.
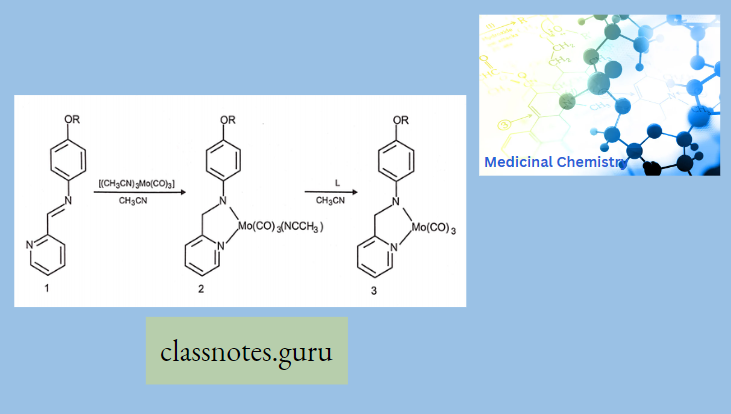
2. Bidentate Schiff Base Metal Conjugates: A solid-phase synthesis approach for molybdenum carbonyl complexes was developed by Heinze.
- Neither peptide coupling nor metallated amino acids are used, because it illustrates that complex organometallic transformations are possible on solid support.
- A specific resin and linker system allows coordination under solid-phase reaction conditions and the cleavage of the metal complex from the solid support.
- Bidentate Schiff base 1 was used as the ligand.
- The phenolic hydroxyl group allows the attachment to the solid support. A silyl ether-based linker was chosen due to its stability under basic and acidic conditions and the possibility to cleave with fluoride ions, which are expected to be unreactive towards most metal complexes.
- In solution high temperature and rather harsh oxidative reaction conditions are necessary to synthesize the desired tricarbonyl compounds.
- Such harsh conditions have to be avoided in solid-phase chemistry with polystyrene resins as the molybdenum precursors can react with the aromatic residues of the support.
- Heinze and co-workers used [(CH3CN)3Mo(CO)3] as a Mo(CO)3 source and under mild reaction conditions the intensely blue-colored complexes 2 and 3 formed rapidly and had excellent yields.
- However, acetonitrile, a rather poor solvent for resin swelling, had to be used in a mixture with toluene.
- The cleavage was performed with tetra-n- butylammonium fluoride in dichloromethane and resulted in deeply colored solutions of the deprotonated complexes.
Solution Phase Synthesis
Most ordinary synthetic chemistry takes place in the solution phase. The use of solution-phase techniques has been explored as an alternative to solid-phase chemistry approaches for the preparation of arrays of compounds in the drug discovery process.
- Solution-phase work is free from some of the constraints of solid-phase approaches but has disadvantages concerning purification.
- In solution phase synthesis we use soluble polymer as support for the product.
- PEG is a common vehicle that is used in solution phase synthesis it can be liquid or solid at room temperature and shows varying degrees of solubility in aqueous and organic solvents.
- By converting one OH group of PEG to methyl ether (MeO-PEG-OH) it is possible to attach a carboxylic acid to the free OH and use in solution phase combinatorial synthesis.
- Another common support that is used in solution phase synthesis is liquid Teflon consisting mainly of a long chain of (-CF2) groups attached to a silicon atom. When these phases are used as a soluble support for synthesis the resulting product can be easily separated from any organic solvent.
The reaction proceeds in Solution. Can be used to produce libraries that consist of single compounds or mixtures.
- Single compound libraries are prepared using parallel synthesis. ‘Easy characterization of intermediates as well as end product.
- No limitations in the attachment point.
- Faster validation times relative to solid phase synthesis. ‘Standard analytical protocols can be used to characterize products between each reaction step ‘Difficult to drive the reaction towards the product, extensive purification is needed.
Solution Phase Synthesis Application
Synthesis of Polymer By Solution Phase Combinatorial Chemistry
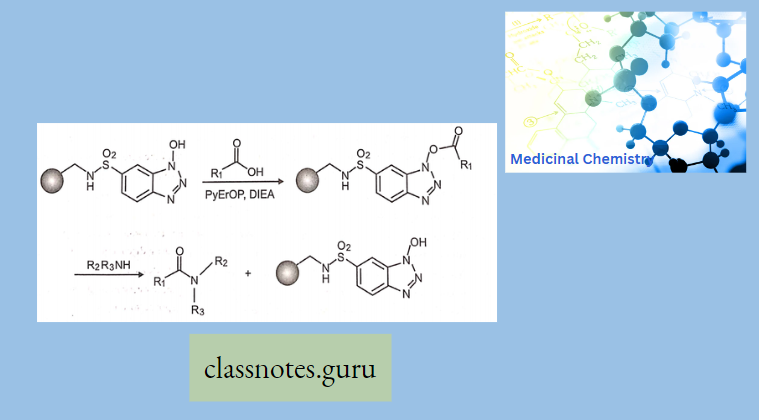
- Tartar and co-workers reported the synthesis of polymer-supported 1-hydroxybenzotriazole. Reaction of the reagent with a carboxylic acid in the presence of an activating agent afforded the polymer-bound activated ester which was reacted with amines to liberate the amide in solution.
- Supported electrophilic, nucleophilic, or ionic reagents used to remove impurities from solution have been termed scavenger reagents; polymer-supported quenching reagents (PSQ), or complementary molecular reactivity or molecular recognition polymer (CMR/R polymer).
- The use of such reagents provides a versatile counterpart to the approach.
- Booth and Hodges utilized a high-loading amine resin derived from chloromethyl polystyrene and tris (2 – aminoethyl)amine in the preparation of ureas, thioureas, sulphonamides, and amides.
Solution Phase Synthesis of Biologically Important Oligosaccharides
- We departed from the traditional goal of oligosaccharide total synthesis striving for maximum convergency, and followed a linear synthesis approach based on monosaccharide
building blocks. - Using this method similar to that practiced for peptides and oligonucleotides we assembled several complex structures.
Solution Phase Synthesis Importance
Combinatorial chemistry continues to provide an important technique, particularly to the medicinal chemist engaged in lead optimization work.
- Combinatorial chemistry and parallel synthesis can greatly benefit from the unique features offered by new synthetic technology.
- These include the possibilities of high-speed parallel processing of chemical transformations in the context of library production, and the rapid optimization of reaction conditions.
- Among the solid and solution phase synthesis, Solid-phase organic synthesis (SPOS) is the most important method for the production of combinatorial libraries because all the synthetic transformations are successfully applied to a solid phase, and with the development of high-throughput screening, libraries are widespread in pharmaceutical and agricultural chemistry.
Combinatorial Chemistry Short Question And Answers
Question 1. What is the combinatorial chemistry?
Answer:
Combinatorial Chemistry: The synthesis of chemical compounds as ensembles (libraries)and the screening of those libraries for compounds with desirable properties ‘Potentially speedy route to new drugs, catalysts, and other compounds and materials.
Question .2 What is the concept of combinatorial chemistry?
Answer:
The Concept Of Combinatorial Chemistry: The use of combinatorial chemistry techniques has been explored as an alternative to conventional approaches for the synthesis of compounds in the drug discovery process.
- This technique is the starting point for the development of synthesis concepts that were intended to cover and explore the chemical space without having to prepare every individual compound.
- Combinatorial Chemistry technologies were developed in response to the increased screening capacities that are available when drug discovery changed its screening paradigm from a pharmacology-based approach to target-oriented lead finding.
