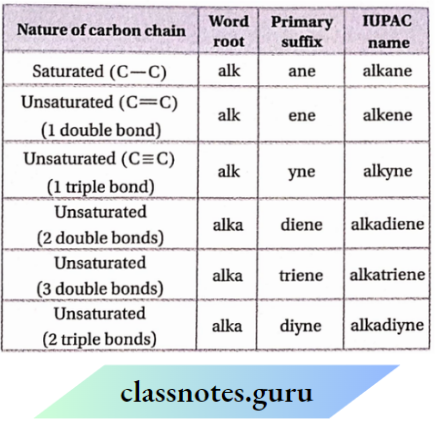
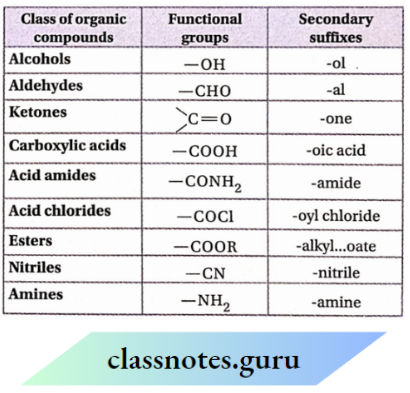
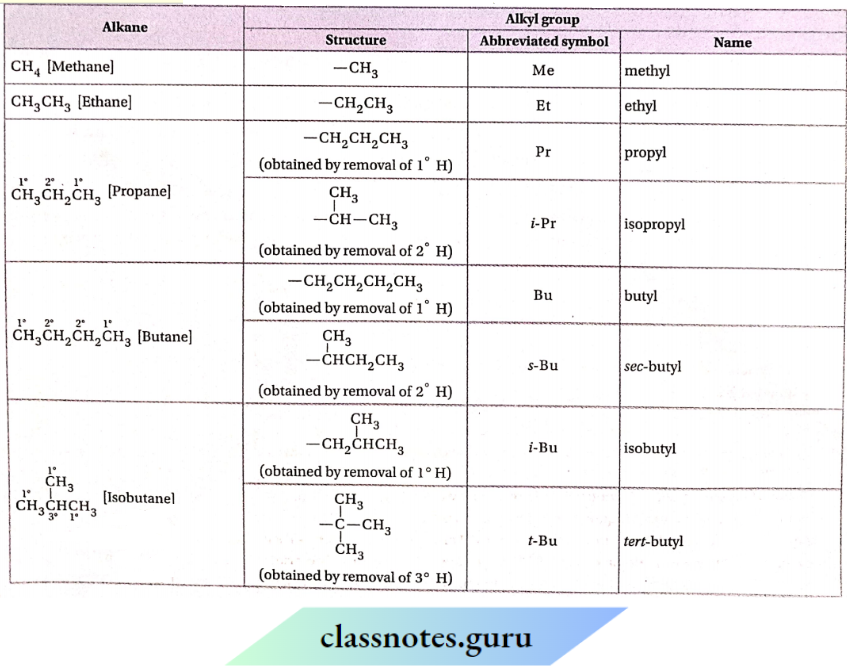
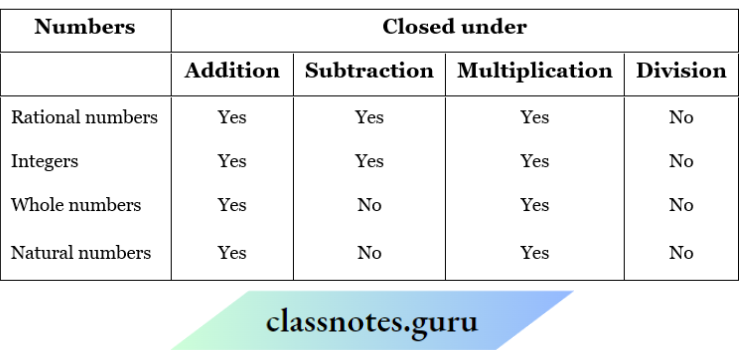
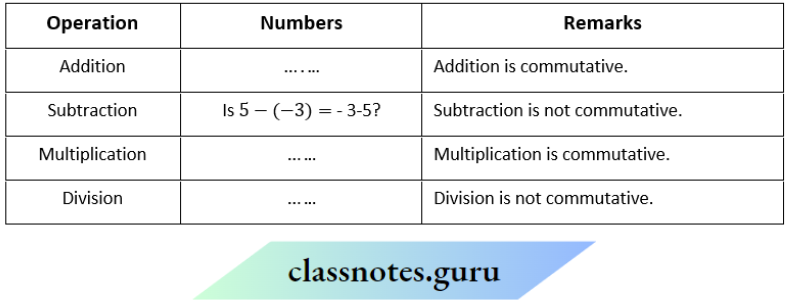
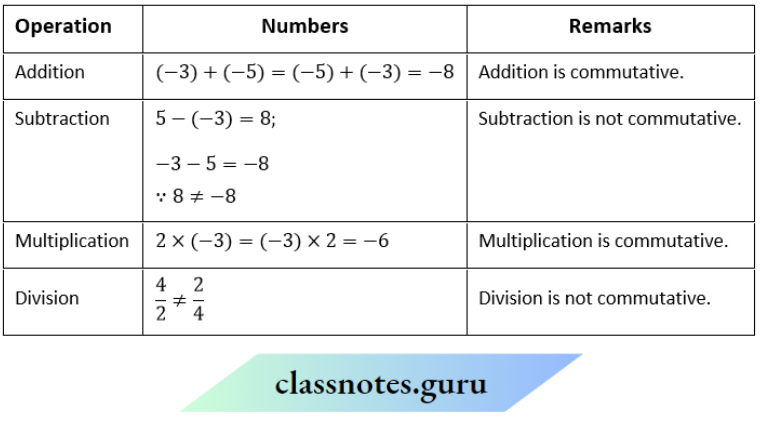
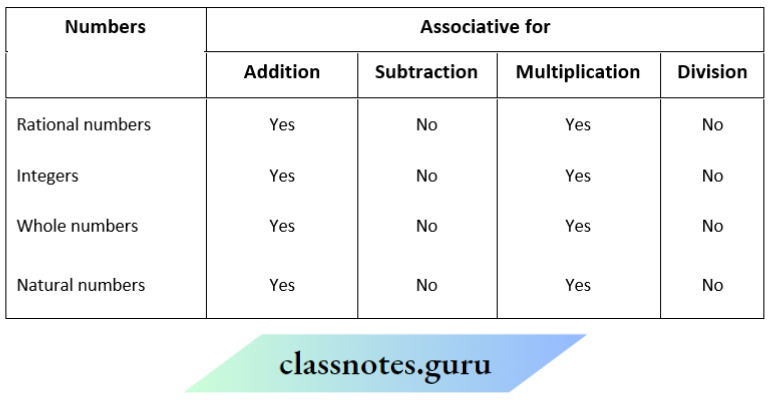
NCERT Solutions For Class 11 Chemistry Chapter 12 Organic Chemistry Basic Principles And Techniques Long Question And Answers
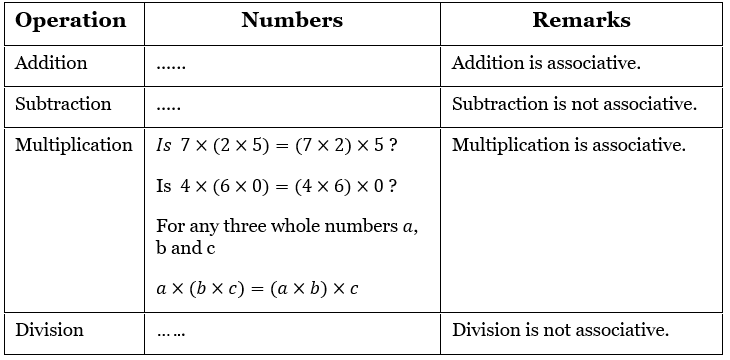
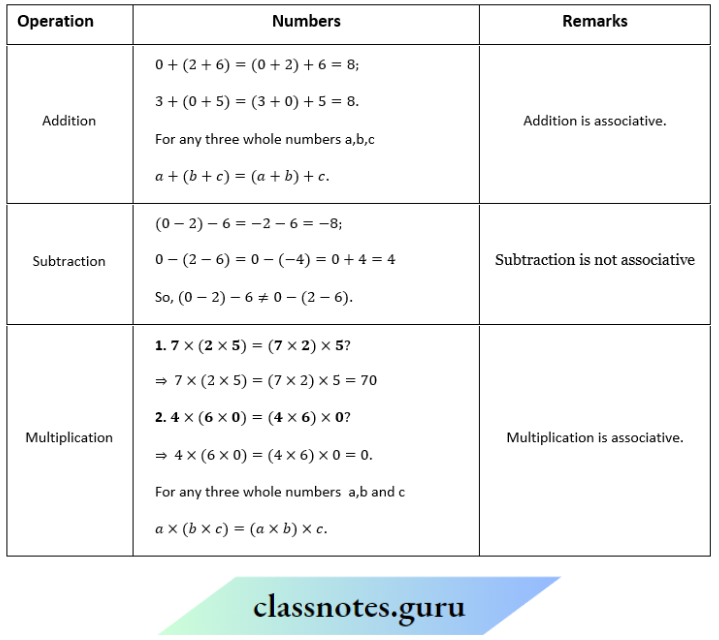
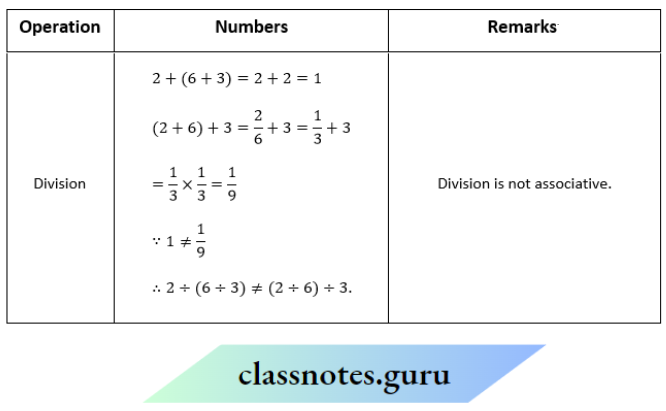
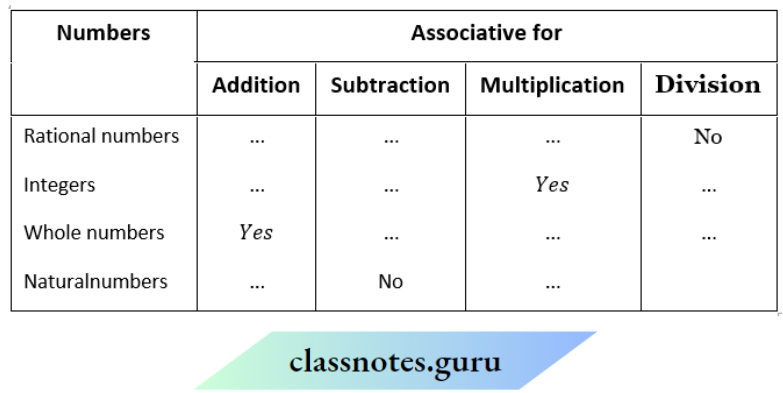
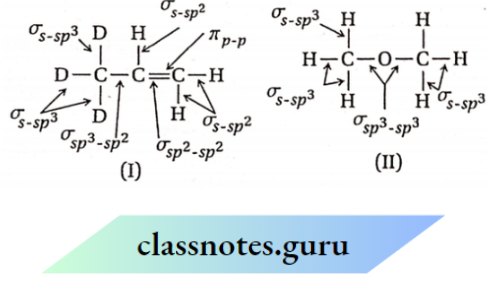
Question 1. Depict the bonding in the following compounds In terms of atomic orbitals involved and predict all the bond angles:
- CD3CH=CH2
- CH3OCH3
Answer:
1. The 3 carbon atoms are sp³ , sp² and sp² -hybridised respectively. Therefore the bond angles about these carbons are 109.5°, 120° and 120° respectively corresponding to tetrahedral and trigonal planar geometries.
2. The carbon and oxygen atoms are all sp³ -hybridized. So, the bond angles are nearly 109.5° corresponding to tetrahedral geometry.
Read and learn More NCERT Class 11 Chemistry
Class 11 Chemistry Chapter 12 Organic Chemistry Long Questions
Question 2. Mention the number of primary (1°), secondary (2°), and tertiary (3°) hydrogen atoms in the following

Answer:
Hydrogen Atoms Given in the table:

Question 3. How many alkyl groups can be derived from the alkane, (CH3)2 CHCH2 CH(CH3)2 and why? Write their IUPAC names.
Answer:
Since this hydrocarbon molecule contains 3 types of nonequivalent hydrogen atoms, the removal of these hydrogen atoms gives 3 different alkyl groups.
These are as follows:

Question 4. Write the IUPAC names of the following compounds

Answer:
- 3-ethyl-4-methylhept-5-en-2-one
- 3,3,5-trlmethylhex-1 -en-2-ol.
- l-bromo-4-metlvylheptan-3-on«.
- l-etliyl-4-methylcyclohexane.
- Cyclohexylcyclohexnne.
- 1,3-dlcyclopropylpropanc.
- 2-metliyl-2-cyclopropylpropnne.
- N -ethyl- N -methylpropan-2-nmine.
- 4-hydroxy-4-methylpontan-2-one.
- 3-methylpent-l-ene.
Question 5. Arrange the given carbocations To increase stability and explain two-order

Answer:
The order of increasing stability of these carbocation Is :

Being an aromatic one [(4 n + 2)n -electron system, where n = 1], the carbocation (I) is the most stable. The carbocation (II) is effectively resonance stabilized. So, its stability is greater than that of (ill) and (IV) (which are not resonance-stabilized) but less than that of (I).
The carbocation (III) is stabilized by +1 and the hyperconjugation effect of the methyl group and its stability is less than (II). The carbocation (IV) is destabilized by the stronger -I effect of the — CF3 group, so it is the least stable one.

Question 6. Terf-Butyl chloride (Me3CCl) does not participate in D+ SN2 reaction—explain with reasons
Answer:.
Due to severe steric hindrance caused by three methyl groups, the backside attack on the central carbon by the nucleophile becomes completely inhibited and it is for this reason, that terf-butyl chloride does not participate in the SN2 reaction..

Question 7. Write the resonance structures of CH2=CH —CHO and compare their stabilities.
Answer:
The compound is a resonance hybrid of three structures:

The stability order of these structures is I > II > III.
The uncharged structure (I) is the most stable one. The charged structure (II) is moderately stable because the more electronegative oxygen atom bears the negative charge and the less electronegative carbon atom bears the positive charge.
Also, the octet of carbon is not filled up. The charged structure (III) is the least stable because the more electronegative O-atom bears the positive charge and the less electronegative C-atom bears the negative charge. Also, the octet of the O-atom is not filled up
Question 8. Which is more stable and why: (CH3)3C, (CD3)3C
Answer:
Since D is more electron-releasing than H, — CD3 is more electron-releasing than — CH3. So, (CD3)3C+ is expected to be more stable than (CH3)3C+. But actually, this is true and this can be explained in terms of hyperconjugation. Since the C—H bond is weaker than the C— D bond the hyperconjugative stability of (CH3)3C+ is greater than that of (CD3)3C+

Question 9.
- How many stereoisomers of formula, CH3 would be possible if methane was a pyramid with a rectangular base? Draw them.
- How many stereoisomers of formula, CH2YZ would be possible if methane was a pyramid with a square base? Draw them.
- What is the relationship (diastereoisomers, enantiomers, conformational isomers, homomers i.e., identical structures or constitutional isomers) between the members of given pairs of structures?

Answer:
1. Two stereoisomers Mirror (enantiomers) are Q possible if methane H / was a pyramid with a rectangular base.
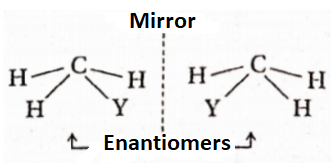
2. Three stereoisomers (1, 2, and 3) are possible if methane is a pyramid with a square base. (1 and 2) are enantiomers. (1 and 3) and (2 and 3) are two pairs of diastereoisomers.

3.
- . Diastereoisomers
- Homomers
- Geo¬ metrical isomers
- Constitutional isomers
- Conformational isomers
- Enantiomers.
Question 10. Although Quorine is more electronegative than iQfi chlorine, fluorobenzene has a lower dipole moment (p = 1.63D ) than chlorobenzene (μ = 1.75D ).
Ans.
Both fluorine in fluorobenzene and chlorine in structure) chlorobenzene withdraws electrons from the ring by the -I effect and donates electrons to the ring by the + R effect. Because of the smaller size of fluorine, the +R effect involving orbitals of similar sizes (2p of both F and C) is much stronger. So, the moment due to the stronger -I effect of fluorine is considerably neutralized by the moment due to the +R effect.
Hence, fluorobenzene possesses a net dipole moment which is relatively low (1.63D). On the other hand, because of the larger size of chlorine, the + R effect involving orbitals of dissimilar sizes (3p of Cl and 2p of C) is much weaker than the -I effect which is somewhat lower due to lower electronegativity of chlorine. So, the moment due to the +R effect is much smaller than the moment due to the -I effect. Hence, chlorobenzene possesses a relatively high net dipole moment (1.75D

Question 11. The negatively charged carbon atom in the structure
- Is sp² -hybridized while the negatively charged carbon atom in
- Is sp³ -hybridised —Explain.
Answer:
The negatively charged carbon atom of a resonance-stabilized carbanion is sp² -hybridized. The carbanion (I) is resonance stabilized. So the negative carbon atom Is sp².
hybridized

On the other hand, the carbanion (2) Is not resonance stabilized because a double bond cannot be formed at the bridgehead position of small bicycle systems (Hrodt’s rule). Hence, the negatively charged carbon of the carbanion (II) is sp³ -hybridized.

Question 12. Mention the state of hybridization of the starred (*) carbon atoms in each of the following compounds.

Answer:
- sp²
- sp
- sp²
- sp
- sp
- sp³
Question 13. How many σ and π -bonds are present in each of the following molecules?
1. CH3-C≡-CH= CH2
2. CH2=CH-CH=C=CHCH3
3.

Answer:
- σ -bonds =10, π -bonds =3
- σ -bonds = 13 , π -bonds = 3
- σ -bonds =10, π -bonds = 3
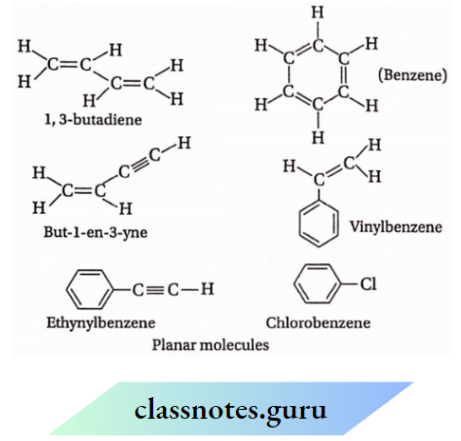
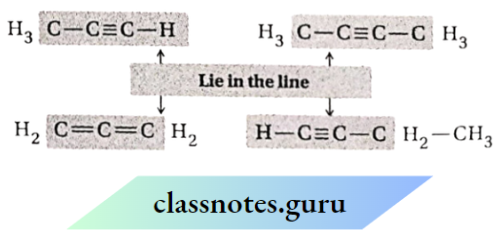
Question 14. Which atoms in each of the following molecules remain in the same plane and why?
- CH3CH= CH3
- C6H5C≡ CCH3
- CH3CH=C=C=CHCH3
- CH3COCH2CH3
Answer:
sp²-carbon atoms and the atoms attached to them lie in one plane.

sp² -carbon atoms and the atoms attached to them lie in one plane. Also, sp carbon atoms & the atoms attached to them lie not only in one plane but also in one line.

Lie in one plane

Lie in one plane

Question 15. Mention the number of primary (1°), secondary (2°), tertiary (3°), and quaternary (4°) C -atoms present in the given molecules: °
Answer:

Answer:

Question 16. Write down the IUPAC name of a hydrocarbon having a 4° C-atom with molecular formula, C6H14. How many monochrome derivatives of this hydrocarbon are possible? Write their structures
Answer:
The hydrocarbon corresponding to the molecular formula C6H14 and containing one tertiary carbon atom is CH3C(CH3)2CH2CH3 (C-2 is a quaternary carbon atom). Its IUPAC name is 2,2-dimethylbutane.
Since the alkane contains three types of non-equivalent hydrogen atoms, three monobromo derivatives of the alkane are possible. These are: (CH3)3CCHBrCH3 and (CH3)3CCH2CH2Br
Question 17. Racemic tartaric acid and meso-tartaric add are both optically inactive—why?
Answer:
Racemic tartaric add is an equimolar mixture of (+) and (-)-tartaric adds. In racemic tartaric add, therefore, the rotatory power of the (+) enantiomer is neutralized by the rotatory power of the (-) enantiomer (external compensation) and for this reason, the racemic tartaric add is optically inactive.
On the other hand, the meso-tartaric ad is optically inactive because it has a plane of symmetry and is superimposable on its mirror image. In fact, in this case, the optical rotation of one half of the molecule is exactly canceled by the optical rotation of the other half (internal compensation).
Organic Chemistry Basic Principles and Techniques Long Q&A
Question 18. How many isomers of butene are possible? What type 1 of isomerism do they exhibit?
Ans.
Three structural isomers of butene are possible: CH3CH2CH=CH2 (But-l-ene), CH3CH=CHCH3 (But-2- ene), and (CH3)2C=CH2 (2-methylpropene). Again, but-2- ene exists as two geometrical isomers (diastereoisomers):

Hence, there are in total 4 isomers of butene: but-1-ene, cis but-2-ene, irans-but-2-ene, 2-methylpropene
Question 19. Give examples of
- An optically inactive compound containing an asymmetric carbon atom
- An optically active compound containing no asymmetric carbon.
Answer:
1. Meso-tartaric acid containing two asymmetric carbon atoms is optically inactive because it has a plane of symmetry, l.e., tire molecule is superimposable on its mirror Image 
2. Penta-2,3-diene (an abC=C=Cab type of allene) is optically active because it is not superimposable on its mirror image.

Question 20. Name a compound having two similar asymmetric carbon atoms and give its structure. What type of isomerism does it exhibit? Draw Fischer projection formulas of these isomers and comment on their optical activity. How are they related to each other?
Answer:
Tartaric acid has two similar asymmetric carbon atoms (HOOC — *CHOH —*CHOH —COOH). The compound exhibits optical isomerism.
Fisher projection formulas of its isomers are as follows:

The relations among the isomers are as follows: I and II are enantiomers; I and III are diastereoisomers and II and III are diastereoisomers
Question 21.
- Give the structure and IUPAC name of an optically active alkane having the lowest molecular mass. Is there another alkane of the same molecular mass also optically active?
- Give an example of a compound that exhibits both optical & geometrical isomerism.
Answer:
Such a compound must contain an asymmetric carbon atom which will remain attached to an H-atom and three different alkyl groups (smaller size).
So, the optically active alkane having the lowest molecular mass’ is, 3-methylhexane [CH3CH2 *CH(CH3)CH2CH2CH3 ]. Another optically active alkane with the same molecular mass is 2,3-dimethyl pentane [CH3*CH2CH(CH3)CH(CH3)2] which is a chain isomer of the first one.
Pent-3-en-2-oI [CH3*CH(OH)CH=CHCH3] exhibits both geometrical and optical isomerism because the compound contains an asymmetric carbon atom and each of the doubly bonded carbon atoms is attached to two different groups.
Question 22. The following two isomers may be called diastereoisomers but not enantiomers —why? Explain why these are optically inactive.
Answer:
The given pair of isomers have the same structure but different configurations. They are neither superimposable nor bear mirror-image relationships with each other. So they are related to each other as a pair of diastereoisomers and not as enantiomers. Each of these two isomers has a plane of symmetry, i.e., each is superimposable on its mirror image, so these are optically inactive.
Question 23. p nitrophenol is more acidic than phenol.
Answer:
In p-nitrophenol, the electron-attracting — NO2 group by its stronger -R effect and relatively weaker -I effect makes the oxygen atom relatively more positively polarised compared to the oxygen atom of phenol. As a result, the O — H bond in nitrophenol dissociates more easily to give H+ ions. For this reason, p-nitrophenol is more acidic than phenol.
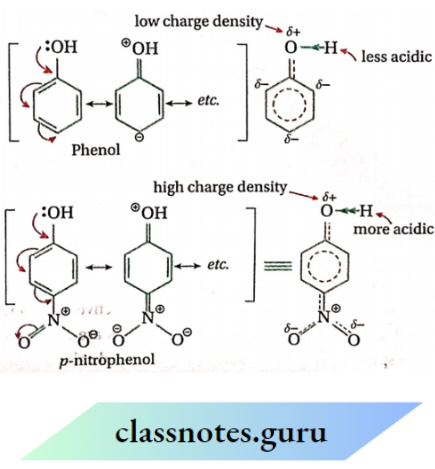
Answer:
In p-nitrosamine, the electron-attracting — NO2 group by its stronger -R effect and relatively weaker -I effect makes the nitrogen atom of the — NH2 group relatively more positive compared to the nitrogen atom of aniline. As a result, the availability of the unshared pair of electrons on nitrogen atoms in p-nitroaniline is highly reduced as compared to the unshared electron pair on nitrogen in aniline. For this reason, p-nitroaniline behaves as a weaker base compared to aniline.

Question 24. The dipole moment of vinyl chloride CH2=CHCI) is less than the dipole moment of ethyl chloride (CH3CH2Cl) —explain.
Answer:
In vinyl chloride, the moment caused by the -I effect of Cl-atom (μσ) is partially neutralized by the moment caused by its +R effect (pn). As a result, the value of the net moment of vinyl chloride decreases and it is lower than that of ethyl chloride in which only the stronger -I effect of chlorine operates.

Question 25. Arrange the following ions in order of increasing basicity and explain the order
- CH3 –CH
- CH ≡–C
- CH2 =–CH
Answer:
The order of increasing basicity of the given ions is:
⇒ \(\mathrm{CH} \equiv \stackrel{\ominus}{\mathrm{C}}(\mathrm{II})<\mathrm{CH}_2=\stackrel{\ominus}{\mathrm{C}} \mathrm{H}(\mathrm{III})<\mathrm{CH}_3 \stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2(\mathrm{I})\)
C -atoms bearing the negative charge in carbanions (1), (2) & (3) are sp³, sp, and sp² -hybridized respectively. Percentages of s -the character of these three hybrid orbitals are 25%, 50%, and 33% respectively.
As the s -the character of hybrid orbital increases, C -atoms bearing the negative charge in carbanions (1), (2) & (3) are sp³, sp, and sp² -hybridized respectively. Percentages of s -the character of these three hybrid orbitals are 25%, 50%, and 33% respectively. As the s -s-character of hybrid orbital increases,
Question 26. Give example:
- A non-nucleophilic anion
- A planar carbocation
- An aromatic carbocation
- An aromatic carbanion
- A reagent which acts as a source of carbanion
- A reaction that does not proceed through intermediate
- An aprotic polar solvent
- An ambident nucleophile
- A neutral electrophile
- A group that stabilizes a carbocation
- A group that stabilizes a carbanion
- An alkyl group which does not supply electrons to a double bond by hyperconjugation
- A carbocation that can be stored for years.
Answer:
1. BF–4
2. Benzyl cation

3. Cyclopropenvl cation

4. Cyclopentadienyl anion

5. Grignard reagents \(\left(\mathrm{R}^{\delta-}-\mathrm{M}^{\delta+} \mathrm{gX}\right)\)
6. SN2 reaction
7. Dimethyl formamide [DMP, Me2NCHO]
8. –CN \((: \stackrel{\ominus}{\mathrm{C}} \equiv \mathrm{N}: \longleftrightarrow: C=\stackrel{\ominus}{\mathrm{N}}:)\)
[Nucleophiles having two or more available sites for nucleophilic attack are called ambident nucleophiles)
9. Dichlorocarbene (: CCl2)
10. \(-\ddot{O}:\mathrm{CH}_3\)
11. —NO2
12. —C(CH3)3
13. Triphenylmethylfluoroborate Ph3+CBF4–
Question 27. Explain the given basicity order in aqueous medium: )2NH(2°) > CH3NH2(1°) > (CH3)3N(3°)
Answer:
The basic strength of amines in the aqueous medium depends on two factors:
Increased electron density on the N-atom makes an amine more basic.
So considering the +1 effect of different numbers of alkyl groups on the N -atom, the basic strength of amines should follow the order:

Again, the basicity of an amine increases as stabilization of the conjugate acid, through solvation, increases. The conjugate acid of primary amine attains maximum stability through intermolecular H -bond formation with three molecules of water, while the conjugate base of tertiary amine attains minimum stabilization through such H-bond formation with only one molecule of water.

Thus based on the stability of the conjugate acids, the basic strength of amines should follow the order: CH3—NH2 > (CH3)2NH > (CH3)3N As a consequence of these two opposite orders of basicity practically we find the given sequence of basicity in aqueous medium: (CH2)2NH(2°) > CH3NH2(1°) > (CH3)3N(3°)
Question 28. Which of the two: 2NCH2CH2O– or CH3CH2O– is expected to be more stable and why?
Answer:
O3 NCH2CH3O– is expected to be more stable than CH3CH2O–. —NO2 group by strong-I effect disperses the negative charge on O-atom in ion and stabilizes it. On the other hand, the CH3CH2— group by its +1 effect tends to intensify the negative charge on the oxygen atom and hence destabilizes it.
Question 29. CH3Cl undergoes hydrolysis more easily than C6H5Cl. Explain.
Answer:
Unshared electron-pair on chlorine atom in chloro-benzene becomes involved in resonance interaction with the n -electrons of a benzene ring. As a result, the C — Cl bond assumes some double bond character. Thus, the C — Cl bond becomes much stronger and so the displacement of chlorine atom from the ring becomes difficult, i.e., the compound does not undergo hydrolysis easily.

On the other hand, the C —Cl bend in CH3— Cl gets no opportunity to assume a double bond character. So r undergoes hydrolysis readily under ordinary conditions.

Question 30. Benzyl chloride participates in SN1 action even though it is a primary (1°) substrate. Explain.
Answer:
Carbocation produced from benzyl chloride in the first step (rate-determining step) of the SN1 reaction is stabilized by resonance. Thus, benzyl chloride participates in the SN1 reaction even though it is a primary (1 °) alkyl halide.

Class 11 Chemistry Chapter 12 Organic Chemistry Long Questions
Question 31. The Bond Dissociation enthalpy of the C6H5CH2 – H bond is much less than the CH2-H bond Explain
Answer:
Benzyl radical (C6H55C•H2) produced by homolytic cleavage of the Csp3 —H bond of toluene is considerably stabilized by resonance. But, the stability of methyl radical (•CH3, obtained by homolytic fission of the C—H bond of methane Is not stabilized by any factor and in fact, it is very much unstable. Thus, the C—H bond dissociation enthalpy of toluene is much less than the C —H bond dissociation enthalpy of methane. ,

Question 32. N, N, 2,6-Tetramethylaniline is more basic than N- dimethylaniline. Explain.
Answer:
Because of steric interaction involving two ortho-methyl groups and two methyl groups attached to nitrogen, the unshared electron-pair on N is not involved in resonance interaction with n -electrons (steric inhibition of resonance) o: ring. To avoid steric strain, the — NMe2 group rotates about the C — N bond axis and thereby loses coplanarity with the ring. As a result, nitrogen can easily donate its unshared electron pair to a proton.
On the other hand, no such steric inhibition occurs in N- dimethylaniline because the two ortho H-atoms are relatively much smaller in size. The unshared electron-pair on N-atom becomes involved in resonance interaction with the ring and therefore, is not fully available for taking up a proton. This explains why N, N,2, O-tetramethylaniline is more basic than N, N -dimethylaniline.

Question 33. Chloroform is more acidic than fluoroform. Explain.
Answer:
CF–3 the conjugate base of fluoroform (CHF3 ), is stabilized by the -I effect of 3 F-atoms. But CCl–3, the conjugate base of chloroform (CHCl3 ), is relatively more stabilized by the somewhat weaker -I effect of 3 Cl-atoms along with rf-orbital resonance (Cl has vacant rf-orbital). So chloroform is more acidic than fluoroform.

Question 34. How can you separate benzoic acid and nitrobenzene from their mixture by the technique of extraction using an appropriate chemical reagent?
Answer:
The mixture is shaken with a dilute sodium bicarbonate solution when benzoic acid gets converted to sodium benzoate and dissolves in water leaving nitrobenzene behind. The mixture is extracted with ether or chloroform when nitrobenzene goes into the organic layer. After separating the organic layer, it is distilled to get nitrobenzene. The aqueous layer is acidified with dilute HC1 when benzoic acid gets precipitated. It is obtained by filtration.
C6H5COOH + NaHCO3→C6H5COONa(Sodium benzoate (Soluble)) + CO2 + H2O
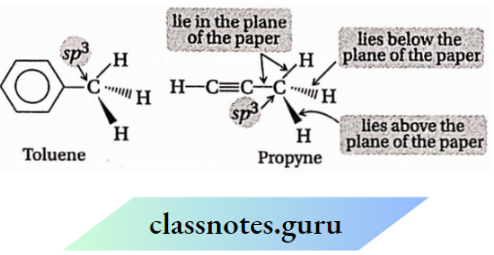
Question 35.
- Which atoms in a toluene molecule always remain in the same plane and why?
- Which atoms in a propyne molecule remain in a straight line and why?
Answer:
1. An sp² is a hybridized carbon atom and the atoms directly attached to it always remain in the same plane. Therefore, in toluene ), all the atoms except 3 H-atoms of the methyl group {i.e., seven C – and five H -atoms) remain in the same plane.
2. An sp -sp-hybridized carbon atom and the atoms directly attached to it remain in a straight line. Therefore, in the propyne molecule (CH3—C = CH), all the atoms except the 3 hydrogen atoms of the methyl group remain in the same straight line.
Question 36. Write the state of hybridization of C -atoms in the following compounds and predict the shape of each of the molecules :
- H2C=O
- CH2Cl
- HC = N
- CH2=C=CH2
- CH2=C=C=CH2
Answer:
- sp² -hybridised C-atom, trigonal planar;
- sp² hybridized C-atom, tetrahedral;
- sp -hybridized C-atom, linear;
- sp², sp and sp² -hybridized C -atoms respectively, elongated tetrahedron;
- sp², sp , sp and sp² -hybridised C atom respectively, planar
Question 37.
1. Expand each of the following condensed formulas into their complete structural formulas:
- HOCH2CH2NH2
- CH3CH=CHCOCH3
- CH3C ≡ CCH2COOH
2. Write bond-line formulas of the following two compounds
- CH3CH2CH2CH2CHBrCH2CHO
- (C2H5)2CHCH2OH
Answer:
1. Condensed Formulas:

2. Bond Line Formulas:

Question 38.
1. Expand each of the following bond-line formulas to show all the atoms including C and H.

2. How many σ and π -bonds are present in
- CH2=CH—CN and
- CH2=C=CHCH3
Answer:
1. Bond-line formulas:

2. σ and π -bonds:
1. σ C -H = 3: σ C -C = 2: π C= C = 1: σ C – N = 1
π C= N= 2 i.e., total σ -bond = 6 and total π -bond = 3
2. 1. σ C -H = 6: σ C -C = 3: π C= C = 2:
i.e total σ – bond = 9 and total π – bond = 2
Question 39. Which of the given compounds may exist as two or more isomeric forms? Give the structures and names of the possible isomers.
- CHBr3
- C2H2Cl4
- C3H8
- C2H5F
- C2H4Br2
- C6H4Cl2
Answer:
1. No isomer is possible
2. Two isomers are possible : ClCH2CCl3 (1,1,1,2-tetrachloroethane), Cl2CHCHCl2 (1, 2,2-tetrachloroethane)
3. No isomer is possible
4. No isomer is possible;
5. Two isomers are possible : BrCH2CH2Br (1, 2- dibromoethane), CH3CHBr2 (1,1-dibromoethane)
6. Three isomers are possible:

Question 40. Write the structures and IUPAC names of the H—C—C=C-C—C-OH compounds with the molecular formula, C4H8O2. H (in) H
Answer:
The molecular formula, C4H8O2conforms to the general formula of monocarboxylic acids and esters. Therefore the following structures of monocarboxylic acids and esters can be written with the given formula.

Question 41. Write the structures and IUPAC names of the compounds with molecular formula, C4H10.
Answer:
The molecular formula, C4H10 conforms to the general formula of monohydric alcohols and ethers. Therefore, the structures of the following monohydric alcohols and ethers can be written with the given molecular formula.

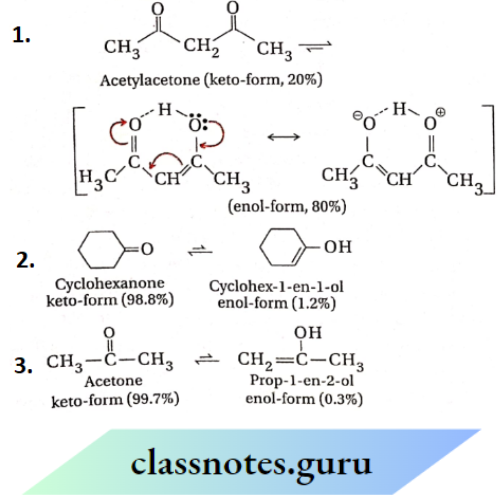
Question 42. Which of the following compounds will exhibit tautomerism and which do not? Give reasons.
- CH3COCH3
- C6H5COC6H5
- C6H5COCH3
- C6H5CHO
- Me3CCOCMe3
Answer:
Tautomerism is possible for those carbonyl compounds which contain at least one α-H atom (the H-atom attached to a carbon atom adjacent to the C= O group) Therefore, compounds 1 and 3 containing a-H atom exhibit tautomerism while compounds 2, 4, and 5 containing no α -H atom do not exhibit tautomerism
Question 43. Designate the following pairs as metamers, chain isomers, position isomers, functional isomers, and stereoisomers. Also, indicate which are not isomers at all
Answer:
1. (CH3)2CHC(CH3)3, (CH3)4C
2. CH3CH2CH2OH, CH3OCH2CH3 CH3
3.

4. (CH3)CHCOCH3, (CH3)2CHCH2CHO COOH
5. CH3OCH2CH2CH3, CH3CH2OCH2CH3
6.
![]()
Answer:
- The molecular formulas of these two compounds are not identical. Thus, these two are not isomers.
- Functional isomers
- Position isomers
- Functional isomers
- Metamers
- Stereoisomers (geometrical isomers)
Question 44. Which of the following compounds will exhibit geometrical isomerism and why?

Answer:
- One of the doubly bonded carbon atoms is attached to two identical atoms (Cl). Therefore, the compound will not exhibit geometrical isomerism.
- Each doubly bonded C-atom is attached to two different groups (C-2 is attached to CH3 and H, while C-3 is attached to Cl and C2H5 ). So, it will exhibit geometrical isomerism.
- The compound will not exhibit geometrical isomerism because each of the two terminal doubly bonded carbon is attached to two identical atoms (H).
- The compound will exhibit geometrical isomerism because each of the two ring carbons is attached to two different groups (H and CH3).
- The compound will exhibit geometrical isomerism because each of the two ring carbons is attached to two different groups (H and CH3 ).
- The compound will not exhibit geometrical isomerism because one of the two doubly bonded carbon atoms is attached to two identical groups (ring moiety.
Question 45. Which of the following compounds are optically active and why?

Answer:
- The compound contains one asymmetric C-atom (CH3CHBrCH2CH3). So, it is not superimposable on its mirror image and hence, it is optically active.
- The molecule has a plane of symmetry and it is superimposable on its mirror image. Therefore, it is optically inactive.
- The molecule is not superimposable on its mirror image. So, it is optically active.
- The molecule is not superimposable on its mirror image. So, it is optically active.
- This planar compound is superimposable on Its mirror image. So, it is optically inactive.
Question 46. Which type of stereoisomerism is exhibited by the compound, CH3CH=CH —CH=CHC2H5? How many stereoisomers are possible? Draw the structures and designate them as E/Z.
Answer:
The compound exhibits geometrical isomerism because the groups attached to each of the terminal doubly bonded carbon atoms are different. The number of geometrical isomers = 2n (n = number of double bonds) =2² = 4. These are as follows:

NCERT Solutions Class 11 Chemistry Chapter 12 Long Questions
Question 47. Name a compound having; two dissimilar asymmetric carbon atoms and write Its structure. What type of isomerism does It exhibit? Draw Fischer projection formulas of the Isomers and comment on their optical activity. How are (lie Isomers related to each other? m Explain the orders of acidity of carboxylic acids:
Answer:
Compound containing two dissimilar asymmetric carbon atoms \(\left(\mathrm{CH}_3 \stackrel{*}{\mathrm{C}} \mathrm{HOH} \stackrel{*}{\mathrm{C}} \mathrm{HBr} \mathrm{CH}_3\right)\).
The compound may have 2n(n = no. of dissimilar asymmetric carbon atom)22 = 4 possible stereoisomers. Projection formulas of these Isomers are as follows

The relations among the isomers are as follows: (1, 2) and (3, 4) are two pair of enantiomers whereas (1, 3), (1, 4), (2, 3), and (2, 4) are four pairs of diastereomer.
Question 48. Arrange cis-but-2-ene, trans-but-2-ene, and but-I-ene H3C in increasing order of their stability and give reason.
Answer:

But-2 -ene (CH3CH=CHCH3) contains sixa-H atoms while but-l-ene (CH3CH2CH=CH2) contains only two o-H -atoms capable of participating in hyperconjugation. Therefore, because of more effective hyperconjugation, but-2- ene is thermodynamically more stable than but-l-ene.
Again, due to steric interaction between two methyl groups on the same side of the double bond in cis-but-2-ene, it is relatively less stable than the trans-isomer in which no such steric interaction operates between the methyl groups situated on the opposite sides of the double bond.
Question 49. CH3Cl is unreactive towards SN1 reaction—why?
Answer:
The stability of the carbocation obtained in the first step (rate¬determining step) of an SN1 reaction determines whether the reaction will take place or not. Since methyl cation [+ CH3 ] is a very unstable one, methyl chloride is unreactive toward the SN1 reaction

Question 50. Explain the orders of acidity of carboxylic acids
- Cl3CCOOH > Cl2CHCOOH > ClCH2COOH
- CH3CI2COOH>(CH3)2CHCOOH >(CH3)3CCOOH
Answer:
1. -I effect explains this order of acid strength. As the number of halogen atoms on the a -carbon decreases, the overall -I effect decreases and as a consequence, the acid strength decreases

2. + 1 effect explains the given order of acid strength. As the number of methyl groups attached to the a -carbon atom increases, the overall +1 effect increases, and consequently, the acid strength decreases

Question 51. Explain why an organic liquid vaporizes below its boiling point when it undergoes steam distillation.
Answer:
In steam distillation, the sum of the vapor pressures of water and organic liquid becomes equal to the atmospheric pressure. This means that both of them distill at a pressure much lower than the atmospheric pressure, i.e., both of them will vapourize at a temperature that is less than their normal boiling points.
Question 52.
1. Write the state of hybridization of C -atoms mentioned In each of the following compounds:
- C-4 of Pcnt-l-cn-4-one
- C-l of I’ropanoic acid
- C-3 of Penta-2,3-diet,
- C-3 of Pcntan-3-one and
- C. -3 of 3,3-dietliylpcntane
2. Which atoms of each of the following molecules/ions always remain in the same plane?
1. CH3CH = CHCH3
2. C6H5C ≡ C—CN
3. C6H5CH3
4. CH2=C=C=CH2
5. CH3COCH3
6. CH3CONH2
7. Cl3 C —CH=CH—–CH2
8. (CD3)3C+


11. –CH2COCH2CH3

13. (CH3)2+CH — NH2
Answer:
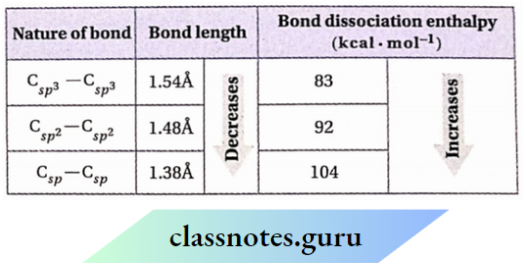
1. If four valencies of carbon atoms are satisfied by four single bonds, then it is sp³ -hybridized. Iffour valencies are satisfied. By one double bond and two single bonds, then it is sp² -hybridized. Iffour valencies are satisfied by one triple bond and one single bond or by two double bonds, then it is sp -hybridized.

2. An sp² -hybridized C -atom and the atoms directly attached to it remain in the same plane. Again, an sp -sp-hybridized C atom along with die atoms with which it is directly attached remain in a straight line. Therefore, in a molecule containing bond sp² -and sp -hybridized carbons, all the atoms remain in the same plane (except 1, 2-dienes).
A negatively charged C -atom (or a heteroatom containing lone pairs of electrons such as N, O, etc.) adjacent to a double bond is sp² – hybridized, and tire atoms attached to that carbon or heteroatom remain in the plane of the system containing the double bond.
Therefore, the atoms in the given molecules/ions that remain in the same plane are as follows:

The boiling point of its pure organic liquid is 70°C. There are two samples of tills liquid having boiling ranges:
- 76-78°C and
- 69-78°C respectively.
Question 53.
- The electronic configuration of C-atom is: ls²2s²2p², yet its valency is four —why?
- The four C —H bonds of methane molecule are equivalent — explain with reasons.
Answer:
During a chemical reaction, the carbon atoms gain energy and promote one of the two electrons of the 2s -orbital to the higher 2pz -orbital. Thus in the excited state, the electronic configuration of carbon becomes ls²2s¹2p¹x2p¹y2p¹z.
At this condition, the valence shell of the C-atom contains four unpaired electrons. Thus, the C -atom can form four covalent bonds using four unpaired electrons. This explains why carbon having electronic configuration, ls²2s²2p² is tetravalent.

The equivalency of four C—H bonds in methane (CH4) be explained by the concept of hybridization of orbitals. In the excited state, the four valence orbitals of carbon, i.e., one 2s and three 2p orbitals possessing slightly different energies mix up and result in the formation of four equivalent sp3 -hybrid orbitals. These hybrid orbitals overlap with the four Is -orbitals of four H -atoms to form four C—H bonds which are also equivalent (same bond length and bond strength)

Question 54.
1. Arrange sp, sp² & sp³ -orbitals in increasing order of:
- Bond length
- Bond angle
- Bond energy
- Size of orbitals and
- S -character.
2. Organic compounds are usually water-insoluble. Why?
3. Write the structure of the smallest hydrocarbon having the empirical formula C2H. What is the shape of the molecule?
4. Draw the p -p-orbitals involved in forming n -n-bonds in the molecule, CH2=C =CH2, and predict whether the molecule is planar or not.
Answer:
1.
- sp—sp<sp²—sp²<sp³—sp³
- sp³ < sp² < sp
- sp³—sp³ < sp²—sp² < sp—sp
- sp < sp² < sp³
- sp³ < sp² < sp
2. Organic compounds are covalent. Thus they do not get ionised. Moreover, they are usually less polar or non-polar compounds and hence do not dissolve in highly polar solvents, or water.
3. The compound is (C2H)2 or C4H2 and its structure is HC = C —C = CH. The shape of the molecule is linear because all the C -atoms are sp -hybridized.

The molecule is non-planar because the two planes containing one C-atom and two H-atoms are perpendicular to each other
Question 55. Give the IUPAC names of the following compounds:
1. CH3CHClCHBrCH3
2. CH3CHFCOCH2CH3
3. (CH3)2CHCH2OH
4. CH3COOCH(CH3)2
5. CH3CHBrCH(CH3)COOII
6. CH3CHOHCH2CHO
7.

8.

9. HC ≡ CCH(CH3)CH=CH2
10. CH3OCH(CH3)CH2CH3
11. CH3CHClCH2CONH2
12. BrCH2CBr2(CH2)3CHCl2
13.

14. CH3COCH2COCH3
Answer:
- 2-bromo-3-chlorobutane
- 2-fluoropentan-3-one
- 2-methyldopa-l-ol
- Isopropyl ethanoate
- 3-bromo-2-methyl butanoic acid
- 3-hydroxy butanal
- 2,3,5-trimethyl-4-propylheptane (the chain containing a maximum number of substituents is considered as the principal chain).
- 3-ethyl-2, 4, 5-trimethyl heptane
- 3-methyl pent-l-en-4-one
- 2-methoxyflurane
- 3-chloro-butanamide
- 5,5,6-tribromo-l,l-dichlorohexane
- 5-sec-butyl-4-isopropyl decane or 4-(l-methyl ethyl)-5- (1-methyl propyl) decane
- Pentane-2,4-dione
Question 56. Write structures of the following:
- Hept-5-en-1-one
- 1-bromo-2-ethoxyethane
- 3-chloropropanoyl bromide
- 1-chloroprocaine-2-amine
- 4-iodo-3- nitro butanal
- 3-phenyl prop-2-enoic acid
- Ethanoic methanoic anhydride
- 2-carbomoylpropanoic acid
- Pentane-2,4-dione
- 5-formyl-3-oxo pentanoic acid
- Ferf-butyl alcohol
- But-2-ene-1,4-dioic acid
- Trimethylacetic acid
- Diethylbutane-1,4-dioate
- 3-(carboxymethyl) pentanoic acid
- 1,3-dimethyl cyclo hex-l-ene
Answer:

Organic Chemistry Chapter 12 NCERT Long Question and Answers
Question 57. Draw resonance structures of the following compounds.
- C6H5NO2
- CH3CH=CHCHO
- C6H5CHO
- C6H5CH2
- CH3CH=CHCH2

Question 58.
- A mixture of ether and water can be separated by simple distillation.
- Water present in rectified spirit can be removed by azeotropic distillation.
- Benzoic add can be extracted from its aqueous solution using benzene.
- Sugar containing NaCl as an impurity can be purified by crystallization using ethanol but not water.
Answer:
- There is a considerable difference between the boiling points of ether and water Hence, at the boiling point of more volatile ether, the vapors almost exclusively consist of ether and at the boiling point of less volatile water, the vapors almost entirely consist of water Thus, these can be separated by simple distillation.
- A mixture of water and rectified spirit forms an azeotropic mixture, i.e., the constituents of this mixture cannot be separated into their components by fractional distillation. So, azeotropic distillation is required to remove the water-rectified spirit.
- Benzene is immiscible ’with water but benzoic add is highly soluble in benzene, Hence, benzoic add can be extracted from its aqueous solution using benzene.
- Sugar is soluble in hot ethanol whereas common salt remains insoluble. Thus, impure sugar can be purified by crystallization. However, purification is not possible using water as a solvent because both components become readily soluble in water
Question 59. In the Lassa goe’s test, NH2OH.HCl responds to the test for the element chlorine but not for the element nitrogen, explain
Answer:
As there is no carbon (C) atom in NH2OH.HCl, NaCN is not produced in the first step by the reaction between sodium (Na) and C.
Hence, the formation of sodium ferrocyanide and ferric ferrocyanide (Prussian blue) in the subsequent step does not take place. Therefore NH2OH HCl does not respond to Lassaigne’s test for nitrogen.
But chlorine (Cl) present in NH2OH-HCl combines with Na metal to form soluble NaCl which reacts with AgNO3 in the subsequent step to produce a white precipitate of AgCl which is soluble in ammonium hydroxide
Na + Cl → NaCl ; NaCl + AgNO3→AgCl ↓(white) + NaNO3
AgCl + 2NH4OH → [Ag(NH3)2]Cl (water soluble) + 2H2O
Question 60. What are the hybridization states of each C- atom in the compounds:
- CH2=C= O
- CH3CH=CH2
- (CH3)2CO
- CH2=CHCN
- C6H6
Answer:

Question 61. Indicate the σ and π bonds in the following Heptan-4-one molecules:
- C6H6
- C6H12
- CH2CI2,
- CH2=C=CH2
- CH3NO2
- HCONHCH3
Answer:

Question 62. Write bond-line formulas for:
- Isopropyl alcohol,
- 2,3-dimethyl butanal
- Heptan-4-one
Answer:

Question 63. Give the IUPAC names of the following compounds:

Answer:
- Propylbenzene
- 3-methylpentanenitrile,
- 2,5-dimethyl heptane
- 3-bromo:3-chloroheptane,
- 3-chloro-propanal
- 2,2-dichloroethanol.
Question 64. Which of the following represents the correct IUPAC CHO name for the compounds concerned?
- 2,2-dimetliyIpentane or 2-dimethyl pentane
- 2,4,7-trimethylolethane or 2,5,7-trimethylolethane
- 2-chloro-4-methyl pentane or 4-chloro-2-methyl pentane
- But-3-yne-l-ol or But-4-ol-l-yne.
Answer:
- 2,2-dimethyl pentane (two alkyl groups are on the same carbon and hence the locant is repeated twice).
- 2,4,7-trimethyloctane (since 2,4,7 locant set is lower than the set 2,5,7).
- 2-chloro-4-methylpentane (alphabetical order of substituents is maintained).
- But-3-one-l-ol (using lower locant for the principal functional group)
Question 65. Draw formulas for the first 5 members of each homologous series beginning with the given compounds:
- HCOOH
- CH3COCH3
- H-CH=CH2
Answer:
1. HCOOH , CH3COOH, CH3CH2COOH,CH3CH2CH2COOH,CH3CH2CH2CH2COOH
2. CH3COCH3 , CH3COCH2CH3, CH3COCH2CH2CH3 ,CH3CH2COCH2CH3, CH3COCH2CH2CH2CH3
3. CH=CH2 , CH3CH=CH2 , CH3CH2CH=CH2 , CH3CH2CH2CH=CH2, CH3CH2CH2CH2CH=CH2
Question 66. Give condensed and bond line structural formulas i and identify the functional group(s) present, if any, I for:
- 2,2,4-trimethylpentane
- 2-hydroxyl, 2,3-propane tricarboxylic acid
- Hexanediol
Answer:

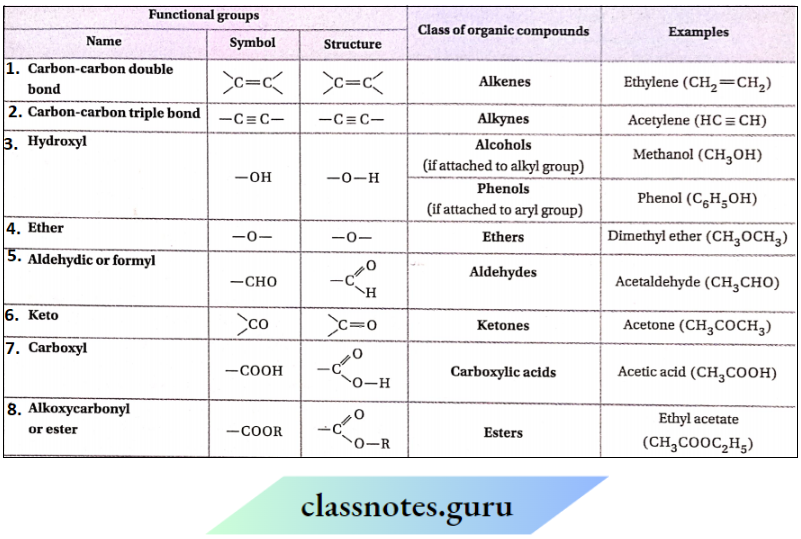
Question 67. Identify the functional groups in given compounds:

Answer:
1. —OH (phenolic hydroxyl), —CHO (aldehyde), — OMe (methoxy).
2. — NH2 [1° amino (aromatic)], —CO2—CH2— (ester), -N(C2H5)2 (3° amino)
3. —CH=CH— (ethylenic double bond), — NO2 (nitro)
Long Answer Questions Organic Chemistry Class 11 Chapter 12
Question 68. 0.4 g of an organic compound containing N was Kjeldahlised and NH3 obtained was passed into 50 mL (N/2) H2SO4 solution. The volume of the acid solution was increased to 150 mL by adding distilled water. 20 mL of this acid solution required 31 mL (N2O) NaOH for complete neutralization. Calculate the percentage of N.
Answer:
20 mL of (partially neutralized) diluted acid solution
= 31mL \(\frac{1}{20}\) NaOH solution.
= 15.5
Strength of(partially neutralized) diluted acid solution
= \(31 \times \frac{1}{20} \times \frac{1}{20}(\mathrm{~N})=\frac{31}{400}(\mathrm{~N})\)
Amount of H2SO4 present in 150 mL (partially Amount of H2S04 present in 150 mL (partially
= \(\frac{31 \times 150}{400 \times 1000}\)
Now, 50mLof \(\frac{1}{2}(\mathrm{~N}) \mathrm{H}_2 \mathrm{SO}_4\) solution contains = \(\frac{1 \times 50}{2 \times 1000}\) g-equiv. H2SO4
NH3 produced by decomposition 0.4 g of the organic compound = \(\left(\frac{50}{2000}-\frac{31 \times 150}{400000}\right)\)
= 0.013375 g-equivalent
= 0.013375 X×17 g
Now, 0.013375 x 17 g NH3 = \(\frac{14}{17} \times 0.013375\)
% of the nitrogen in the organic compound
= \(\frac{14 \times 0.013375}{0.4} \times 100\)
= 46.81
Question 69. Expand each of the following condensed formulas into their complete structural formulas:
- CH3CH2COCH2CH2CI
- CH3CH= CH(CH2)4CH3
- BrCH2CH2C=CCH2CH3
Answer:

Question 70. Write down the condensed structural formula and bond¬ line structural formula for each of the following molecules:
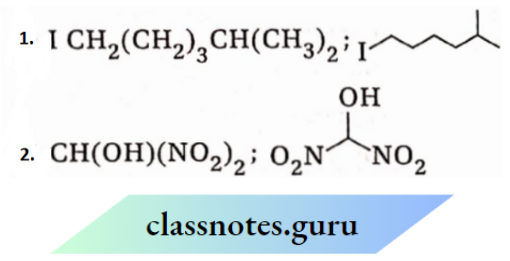
1. ICH2CH2CH2CH2CH(CH3)CH2
2.

Answer:
Question 71. Expand each of the following bond-line formulas to show all the atoms including carbon and hydrogen:

Answer:
 .
.
Question 72. Explain why alkyl groups act as electron donors when attached to a n system.
Answer:
Due to hyperconjugation (cr, n conjugation), alkyl groups act as electron donors when attached to a n -system. This is shown in the case of propane—

Question 73. Draw the resonance structures for the following compounds. Show the electron shift using curved arrow notation:
- C6H5OH
- C6H5NO2
- CH3CH=CHCHO
- C6H5— CHO
- C6H5—CH2
- CH3CH=CHCH2
Answer:

Question 74. Identify the reagents underlined in the following ey=o + H2O equations as nucleophiles or electrophiles

Answer:
- Nucleophile OH–
- Nucleophile (–CN)
- Electrophile (CH3+CO)
Question 75. Classify the following reactions in one of the reaction types studied in this unit.

Answer:
- Nucleophilic substitution
- Electrophilic addition
- P -Elimination
- Nucleophilic substitution & rearrangement
Question 76. What is the relationship between the members of the following pairs of structures? Are they structural or geometrical isomers or resonance contributors?

Answer:
- Structural isomers (position isomers as well as metamers)
- Geometrical isomers
- Resonance contributors
Question 77. For the given bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbonation, and carbanion.

Answer:

Question 78. Write down the IUPAC names of the alkyl groups having the molecular formula, C4H6.
Answer:
Four alkyl groups are possible. These are

Question 79. Give the structural difference O aldehyde C & ketonic groups.
Answer:
An aldehyde group (—CHO) is a carbonyl group in which one valency of the carbonyl carbon is satisfied by a H-atom, and the other valency is satisfied by another atom or an alkyl group. On the other hand, the keto group is also a carbonyl group in which two valencies of the carbonyl carbon are satisfied by two alkyl groups.

Question 80. Both formic acid (HCOOH) and acetic acid (CH3COOH) contain the same functional group, yet there are some differences in their chemical properties—explain.
Answer:
The structural formula of formic acid is such that it can be said to contain said to contain a  as well as
as well as 
So it exhibits the properties of both —CHO and
—COOH groups. But acetic acid contains only
—COOH group and hence it exhibits the properties of compounds containing only carboxyl group

Question 81. Label the primary (1°), secondary (2°), tertiary (3°), and quaternary (4°) carbon atoms in the following compound:

Answer:

NCERT Class 11 Organic Chemistry Chapter 12 Long Answer Solutions
Question 82. Write down the IUPAC and common names of each of the given compounds:
- CH3CH= CH2
- CH3C=CCH3
- CH3CHOHCH3
- CH3OCH2CH2CH3
- CH3CH2CHO
- CH3COC2H5
- C2H5COOH
- C2H2COCl
- CH3CONH2
- CH3CO2C2H5
- CH3CH2NH2
- CH3NHCH2CH3
- (CH3)2NCH2CH3
- CH3CH2CN
Answer:
- Propene; Propylene
- But-2-yen; Dimethylacetylene
- Propan-2-ol; Isopropyl alcohol
- 1-methoxy propane; Methyl /t-propyl ether
- Propanal; Propionaldehyde
- Butan-2-one; Ethyl methyl ketone
- Propanoic acid; Propionic acid
- Propanoyl chloride; Propionyl chloride
- Propanamide; Propionamide
- Ethyl ethanoate; Ethyl acetate
- Ethanamine; Ethylamine
- Methylethanamine; Ethylmethylamine
- N, N-dimethylethanolamine; Ethyldimethylamine
- Propanenitrile; Ethyl cyanide
Question 83. Write down the structures of the following compounds:
- 2-Iodopropane
- Hex-3-one
- Pent-l-ene
- 2,2-Dichloropropane
- 1, l, 1, 2-Tetrachloroethane
- Propan-2-ol
- Propane-1,3-diol
- Butane-1,2,3-triol.
- 2-Methoxypropane
- 2-Methylpentanoic acid
- 2,2-Dimethylbutanal
- Pentan-3-one
- Butanoyl chloride
- Aceticformic anhydride
- Ethylmethanoate
- N-Methylmethanamine
- N-Ethyl-N-methylhexanamine
- Butanenitrile.
Answer:
- CH3CHICH3
- CH3CH2C=CCH2CH3
- CH3CH2CH2CH=CH2
- CH3CCl2CH3
- CH2Cl CCl3,
- CH3CH(OH)CH3
- HOCH2CH2CH2OH
- CH3CH(OH)CH(OH)CH2OH
- CH3CH(OCH3)CH3
- CH3CH2CH2CH(CH3)COOH
- CH3CH2C(CH3)2CHO,
- CH3CH2COCH2CH3
- CH3CH3CH2COCl
- CH3COOCHO
- HCOOCH2CH3
- CH3NHCH3
- CH3CH2N(CH3)CH2CH3
- CH3CH2CH2CN
Question 84. Write down the IUPAC names of the following compounds:

Answer:
- 3-ethyl-5-methyl heptane,
- 2,2-dimethylbutane
- 2,2,4-trimethylpentane,
- 3-ethyl-2,2,4-trimethylpentane
- 4-(1,1-dimethyl ethyl)heptane
- 3,4-diethyl hexane
- 6-ethyl-2-methyl-5-(1,1-dimethyl ethyl)octane
Question 85. What is wrong with the following names? Draw the structures they represent and write their correct names.
- 1,1-dimethyl hexane
- 3-methyl-5-methyl heptane
- 4, A-dimethyl-3-ethyl pentane
- 3, 4,7-trimethylolethane
- 3,3-diethyl-2, A, Atrimethylpentane
Answer:
- (CH3)2CHCH2CH2CH2CH2CH3 – 2-methylheptane
- CH3CH2CH2C(CH3)2CH2CH2CH2CH3 – 4,4-dimethyl octane
- CH3CH2CH(CH3)CH2CH(CH2CH3)2– 3-ethyl-5-methyl heptane
- CH3C(CH3)2CH(CH2CH3)2 – 3-ethyl-2, 2-dimethyl pentane
- CH3CH2CH(CH3)CH(CH3)CH2CH2CH(CH3)2 – 2,5,6-trimethylolethane
- (CH3)2CHC(CH2CH3)2C(CH3)3 – 3,3-diethyl-2,2,4-trimethylpentane
Question 86. Give the IUPAC name of the following alkane containing complex substituents:

Answer: 3-ethyl-7,7-fels(2,4-dimethylhexyl)-5,9,11-trimethyltridecane
Question 87. Write the IUPAC names of the following compounds:

Answer:
- 3-ethyl pent-1-ene,
- 3-ethyl hex-1-en-5-one
- 2-ethyl-3,3-dimethyl but-1-ene
- Pent-3-en-1-one
- 3-methyihexa-1,5-diene
- 3-isobutylhept-1-en-4-yne
- 3-propylhept-l-ene,
- 3-methyl-4-methylidenehept-1-en-6-yne
- Hexa- 1,3-dien-5-one,
- 5-methylhepta-1,2,6-triene
Question 88. Write down the structures of the following compounds
- Pent-3-en-1-one
- 3-methylpenta-1, 4-diyne
- 3-(2-methylpropyl)hept- 1-en-4-yne
- 3-ethylpenta-1,3-diene
- 5-ethynylhepta-1,3,6-triene
- 4-ethyl-4-methylhex-1-one
Answer:

Question 89. Write down the IUPAC names of the following compounds:

Answer:


Question 90. Write down the structures of the following:
- 2-methyl butanol chloride
- 5-chloro-3-ethylpentan-2-one
- Diethyl butane-1, A-date
- Methyl-2-methyl prop- 2-en-1-rate
- 3-phenyl prop-2-enoic acid
- Propane- 1,2,3-tricarboxamide.
Answer:

Question 91. Give the IUPAC names of the following compounds:
- CH3COCH2COOC2H5
- H2NCH2CH2CH2COOH
- CH3CH(CN)CH2COCH3
Answer:

Question 92. Write down the structures of the following compounds:
- 3-formylpentanoic acid
- 3-hydroxyl-oxopentanal
- 2, 3-dihydroxy butane dioic acid
- 3-hydroxy cyclohexanone
- 3-hydroxy-3-methyl butane-2-one
Answer:

Organic Chemistry Techniques Long Answer Class 11 NCERT
Question 93. Write the structures of the following compounds:
- 2-chloro-2-methyl butane-1-ol
- 4-amino-2-ethyl pent-2-enal
- Hex-A-in-2-one
- 1-bromo-3-chloracyclohex-1-ene
- But-2-ene-l, 4-dioic acid
- 4-nitrogen-l-one Ethyl 3-methoxy – 4-nitro butanoate
Answer:

Question 94. What type of structural isomerism is exhibited by the following pairs of isomers?
1. CH3CHCOOH and CH3COOCH3
2. CH3 —C≡C — CH3 and CH3CH2C≡CH
3. CH2= CHOH and CH3CHO
4. CH2 = CH(CH2)3CH3 and C6H6

6. CH3CH2CH2OH and (CH3)2CHOH
Answer:
- Functional group isomerism
- Position isomerism
- Tautomerism (special case of functional group isomerism)
- Ring-chain isomerism
- Position isomerism
- Position isomerism
Question 95. Which two of the following compounds are
- Position isomers
- Tautomers
- Ring-chain isomers
- Metamers
- Chain isomers and
- Functional isomers
Answer:
- Position isomers: (h) and (k),
- Tautomers: (a) and (f)
- Ring-chain isomers: (e) and (j)
- Metamers : (c) and (g)
- Chain isomers: (b) and (i)
- Functional isomers: (d) and (l)
Question 96. Identify the optically active and optically inactive compounds:
1. CH3CHOHC2H5
2. CH3CH2OH
3. C2HgCHBrCH(CH3)2
4.

5. CH3CH=CHC2H5
Answer:
(1), (3), and (4) will be optically active as each of these molecules contains one asymmetric center.
But (2) & (5) are optically inactive as they do not have a symmetric center.
Question 97. Which of the following will exhibit geometrical or cis-trans isomerism and which of them will not? Give reasons.
1. CH3CH=CBr2
2. BrCH=CHCH2CH3
3. CH2=CH —CH=CH2
5. CH2=CHCH=CHCH=CH2

Answer:
(2), (5), (6), (7), and (8) exhibit geometrical isomerism.
But (1), (3), and (4) do not exhibit geometrical isomerism.
Question 98. Draw the Fischer projectionformulas of all stereoisomers CH3CHBrCHClCOOH. Mention how they are related to each other
Answer:

Enantiomers: 1 and 2, 3 and 4
Diastereomers: 1 and 3; 1 and 4; 2 and 3; 2 and 4
Question 99. Write down the structure and IUPAC names of two isomeric optically active alkanes having the lowest molecular mass.
Answer:

Question 100. Which of the following compounds are meso-compounds and which are not? Give reasons

Answer:
(2) and (3) are meso compounds (they are optically inactive due to the presence of a center of symmetry). (1) is optically active. It contains two asymmetric centers and it is not superimposable on its mirror image.
Question 101. Arrange in order of decreasing basic strength and show(XI) Pari-n reasons: CH3—CH=NH, CH3—C=N, CH3 — NH2
Ongoing from 
in CH3NH2, CH3CH=NH and CH3C=N, the unshared electron pairs are in sp³ , sp² and sp -orbitals respectively. As the s -character of the hybrid orbital (containing lone pair) of N -atom increases, the electrons are drawn closer to the nitrogen nucleus, and hence electron donating ability of the amino nitrogen decreases causing a decrease in basicity.
Thus basic strength decreases in the sequence:

Question 102. Arrange in order of increasing acidity and give reasons: CH3CH2OH, (CH3)3 COH, CH3OH, (CH3)2CHOH
Answer:
Since alkyl groups have a +1 effect, there will be an increased electron displacement towards the oxygen atom on going from primary to secondary to tertiary alcohol. This may be represented (qualitatively) as follows:

Question 103. Arrange the following anions in order of increasing stability and give reasons: CH2=–CH, CH3–CH2, CH = C–
Answer:
The greater the -ve charge on the oxygen atom, the closer the displacement of the covalent pair in the O —H bond to the hydrogen atom, hence separation of a proton becomes increasingly difficult. Thus the acid strengths of alcohols will be in the following order:
(CH3)3COH < (CH3)2CHOH < CH3CH2OH < CH3OH
Ongoing from \(\mathrm{CH}_3 \stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2→\mathrm{CH}_2=\stackrel{\ominus}{\mathrm{C}} \mathrm{H}→\mathrm{HC} \equiv \stackrel{\ominus}{\mathrm{C}}\) it is seen that the unshared electron pairs of the carbanion carbons are in sp³, sp² and sp -hybrid orbitals respectively. As the s -s-character of the hybrid orbitals increases, the electrons are drawn closer to the nucleus of the carbanion carbon, and hence the ability to hold the electron pair increases, causing successive increases in stability. Stability order
⇒ \(\mathrm{CH}_3 \stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2<\mathrm{CH}_2=\stackrel{\ominus}{\mathrm{C}} \mathrm{H}<\mathrm{CH} \equiv \stackrel{\ominus}{\mathrm{C}}\)
Question 104. Which of the following pairs do not represent two resonance structures and why?

Answer:
The two structures which differ in the positions of atoms are not resonance structures. Thus the pair of structures given in (1), (2), and (5) do not represent resonance structures.
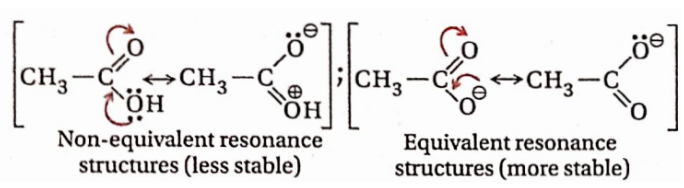
Question 105. In between CH3COOH and CH3COO–, which one is more resonance stabilized and why?
Answer:
Both CH3COOH and CH3COO– are resonance hybrids of two canonical forms. But one of the resonance structures of CH3COOH involves separation of charge, while none of the resonance structures of CH3COO– involves any separation of charge (also these structures are equivalent). Hence CH3COO– is more resonance stabilized compared to CH3COOH.
Question 106. Which N -atom of guanidine  is more basic and why
is more basic and why
Answer:
Protonation on tire doubly bonded N -atoms produces a cation (conjugate acid) which is stabilized by resonance involving three equivalent canonical forms. On the other hand protonation on either of the singly bonded N-atoms produces a cation (conjugate acid) which is not stabilised by resonance. Thus the doubly bonded N-atom of guanidine is more basic.

Question 107. Which of the two N atoms of the following compound undergoes protonation and why?
Answer:
N-atom ofthe ring ‘A’ undergoes protonation because the resultant cation (conjugate acid) is stabilized by resonance. The n-atom of the ring ‘B’ does not undergo protonation because in that case the resulting cation will not be stabilized by resonance.

Question 108. Which resonance structure in each of the following compounds contributes more towards the hybrid and why?
Answer:
- The 1st structure contributes more because both C and N have octets of electrons in their valence shells.
- The 1st structure contributes more because it involves no separation of charge.
- The 2nd structure contributes more as the -ve charge is on the more electronegative O-atom.
- The 1st structure contributes more because there is separation of charge. The 2nd structure involves the separation of charge; also the +ve charges, on the adjacent atoms, repel each other.
- The 1st structure contributes more compared to the 2nd structure. The 2nd structure is highly unstable as it contains a negative N-atom.
Question 109. Which of the following compounds can be represented as a resonance hybrid and which of them can the? Give reasons.
1. CH3CH2OH,
2.CH3CONH2
3. CH3CH=CHCH2NH2
4. H2N—CH=CH— NO2
5.

Answer:
Structures and (3) can not be represented as resonance hybrids because lone pairs on the O-atom or N-atom can not undergo delocalization. However, structures (2), (4) and (5) can be represented as resonance hybrids.

Question 110. Why are the three carbon-oxygen bonds In carbonate (CO3-2) ion equal in length?
This is so because the CO3-2 ion is a resonance hybrid of three equivalent canonical forms.

Question 111. Which one between phenol and cyclohexanol is more acidic and why?
Answer:
Phenol is a stronger acid than cyclohexanol.
It can be explained as follows:
1. Due to resonance, the O-atom of the OH group acquires a +ve charge and so the release of the proton is facilitated

2. When phenol ionizes, the formed phenoxide ion is also a resonance hybrid, but it is more stabilized by resonance than a unionized phenol molecule because of the spreading of a negative charge only. In the unionized molecule, resonance involves the separation of charge

Such effects are not possible in the case of cyclohexanol and hence proton release is not facilitated.
Long Questions for Organic Chemistry Class 11 Chapter 12 NCERT
Question 112. Arrange the following ions in order of increasing stability and give your reasons
Answer:

Question 113. Which one between 2-methylbut-2-ene and 2-methylbut-1- ene has higher heat of hydrogenation and why?
Answer:
In structure 3+ve charge on the carbon is involved in I delocalization with the benzene ring.

In structure (1), +ve charge on the carbon is involved in delocalization not only with the benzene ring but also with N-atom of the \(\ddot{\mathrm{N}}\)Me2 group, thereby making this structure more stable than (III).

Structure (2) is the least stable because +ve charge on the carbon can not be involved in delocalization with the aromatic ring (steric inhibition of resonance)

Question 114. Arrange the following ions in order of increasing stability and give your reasons
Answer:
2-Methylbut-2-ene contains nine hyperconjugable α-H -atoms, so this molecule is involved in effective hyperconjugation. As a result, this molecule gains extra stability and it has relatively lower heat of hydrogenation. On the other hand, 2-methylbut-1-ene contains only five hypercoagulable α-H -atoms so the effect of hyperconjugation stabilizing this molecule is relatively less. Thus it has a relatively higher heat of hydrogenation.

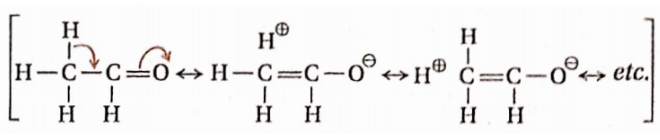
Question 115. The C—C bond in acetaldehyde (CH3CHO) is shorter than that in ethane while the C— C bond in trifluoro acetaldehyde (CF3CHO) is essentially the same as that in ethane. Explain
Answer:
Acetaldehyde molecule contains three α-H -atoms. These H-atoms are involved in hyperconjugation with the double bond of the carbonyl group. So C—C bonds in acetaldehyde have a partial double bond character. In ethane the C—C bond has pure single bond character.
Thus C — C bond in acetaldehyde is shorter than that in ethane. Trifluoroacetaldehyde does not have a-H -atoms. So hyper-conjugation is not possible in CF3CHO.
Thus, the C — C bond in CF3CHO is essentially the same as that in ethane.
Question 116. Arrange the following isomeric alkenes in order of increasing stability and give your reasons:
- (CH3)2C=C(CH3)2 (1)
- CH2=CHCH2CH2CH3 (2)
- CH3CH=CHCH(CH3)2 (3)
- CH3CH =C(CH3)CH2CH3 (4)
Answer:
The stability of an alkene is determined by the number of hyperconjugative structures, which in turn is dictated by the number of α-H -atoms (concerning the olefinic carbons) present in the molecule. The greater the number of hyperconjugative structures, the higher the stability of the alkene Now the number of ar-H -atoms in the alkene 1, 2, 3, and 4 are 12, 2, 4, and 8 respectively. Thus stability of the alkanes follows the sequence: 2 < 3< 4 < 1.
Question 117. Which one of the following two conformations of butane is more stable and why?

Answer:
Eclipsed conformation I, in which methyl groups on two adjacent carbons are just opposite to each other. In this conformation steric strain and bond opposition strain are maximum, hence this conformation is most unstable.
Anti-conformation II, in which methyl groups are as far apart as possible, is most stable due to minimum repulsion between methyl groups. Note that, there is no bond opposition strain in this conformation.
Question 118. Which of the 2 geometric isomers I of Me3CCH=CHCMe3 has a higher heat of combustion and why?
Answer:
Cis-isomer is less stable because of a very large steric hindrance between two bulky t-butyl groups lying on the same side of the double bond. On the other hand, transisomer is more stable because the bulky f-butyl groups are on the opposite sides of the double bond. Thus cis isomer has a higher heat of hydrogenation.

Question 119. Which one between C6H5CH3 and CH4 has lower Csp³—H bond dissociation enthalpy and why?
Answer:
The bond dissociation enthalpy of C6H5CH2—H is less than (II) that of H3C — H as C6H5CH2 is more stable (stabilized by resonance) than that of •CH3 (which has no resonance stabilization).

Question 120. Arrange the following carbocations in order of increasing stability and explain the order:

Answer:
In cation in,(3) +ve charge is not delocalized due to steric inhibition of resonance. However, +ve charge is delocalized in both the cations I and But the extent of delocalization of +ve charge is higher in H due to the additional effect involving the methoxy group.

Question 121. Arrange the following carbanions in order of increasing stability and explain the order

Answer:
The stability of carbanions increases as the extent of delocalization of the -ve charge increases. Carbanion I is the most stable as the -ve charge is delocalized not only by the benzene ring but also by the -NO2 group. Carbanion HI is moderately stable as the -ve charge is delocalized only by the benzene ring. Carbanion U is the least stable because of the +R effect of the -OMe group (although the -ve charge is delocalized by the benzene ring). Thus the sequence of stability is 2 < 3< 1

Question 122. Classify the following species as electrophile or nucleophile and explain your choice:

Answer:
Nucleophile : CH3C– , CHgCOO– , CH2=CH2
Electrophile : Cl+ , BF3 , (CH3)3C+ , R— X
CH3O–– and CH3COO– are negatively charged species having available unshared electron pairs on the O-atom. So these are nucleophiles. CH2=CH2 can also act as a nucleophile as it contains loosely bound or -electrons.
In the species Cl+, BF3, and Me3C+, there is electron deficiency (having sex tet of electrons) on the valence shells of Cl, B, and C-atom respectively. So these are electrophiles. In the alkyl halides (R—X) there is electron deficiency on the a -carbon due to the strong -I effect of the halogen atom. So RX can act as electrophile.
Question 123. Formulate the following as a two-step reaction and designate the nucleophile and electrophile in each step: CH2= CH2 + Br2 → BrCH2CH2Br
Answer:

Question 124. CN– and NO2– are called ambient nucleophiles. Explain
Answer:
Nucleophiles that have more than one (generally two) suitable atoms through which they can attack the substrate are called ambident nucleophiles. Each of the –CN ions and NO2– contain two atoms through which they can be involved in nucleophilic attack (these atoms are indicated by arrows). So these are ambident nucleophiles.

Question 125. Mention the type of each of the following reactions
Answer:

Answer:
- Nucleophilic substitution (SN2)
- Electrophilic addition
- Free-radical substitution
- Elimination reaction (E2)
- Rearrangement reaction
Question 126. Calculate the double bond equivalent (DBE) of each of the given compounds:
- C13H9BrS
- C12H16N2O4
Answer:
Double bond equivalent (DBE) of C13H9BrS
= \(\frac{13(4-2)+9(1-2)+1(1-2)+1(2-2)}{2}+1\)
= 9
DBE of C12H16N2O4
= \(\frac{12(4-2)+16(1-2)+2(3-2)+4(2-2)}{2}+1\)
= 6
Question 127. Calculate the double bond equivalent (DBE) of a compound having molecular formula, C5H8. On catalytic hydrogenation, the compound consumes 1 mol of hydrogen. Write the structures of all the possible isomers of the compound
Answer:
DBE of C5H8 \(=\frac{5(4-2)+8(1-2)}{2}+1\) = 2
On hydrogenation, it consumes 1 mol of H2. So it contains one double bond and one ring.
Thus possible structures of the compounds are:

Question 128. Arrange the following carbocations in order of increasing stability and explain the order:
Answer:

The sequence of stability: (4) > (2) > (3) > (1) Carbocation (4) is a primary carbocation, but it is most stable due to resonance.

Question 129. Which of the carbocations is the most stable?
- CH3CH2+CH2,
- CH2=CH+CH2,
- C6H5 +CH2
- All are equally stable.
Answer:
(1), (2), and (3) are all primary carbocations. Cations (1), (2), and (3) have 0, 2, and 4 resonance structures respectively. So carbocation (3) is the most stable

Class 11 Chemistry Organic Chemistry Chapter 12 Long Questions & Answers Solutions
Question 130. Which one between the two CH3CO– and CH3 CH3COC– HCOCH3 is more stable and why?
Negative charge CH3COC– H2 is involved in delocalization with the n electrons of only one carbonyl group. On the other hand, the carbonation-carbon of on the carbanion-carbon of charge e -ve on n CH3COC–HCOCH3 is involved in delocalization with the π -electrons of two carbonyl groups. Thus the second carbanion is more stable.


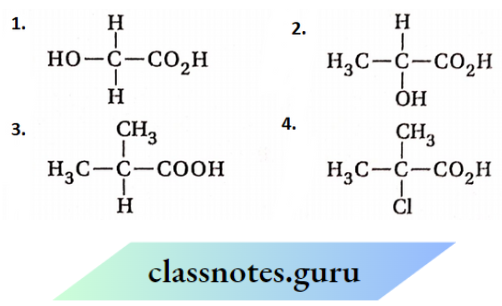

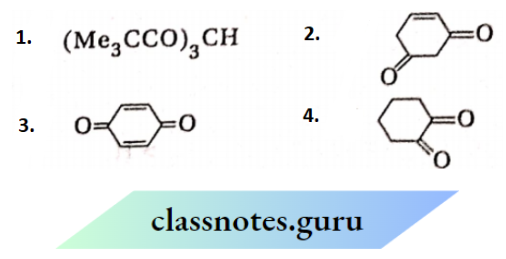













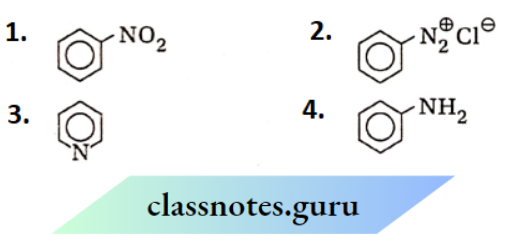











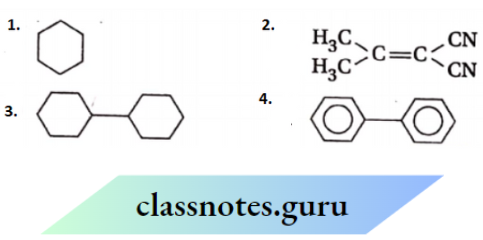






















 is substituted by chlorine is—
is substituted by chlorine is—







 is_________
is_________ 






















 , CH3 COCH2COCH3
, CH3 COCH2COCH3









 and Azulene
and Azulene