CBSE Class 11 Chemistry Notes For Chapter 10 S Block Elements Group 1 Elements (Alkali Metals) Introduction
All the alkali metals have one valence electron ( ns¹ ) outside the noble gas core. The loosely held s -electronin the outermost valence shell makes them the most electropositive metals.
- To get the stable electronic configurations of noble gases, they readily lose the valence electron to generate the monovalent (M+) ions. Hence, they are never found in a free state but in the combined state of nature.
- Since the last electron enters ns -orbital, these are called s -block elements.
- Since all these elements have similar valence shells or outer electronic configurations, all the alkali metals exhibit a striking resemblance in their physical and chemical properties and they are placed in a definite group (Gr-1).
- Lithium shows some abnormal behavior as its electronic configuration is slightly different from the rest of the members of Gr-1 and also because of its extremely small atomic and ionic radii.
Again, lithium shows some similarities with magnesium present in the group- 2 of the third period
Electronic configuration of alkali metals:

Occurrence Of Alkali Metals
- Since the alkali metals are highly reactive, they do not exist in a free state. In nature, they mostly occur as compounds like halides, oxides, silicates, borates, and nitrates.
- According to abundance, lithium is placed at the 35th position. It mainly occurs in nature in the tire form of silicates,
- For example: Spodumene: LiAl(SiO3)2 and Lepidolite: Li2Al2(SiO3)3(F, OH)2
- Sodium and potassium are respectively placed at 7th and 8th position in order of their abundance. Sea water is a major source of NaCl and KCl.
- Sodium is abundantly present in the form of rock salt (NaCl). Other important minerals are Chile salt petre: NaNO3, borax: Na2B4O7.10H2O, mirabilite: Na2SO4, and trona: Na2CO3-NaHCO3-2H2O.
- Important ores of potassium are sylvite: KCl, carnallite: KCl-MgCl2-6H2O, and feldspar: (K2O-Al2O3-6SiO2).
- Rubidium and cesium are much less abundant than lithium. Radioactive francium does not occur appreciably in nature. It is obtained from the radioactive decay of actinium
- 227Ac 89 → 223Fr87 +4He2 Its longest-lived isotope 223Fr87 has a half¬life period of only 21 minutes.
- Since most of the compounds of alkali metals are water soluble, they are found in adequate amounts in seawater.
CBSE Class 11 Chemistry Notes Chapter 10 S Block Elements
General Trends In Atomic And Physical Flv Properties Of Alkali Metals
The alkali metals show regular trends in their physical and chemical properties with an increase in atomic number. Some important atomic and physical properties of alkali metals are given in the following table:
Atomic and physical properties of alkali metals:

General trends in different atomic and physical properties of alkali metals and their explanations:
1. Atomic and ionic radii
The atomic and ionic radii of alkali metals are the largest in their respective periods and these values further increase on moving down the group from Li to Cs.
Atomic and ionic radii Explanation:
On moving from left to right in a period, the number of electronic shells remains the same but the nuclear charge increases with each succeeding element Thus, the valence shell electrons experience a greater pull towards the nucleus and this results in successive decreases in atomic and ionic radii with an increase in atomic number
- Thus, the atomic and ionic radii of alkali metals are the largest in their respective periods.
- On moving down the group, a new electronic shell is ) added to each element and the nuclear charge increases with an increase in atomic number.
- The addition of an electronic shell tends to increase the size of the atom but the increase in nuclear charge tends to decrease the atomic radii by attracting the electron cloud inward. Thus, the two factors oppose each other.
- However, the increase in the number of shells increases the screening effect of the inner electrons on the outermost s -electron and as the screening effect is, quite large, it overcomes the contractive effect of the increased nuclear charge.
- The net result is an increase in atomic and ionic radii down the group from Li to Cs.
2. Ionization enthalpy
The first ionization enthalpies of alkali metals are the lowest in their respective periods. Explanation: Since the alkali metal atoms are largest in their respective periods, their outermost electrons being far away from the nucleus experience less force of attraction and hence, can be removed easily.
1. Ionization enthalpy of group-1 alkali metals decreases down the group.
Explanation:
Since the alkali metal atoms are largest in their respective periods, their outermost electrons being far away from the nucleus experience less force of attraction and hence, can be removed easily.
2. Ionisation enthalpy7 of group-1 alkali metals decreases down the group
Explanation:
On moving down the group from Li to Cs, the distance of the valence s-electron from the nucleus progressively increases due to the addition of a new shell with each succeeding element With an increase in the number of inner shells, the screening effects progressively increase and as a result, the effective nuclear charge experienced by the valence electron progressively decreases and hence, the ionization enthalpies decrease down the group.
3. The second ionization enthalpies of alkali metals are very high.
Explanation:
The monovalent cation formed by the removal of an electron from the alkali metal atom has a very stable noble gas configuration,
For example –
- Li+: 1s2 or [He], Na+: 1s22s22p6
- [Ne], k+: 1s22s22p63s23p6 or [Ar] etc.
Removal of another electron from the monovalent ion having a stable noble gas configuration is very difficult and requires a huge amount of energy. For this reason, the second ionization enthalpies of alkali metals are very high.
3. Hydration of ions, hydrated radii, and hydration enthalpy
The salts of alkali metals are generally ionic and are soluble in water because the cations get hydrated in water to form hydrated cations: M+ + aq —> [M(aq)]+.
1. The degree of hydration of ions and the hydrated radii decrease as we move down the group
Explanation:
The smaller the cation, the greater its degree of hydration. Since ionic radii increase down the group, the degree of hydration decreases, and consequently, the radii of die-hydrated ions decrease from Li+ to Cs+.

2. The order of mobilities of die alkali metal ions in aqueous solution is: Li+ < Na+ < K+ < Rb+ < Cs+
Explanation:
Smaller ions are more easily hydrated. As Li+ is the smallest ion among the given ions, it is most easily hydrated and has the least ionic mobility in an aqueous solution whereas Cs+ is the largest and is least hydrated. So its mobility is die highest.
3. Ionic conductance of the hydrated ions increases from [Li(m7)]+ to [Cs(aq)]+.
Explanation:
The ionic conductance of these hydrated ions increases from [Li(aq)]+ to [Cs(ag)]+ because die size decreases and mobility increases in this order. Hydration of ions is an exothermic process. The energy released when 1 gram-mol of an ion undergoes hydration is called hydration energy or hydration enthalpy,
4. Hydration enthalpy of alkali metal ions decreases from Li+ to Cs+.
Explanation:
The hydration enthalpy of an ion depends upon the ratio of charge to radius (q: r). Since the radii of alkali metal ions increase down the group, the hydration enthalpies decrease from Li+ to Cs+. Li+ ion has the maximum degree of hydration and for this reason, most of the lithium salts are found to be hydrated
For example: LiCl-2H2O, LiClO4-3H2O etc.
4. Oxidation State
Alkali metals exhibit a +1 oxidation state in their compounds and it remains restricted in a +1 state only.
Oxidation State Explanation:
Alkali metals have low ionization enthalpies and by losing their valence s -electrons they acquire the stable electronic configurations of the nearest noble gases. Thus, they have a strong tendency to form M+ ions and exhibit a +1 oxidation state in their compounds.
The second ionization enthalpies required to pull out another electron from M+ ions having very unstable noble gas electronic configuration are very high indeed and are not available under the conditions of chemical bond formation. Hence,v the alkali metals do not form M2+ ions, i.e. their oxidation state remains restricted to +1 state.
5. Metallic character
The elements of this group are typical metals that are soft (can be easily cut with a knife) and light. When freshly cut, they are silvery white but on exposure to air, they turn tarnished. The metallic character, which refers to the level of reactivity of a metal, increases on moving down the group.
Metallic character Explanation:
As the ionization enthalpy decreases down the group, the tendency to lose the valence electron increases, and consequently, the metallic character increases.
6. Photoelectric effect
Alkali metals (except Li) exhibit a photoelectric effect. The emission of electrons from the surface of a metal exposed to electromagnetic radiations of suitable wavelength is called the photoelectric effect.
S Block Elements Class 11 NCERT Notes
Photoelectric effect Explanation:
Due to low ionization enthalpies, the alkali metals exhibit a photoelectric effect. It is to be noted that lithium having the highest ionisation enthalpy does not exhibit a photoelectric effect. Cesium having the lowest ionisation enthalpy possesses the highest tendency to exhibit a photoelectric effect.
Potassium anti-cesium, rather than lithium is used in photoelectric cells:
The ionization enthalpies of potassium and cesium are much lower than that of lithium. For this reason, these two metals on exposure to light easily emit electrons from their surface but lithium does not. Hence, potassium and cesium rather than lithium are used in photoelectric cells.
7. Electronegativity
The alkali metals have low electronegativity which further decreases down the group.
Electronegativity Explanation:
The alkali metals having ns¹ electronic configuration preferably show electron releasing tendency rather than electron accepting. Thus, they have low electronegativities. Since the atomic sizes increase down the group, the tendency of atoms to hold their valence electrons decreases down the group, and consequently, electronegativity decreases down the group.
8. Conductivity
Alkali metals are good conductors of heat and electricity.
Conductivity Explanation:
Due to the presence of loosely bound valence electrons (ns¹) which are free to move throughout the metal structure, the alkali metals are good conductors of heat and electricity.
9. Melting and boiling points
Melting and boiling points: Melting and boiling points of alkali metals are low and decrease down tire group.
Melting and boiling point Explanation:
The cohesive energy that binds the atoms in the crystal lattices of these metals is relatively low (weak metallic bonding) due to the presence of only one valence electron (ns¹) which can take part in bonding. Hence, their melting and boiling points are low. These further decrease down the group as the strength of the metallic bonds and cohesive energy decrease with increasing atomic size.
10. Nature of bonds formed
Alkali metals form ionic compounds and the ionic character of compounds increases down the group from Li to Cs.
Nature of bonds formed Explanation:
For low ionization enthalpies, alkali metals readily form monovalent cations by losing their valence electrons. As ionization enthalpies decrease down the group, the ionic character of the compounds increases down the group.
11. Density
The densities of alkali metals are quite low and increase down the group from Li to Cs.
Density Explanation:
Due to their large atomic size and weak metallic bond, alkali metals have low density. Both the atomic volume and the atomic mass increase down the group but the corresponding increase in atomic mass is not balanced by the increase in atomic volume. As a result, the densities of alkali metals increase down the group. However, the density of K is less than that of Na because the atomic size and atomic volume of potassium are quite higher than that of sodium. As a result, the ratio of mass/ volume decreases.
Li is the lightest metal having a density of 0.53 g. cm-3. It cannot be preserved in kerosene because it floats over it. Generally kept wrapped in paraffin wax.
12. Flame coloration
Alkali metals or their salts on heating in the flame of the bunsen burner, impart characteristic colors and they can be easily identified from the color of the flame

Flame coloration Explanation:
Ionization enthalpies of alkali metals are not much higher. Thus, when an alkali metal or its salt (especially chloride due to its more volatile nature) is heated in a Bunsen burner flame, the electrons in the valence shell get excited and jump to higher energy levels by absorbing energy. When the excited electron drops back to its ground state, the emitted radiation falls in the visible region and as a result, alkali metals or their salts impart color to the flame.
Alkali metals can be detected by flame tests and can be estimated by flame photometry or atomic absorption spectroscopy.
13. Softness
Alkali metals are soft (can be cut easily with die help of a knife) and their softness increases down the group.
Softness Explanation:
The softness of alkali metals is due to their low cohesive energy and weak metallic bonding. Further, on moving down the group, the strength of metallic bonding decreases due to an increase in atomic size and as a result, the softness of the metals increases down the group.
Chemical Properties Of Alkali Metals
Alkali metals are highly reactive. Such reactivity may be attributed to their large atomic size, low ionization enthalpies, and low heats of atomization. –
Action of air and moisture
Alkali metals, being highly reactive, react readily with atmospheric oxygen to form oxides. These oxides further react with moisture to form hydroxides which in turn produce carbonates by reacting with atmospheric CO2
Metal oxides also react with CO2 to form carbonates.

These metals lose their glossiness and become tarnished due to the formation of carbonate layers on their surface. To protect from atmospheric oxygen and moisture, these metals are always stored in inert hydrocarbon solvents such as kerosene, petroleum ether, etc.
Reaction with oxygen
When the alkali metals are heated with oxygen or excess air, they form different types of oxides depending upon the nature of the metal involved. Lithium mainly forms monoxide (Li2O), sodium forms peroxide (Na2O2), and the other alkali metals (K, Rb, and Cs) mainly form superoxides having the general formula MO2. The temperature required for the reaction decreases down the group from Li to Cs.
4Li + O2 → 2Li2 O2 (Lithium monoxide)
2Na + O2 → Na2O2 (Sodium peroxide)
M +O2 → MO2 (Superoxide) [here, M = K, Rb, Cs]
Oxygen Explanation
A smaller cation can stabilize a smaller anion while a larger cation can stabilize a larger anion. If both the ions are similar in size, the coordination number will be high and this results in higher lattice energy.
A cation having a weak positive electric field can stabilize an anion having a weak negative electric field. Li+ ion and oxide ion (O2-) have small ionic radii and high charge densities. Hence, these small ions combine to form a very’ stable lattice of Li2O.
Sodium forms peroxide (Na2O2) but potassium forms superoxide (KO2), even though the peroxide ion, ![]() is larger in size than the superoxide ion,
is larger in size than the superoxide ion, ![]() This can be explained in terms of charge densities. Due to the bigger size
This can be explained in terms of charge densities. Due to the bigger size
Na+ ion has a weaker positive field around It and therefore it can stabilise peroxide Ion which also has a weaker negative field around it. Thus, Na+ forms peroxide. K+ ion is still bigger in size and the magnitude of the positive field around it is much weaker so it can’t stabilize superoxide ion which also has a much weaker negative field around it. Hence, K forms superoxide.
According to valence bond theory, two O-atoms in superoxide ion (O2–) are attached by a 2- 2-electron bond (common covalent bond) and a 2-electron bond. As the unpaired electron is present in the 2-electron bond, the superoxide ion is paramagnetic and all tire superoxides are colored (LiO2 and NaO2 are yellow, KO2 & CsO2 are orange, RbO2 is brown).
According to MO theory, there is an unpaired electron in one of the n antibonding orbitals and because of this, the superoxide ion is paramagnetic. The electronic configuration of O2–

O2– ion present in common oxides [For example, Li2O, NaO2etc.) and the 02~ ion in peroxides (For example, Na2O2), contain no unpaired electron and for this reason, these are diamagnetic and colorless.
Reaction with water
The alkali metals having high negative reduction potentials (E°) can act as a better-reducing agent than hydrogen. Hence, they react with water to form water-soluble hydroxides and liberate hydrogen gas.
2M + 2H2O → 2MOH + H2T [M = alkali metal]
Reactivity with water increases down the group as the electropositive character of the metals increases clown the group. Lithium decomposes water slowly. Sodium reacts with water vigorously. K, Rb, and Cs react with water explosively and the evolved hydrogen gas catches fire.
Alkali metals also react with compounds containing acidic H-atoms
For example: halogen halides (HX), alcohols (ROH), acetylene HC=CH, etc., to form their corresponding salts and H2
2Na + 2HX → 2NaX (Sodium halide) + H2 ↑
Li + 2C2H5OH → 2 C2 H5 OLi (Lithium ethoxide) + H2 ↑
2Na + 2HC=CH → 2NaC = CH (Sodium acetylide)+ H2 ↑
The standard electrode potential of Li is most negative while that of sodium is least negative i.e., in the reaction with water, Li releases a greater amount of heat than sodium. Despite that, Li reacts less vigorously with water than Na. j This can be explained concerning chemical kinetics. Na has a low melting point and the heat of the reaction is sufficient j to melt it. Molten metal spreads out and exposes a relatively large surface to water and as a result, it reacts with water 1 readily and violently. On the other hand, the melting point of Li is much higher and the heat of the reaction is not sufficient to melt it. Hence, its surface area does not increase and it reacts slowly with water.
NCERT Solutions Class 11 Chemistry Chapter 10 S Block Elements
Reaction with dihydrogen
All alkali metals react with dihydrogen at about 673 K (Li at 1073 K) to form colorless, crystalline hydrides (MH). These hydrides have a high melting point.

1. The reactivity of the alkali metals towards dihydrogen decreases down the group.
Explanation:
As the size of the metal cation increases down the group, the lattice energy of the hydrides decreases down the group. Consequently, the reactivity of the alkali metals towards hydrogen decreases down the group,
2. The ionic character of the alkali metal hydrides increases from Li to Cs.
Explanation:
Since the ionization enthalpy of alkali metals decreases down the group, the tendency to form cations as well as the ionic character of the hydrides increases.
Reaction with halogens
All alkali metals react vigorously with halogens to form crystalline halide compounds having the general formula MX. Lithium halides are covalent due to the very high polarising power of the Li+ ion. Halides of other alkali metals are ionic in nature’
⇒ \(2 \mathrm{M}+\mathrm{X}_2 \rightarrow 2 \mathrm{M}^{+} \mathrm{X}^{-}\) ( X = F, Cl, Br or I)
The reactivity of the alkali metals towards a particular halogen increases down the group. Due to a decrease in ionization enthalpies or an increase in the electropositive character of the metals down the group, the reactivity increases down the group.
The reactivity of halogens towards a particular alkali metal decreases in the order:
F2> Cl2 > Br2 > I2
Reducing nature
The alkali metals act as strong reducing agents because of their low ionization enthalpies. Since the ionization enthalpies decrease on moving down the group, therefore, in the free state the reducing power also increases in the same order, i.e., Li < Na < K < Rb < Cs.
The tendency of a metal to lose an electron in solution is measured by its standard electrode potential (E°). The alkali metals have low values (higher negative values) of E° and so they have a strong tendency to lose electrons and can act as strong reducing agents. Lithium, although, has the highest ionization enthalpy, is the strongest reducing agent in solution (E° = -3.04 V). On the other hand, Na is the weakest reducing agent and the reducing character increases from Na to Cs, i.e., Na < K < Rb < Cs.
Explanation of anomalous behavior of lithium
The anomalous behavior of lithium can be explained because the ionization enthalpy is the property of an isolated atom in the gaseous state while the standard electrode potential is concerned when the metal atom goes into solution.
The ionization enthalpy involves the change:
M(g) → M++(g) + e, while the standard electrode potential involves the change: M(s) → M+(aq) + e.
The latter change occurs in three steps as follows:
- M(s)→ M(g) – sublimation enthalpy
- M(g)→ M+(g) + e – ionisation enthalpy
- M+ (g) + H9O → M+(aq) + hydration enthalpy
The overall tendency for the change depends on the net effect of these three steps. Among the alkali metal cations, Li+ ion has the maximum tendency to get hydrated due to its very small size. The high hydration enthalpy compensates the energy required in the first two steps to a large extent and the overall energy required to convert M(s) to M+ (aq) is minimum for lithium.
Thus, small size and high hydration enthalpy are responsible for the strong reducing character of lithium.
The solution in liquid ammonia
Alkali metals dissolve in liquid ammonia to give highly conducting deep blue solutions which are highly reducing and paramagnetic. As the concentration increases (> 3M), the color of the solution changes to copper-bronze. These concentrated solutions are diamagnetic.
Solution in liquid ammonia Explanation:
1. When an alkali metal is dissolved in liquid v ammonia, ammoniated cations, and ammoniated electrons are formed as shown below:
M + (x+ y)NH3 → [M(NH3)x]+ (Ammoniated cation) + [e(NH3)y]–(Ammoniated electron)
2. The blue color of these solutions is due to the excitation of the free ammoniated electrons to higher energy levels by absorbing energy corresponding to the red region of visible light. The transmitted light is blue which imparts a blue color to the solutions.
3. With the increase in the concentration of the alkali metal, the formation of clusters of metal ions starts and because of this, at a much higher concentration (> 3M) the solutions possess metallic luster and attain the color of copper-bronze.
4. These blue solutions are highly conducting because of the presence of ammoniated electrons and ammoniated cations but the conductivity decreases with increasing concentration as the ammoniated cations get attached to the free unpaired electrons.
5. These blue solutions are paramagnetic due to the presence of unpaired electrons. However, tire paramagnetism decreases with increasing concentration due to the association of ammoniated electrons to yield diamagnetic species.
6. The free ammoniated electrons make these solutions very powerful reducing agents.
7. These solutions when kept, form metal amides and release H2. However, these solutions can be stored in anhydrous conditions in the absence of impurities like Fe, Pt, Zn, etc.
M+(am) + e(am) + NH3(l)→ MNH2(am) + ½H2(g)
Where ‘am’ stands for ‘solution in ammonia
2M + 2NH3→ 2MNH2 (metal amide)+ H2
Extraction of alkali metals
Alkali metals cannot be extracted by applying common processes used for the extraction of other metals.
Alkali metals Explanation:
- The alkali metals are strong reducing agents. Hence, they cannot be extracted by reduction of their oxides or other compounds.
- Since they are highly electropositive, the method of displacing them from their salts by any other element is not possible.
- The aqueous solution of their salts cannot be used for extraction by electrolytic method because hydrogen, instead of the alkali metal is discharged at the cathode (discharge potentials of alkali metals are much higher).
- However, by using Hg as a cathode, the alkali metals can be deposited but in that case, the alkali metals readily combine with mercury to form amalgams from which the recovery of metals becomes quite difficult.
- The electrolysis of their fused salts (usually chlorides) is the only successful method for their extraction, Another metal . ‘ chloride is generally added to lower its fusion temperature
General Characteristics Of The Compounds Of Alkali Metals
The compounds of alkali metals are predominantly ionic. Some of the general characteristics of these compounds are described below.
1. Oxides and hydroxides
1. Typical oxides or monoxides of alkali metals
For example: Li2O and Na2O are white ionic solids and basic. These oxides react with water to form strong alkalis (MOH).
Example: Na2O + HO → 2NaOH
2. All peroxides are strong oxidizing agents. They react with water or acid to give hydrogen peroxide (H2O2) and the corresponding metal hydroxide. Na2O2 is widely used as an oxidizing agent in inorganic chemistry.
M2O2 + 2H2O → 2MOH + H2O2
Example: Na2O2 + 2H2O2→ 2NaOH + H2O2
3. Superoxides are stronger oxidizing agents than peroxides and react with water or acid to give both H2O2 and O2 along with metal hydroxide.
2MO2 + 2H2O→ 2MOIH + H2O2+ O2
Example: 2KO2 + 2H2O2 →2KOH + H2O2 + O2
The alkali metal hydroxides (MOH) are all white crystalline solids and corrosive. They are the strongest of all bases and readily dissolve in water. Due to excess hydration, a large amount of heat is released. These hydroxides are thermally stable except Li OH. The basic strength of alkali metal hydroxides increases on moving down the group from Li to Cs.
Explanation:
The ionization enthalpies of alkali metals decrease on moving down the group and this causes a weakening of the bond between the alkali metal and the hydroxyl group (M —OH). This results in an increase in the concentration of hydroxyl ions in the solution, i.e., the basic character of the solution increases on moving down the group.
Thus, the basic strength of the hydroxides follows the order:
CsOH > RbOH > KOH > NaOH > LiOH
2. Halides
The alkali metal halides can be prepared by combining metals directly with halogens or by reacting appropriate oxides, hydroxides or carbonates with aqueous halogen acids (HX).
2M + X2 → 2MX ; M2 O + 2HX↓ 2MX + H2 O
MOH + HX→ MX + H2 O
M2 CO3+ 2HX→ 2MX + HO2 + CO2
The enthalpy of formation (ΔH°f) of alkali metal halides is highly negative. For a given metal, AHj values decrease from fluoride to iodide. These halides are colorless crystalline solids having high melting and boiling points.
1. The melting point of halides of a particular alkali metal decreases as:
Fluoride > Chloride > Bromide > Iodide.
Explanation:
For a particular alkali metal ion, the lattice enthalpies decrease as the size of the halide ion increases.
Lattice enthalpies of NaP, NaCl, Nalir, and Nal are 893, 770,730, and 685 kJ. mol-1 respectively, A.s the lattice enthalpy decreases, the energy required to break the crystal lattice decreases, and consequently, the melting points decrease. Thus, the melting points of NaF, NaCl, NaBr, and Nal are found to be 1201K, 10IMK, 1028 K, and 944 K respectively.
2. For a particular halide ion, the melting point of IJX is less than that of
NaX and thereafter the melting points decrease on moving down the group from Na to Cs.
Explanation:
The melting point of LiCl (887K) is less than that of NaCl (I084K), because LiCl is covalent (for smaller atomic size of Li compared to that of Na), but NaCl is ionic. Thereafter, the order of melting point is:
NaCl(1084K)>KCl(1039K)>RbCI(988K)>CsCl(925K) This is observed because the lattice enthalpies decrease as the size of the alkali metal atom increases.
3. Solubilities of the alkali metal halides (except fluorides) decrease on moving down the group since the decrease in hydration enthalpy is more than the corresponding decrease in the lattice enthalpy.
For example, the difference in lattice enthalpy between NaCl and KCl is 67kJ. mol-1 whereas the difference in hydration enthalpy between Na+ and K+ ion is 76 kj -mol-1 Thus, KCl is relatively less soluble in water compared to NaCl.
Explanation:
The solubility of a salt in water depends on its lattice enthalpy as well as its hydration enthalpy. In general, if hydration enthalpy > lattice enthalpy, the salt dissolves in water but if the hydration enthalpy < lattice enthalpy, the salt does not dissolve.
Further, the extent of hydration depends on the ionic size. The smaller the size of the ion, the more it will get hydrated and the greater will be its hydration enthalpy. LiF, for example, is almost insoluble in water because of its higher lattice enthalpy (-1005 kJ . mol-1 ).
On the other hand, the low solubility of Csl in water is due to smaller hydration enthalpies of the two large ions [-276(Cs+)-305(I–) = -581 kJ.-mol-1]. o Due to the smaller size and relatively higher electronegativity of Li, lithium halides except LiF are predo¬minantly covalent and hence, are soluble in organic solvents such as acetone, alcohol, ethyl acetate etc.
In contrast, sodium chloride, being ionic, is insoluble in organic solvents.
3. Soils of oxoacids
Alkali metals react with c to acids such as carbonic acid (H2CO3), nitric acid (HNO3), sulphuric acid (H8SO4), etc., to form corresponding salts and release H2. Due to the high polarising power and lattice energy of small Li ions, lithium salts behave abnormally.
4. Nature of carbonates and bicarbonates
All alkali metals form carbonates of the type M2CO3. Since the alkali metals are highly electropositive, their carbonates are remarkably stable up to l000°C above which they first melt and then decompose to form oxides. These salts are readily soluble in water. As electropositive character increases down the group, the stability of carbonates increases in the same order:
Cs2CO3 > Rb2CO3 > K2CO3 > Na2CO3 > Li2CO3
Li2CO3 is insoluble in water and unstable towards heat. It decomposes readily to give Li2O and CO2.
Explanation:
1. The very small L ion exerts a strong polarising power on the large carbonate (CO32-) ion and distorts the electron cloud of its nearby oxygen atom.
This results in the weakening of the C—O bond and the strengthening of the Li — O bond. This eventually facilitates the decomposition of Li2CO3 leading to the formation of Li2O and CO2. %
2. The crystal lattice formed by a smaller Li+ ion with a smaller O2 ion is more stable than that 2 formed by a larger CO3 ion and a smaller Li+ ion. This also favors the decomposition of Li2CO3

3. The aqueous solution of carbonates is alkaline. This is because carbonates being the salts of strong bases and weak acids (H2CO3) undergo hydrolysis.
M2CO3 + 2H2O ⇌ 2MOH (strong base) + H2CO3 (weak acid)
4. Bicarbonates or hydrogen carbonates (MHCO3) of the alkali metals except LiHCO3 are obtained in the solid state. These bicarbonates are soluble in water and stable towards heat. On strong heating, all the bicarbonates undergo decomposition to yield carbonates with the evolution of carbon dioxide.
2MHCO3 (heat)→ M2CO3 + CO2 + H2O
As the electropositive character of the metals increases down the group from Li to Cs, the stability of the bicarbonates increases in the same order.
5. Nature of nitrates
The alkali metal nitrates (MNO3) are prepared by the action of HNO3 on the corresponding carbonates or hydroxides. They are ionic crystalline solids having low melting points and are highly soluble in water. On strong heating, they (except LiNO3 ) decompose into nitrites and at higher temperatures oxides.
S Block Elements Chapter 10 NCERT Notes
For example:

LiNO3 decomposes readily on heating to give

6. Nature of sulphates
The alkali metals form sulfates of the type M2SO4. All the sulfates except Li2SO4 are soluble in water. The sulfates when fused with charcoal, form sulphides.
M2SO4.+ 4C→ M2 S + 4CO
Sulfates of alkali metals form double salts with the sulfates of trivalent metals like Fe, Al, Cr, etc. These double salts crystallize with a large number of water molecules to form alum. A typical example is potassium aluminum
[K2SO4→ Al2(SO4)3 -24H2O].
Lithium sulfate (Li2SO4) is not known to form alum.
Anomalous Behaviour Of Lithium (Li) And Similarity Between Li And Mg
Although lithium, the first element of group 1, exhibits most of the characteristic properties of this group, yet it differs from other members of this group in several respects.
Reasons for anomalous behavior of lithium
- Both Li- atom and Li+ ion have very small sizes.
- Much higher polarising power of very small Li+ ion results in increased Points of difference between lithium and other alkali metals covalent character of its compounds.
- Lithium has the lowest electropositive character, the highest ionization enthalpy, and the highest electronegativity compared to the rest of the members.
- Non-availability of d -d-orbital in its valence (outermost) shell.
- Strong intermetallic bonding (cohesive force) due to its small size. On the other hand, lithium shows a diagonal relationship with magnesium
Points of difference between lithium and other alkali metals:


Reasons for the similarities between lithium & magnesium:
Lithium exhibits a diagonal relationship with the 3rd-period group-2 element, magnesium. Reasons for the similarities between lithium and magnesium
- The atomic as well as ionic radii of Li and Mg are almost the same (Li+ = 76 pm and Mg2+ = 72 pm).
- Both lithium and magnesium have almost similar electronegativities (Li = 0.98 and Mg = 1.2).
Similarities between lithium and magnesium:

Uses Of Alkali Metals


Preparation, Properties, And Uses Of Some Important Compounds Of Sodium
1. Sodium carbonate (washing soda), (Na2CO3-10H2O)
1. Manufacture: Ammonia-soda or Solvay process
Sodium carbonate is commonly known as washing soda. It is generally manufactured by the Solvay process or ammonia-soda process.
Principle: When carbon dioxide is passed through an aqueous solution of NaCl (brine, 28% NaCl solution) saturated with ammonia, sodium bicarbonate is formed.
NH3 + CO2 + H2O > NH4HCO3
NH4HCO3 + NaCl ⇌ NaHCO3 + NH4Cl
Due to the common ion effect of Na+ ion, sodium bicarbonate so formed gets precipitated. Such removal of solid NaHCO3 shifts the reaction more and more towards the right. This results in a greater yield of NaHCO3. In this way, a nearly two-thirds portion of NaCl is converted into NaHCO3. The precipitated NaHCO3 is then filtered off, dried, and heated at 150°C to get sodium carbonate.

Evolved CO2 is reused to saturate the ammoniated brine.
Raw materials:
- Brine solution (28% aqueous solution of NaCl),
- Limestone or calcium carbonate (CaCO3) it is the source of CO2 and
- Ammonia.
2. Description of the process
Preparation of ammoniated brine:
1. This process is carried out in the absorption tower made of iron
2. From an overhead tank, brine is allowed to trickle down slowly along the tower and ammonia gas from the ammonia recovery tower which is mixed with a small amount of CO2 is allowed to pass through a tube situated near the bottom of the tower. As a result, the brine solution gets saturated with ammonia while calcium chloride and magnesium chloride are present as impurities in commercial.
3. Sodium chloride gets precipitated as their corresponding insoluble carbonates.
2NH3 + CO3 + H2O → (NH4)2CO3
CaCl2 + (NH4)CO3 → 2NH4Cl + CaCO3↓
MgCl2 + (NH4)2CO3 → 2NH4Cl + MgCO3↓
4. The ammoniated brine is then filtered to remove the precipitated calcium and magnesium carbonates and the filtrate thus obtained is passed into the carbonation tower.

Carbonation of ammoniated brine:
1. This operation is carried out in a long cast iron tower (carbonation or Solvay tower). The tower is fitted with several horizontal plates.
2. The ammoniated brine solution is trickled down from the top of the tower while CO2 gas from the lime kiln is introduced into the tower under high pressure through a pipe fitted at the base of the tower.
3. In this way, CO2 comes in contact with the descending stream of ammoniated brine and they react with each other to form ammonium bicarbonate which subsequently combines with NaCl to produce sodium bicarbonate and ammonium chloride.
NH3 + CO2 + H2O → NH4HCO3
NaCl + NH4HCO3 → NaHCO3↓ + NH4Cl
Separation of sodium bicarbonate:
1. The solution coming out of the carbonation tower contains crystals of NaHCO3. These are separated by passing the solution through vacuum filters.
2. The separated sodium bicar¬bonate is washed with water to remove any sodium or ammonium chloride that may adhere to it and then dried.
3. The filtrate containing NH4Cl and a small amount of NH4HCO3 is taken to the ammonia recovery tower where it comes in contact with Ca(OH)2.
Calcination:
When the dry NaHCO3 is heated strongly in a furnace at 180°C, it decomposes to form anhydrous Na2CO3. It is called soda ash. It is nearly 99.5 % pure.

Evolved CO2 is reused in the carbonation tower or absorption tower
Recovery of ammonia:
The filtrate from the carbonation tower which contains ammonium chloride and a little ammonium bicarbonate is made to flow down the ammonia recovery tower. NH4HCO3 is decomposed by the heat of steam and NH4Cl reacts with calcium hydroxide to form ammonia, carbon dioxide, and CaCl2. The mixture of NH3 and CO2 is used for tire saturation of brine while calcium chloride is obtained as a by-product.
1.

2.

Potassium carbonate (K2CO3) cannot be prepared by the Soh’ay process. This is because unlike sodium bicarbonate, potassium bicarbonate (KHCO3) which is fairly soluble in water does not get precipitated when CO2 is passed through the ammoniated solution of KCl.
Properties of Sodium carbonate:
1. State:
Sodium carbonate is available either as anhydrous salt or as hydrated salt. The hydrated salts are white crystalline substances and are mainly of two types
- Decahydrate (Na2CO3-10H2O) and
- Monohydrate (Na2CO2-H2O).
The decahydrate is also called washing soda. The anhydrous salt commonly known as soda ash, is a white powder. When sodium carbonate is crystallized from water, the decahydrate is obtained as white transparent crystals. These crystals are efflorescent. When exposed to air for a long time, crystals of decahydrate partially lose their water of crystallization and is converted to monohydrate, a powdery substance which is known as crystal carbonate.
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3 \cdot 10 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Na}_2 \mathrm{CO}_3 \cdot \mathrm{H}_2 \mathrm{O}+9 \mathrm{H}_2 \mathrm{O}\)
2. Action of heat:
When the decahydrate is heated up to 100°C, it slowly loses nine molecules of water of crystallization and gets converted into monohydrate. When the monohydrate is heated above 100°C, the anhydrous salt (Na2CO3) is produced as a white powder which melts at high temperatures but never undergoes decomposition.
1.

2.

Anhydrous Na2CO3 or soda ash melts at higher temperatures (melting point 852°C) but does not decompose. It turns to monohydrate when kept in the air.
3. Hydrolysis:
It dissolves in water with the evolution of a considerable amount of heat. Being a salt of a weak acid (HCO3) and a strong base (NaOH), it undergoes hydrolysis in water to give an alkaline solution.
⇒\(\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{H}_2 \mathrm{O} \rightleftharpoons 2\left[\mathrm{Na}^{+}+\mathrm{OH}^{-}\right]+\mathrm{H}_2 \mathrm{CO}_3\)
4. Reaction with acid:
At ordinary temperature, Na2CO3 reacts with dilute mineral acids to form the corresponding sodium salts and water along with the evolution of CO2
Na2CO3 + 2HCl→2NaCl + CO2↑ + H2O
Na2CO3 + 2CH3COOH→2CH3COONa + CO2↑ + H2O
Reaction with slaked lime: When a solution of Na2CO3 is heated with slaked lime (milk of lime) at 80°C, sodium hydroxide with insoluble calcium carbonate is obtained.
Na2CO3 + Ca(OH)2 → CaCO3↓+ 2NaOH
Uses of sodium carbonate:
- Sodium carbonate is mainly used for softening hard water and for washing clothes.
- It is used in fire extinguishers.
- It is largely used in the manufacture of soap, glass, borax, and caustic soda.
- It is used in the paper, paint, and textile industries.
- A mixture of Na2CO3 and K2CO3 is used as a fusion mixture.
- It is used as an important laboratory reagent both in qualitative and quantitative analysis.
2. Sodium bicarbonate or sodium hydrogen carbonate (baking soda), NaHCO3
Preparation of sodium bicarbonate:
Sodium hydrogen carbonate is obtained as the intermediate product in the Solvay process of manufacturing sodium carbonate.
It can also be prepared by passing CO2 through a saturated solution of sodium carbonate. Being less soluble, the white crystals of sodium hydrogen carbonate can be filtered out and dried at room temperature.
Na2CO3 + H2O+ CO2 ⇌ 2NaHCO3↓
Properties of sodium bicarbonate:
1. State: It is a white crystalline solid and is sparingly soluble in cold water. It is also stable in air.
2. Hydrolysis: Being a salt of weak acid (H2CO3) and strong base (NaOH), it hydrolyses to give a faintly alkaline solution.
NaHCO3 + H2O ⇌ [Na+ + OH–] + H2CO3
3. Action of heat: On heating, it decomposes to form CO2, water, and sodium carbonate.

4. Reaction with acids: At ordinary temperature, it reacts with mineral acids to form CO2, water, and the sodium salt of the acid:
NaHCO3 + HCl→ NaCl + CO2 ↑ + H2O
Uses of sodium bicarbonate:
- It is used as an antacid (known as soda bi-carb). It is also used as a mild antiseptic for skin infections.
- It is the chief ingredient of ‘baking powder’ which is used in preparing breads, biscuits, cakes etc.
- It is used in the preparation of soft drinks like soda- water, lemonades, etc.
- It is also used in fire extinguishers.
3. Sodium hydroxide (caustic soda), NaOH
Manufacture of sodium hydroxide:
Sodium hydroxide is industrially prepared by the electrolysis of an aqueous solution of NaCl (brine) in a specially designed cell called the Castner-Kellner cell or mercury cathode cell.
Class 11 Chemistry Chapter 10 S Block Elements Notes PDF
Sodium hydroxide Principle:
When a brine solution is electrolyzed in a cell using a mercury cathode and graphite anode, metallic sodium discharged at the cathode combines with mercury to form sodium amalgam. Now, electrolysis of slightly alkaline water in the cell using sodium amalgam as anode and iron rod as cathode produces NaOH. The reaction between sodium amalgam and water also produces NaOH.
Sodium hydroxide Procedure:

1. The cell consists of a large rectangular iron tank divided into three compartments by two slate partitions which do not touch the bottom of the tank but remain suspended in mercury placed in the grooves
2. The graphite anodes are fixed in the two outer compartments and the cathode which consists of several iron rods is fitted in the central compartment.
3. The layer of mercury at the bottom serves as an intermediate electrode as a cathode in the outer compartment and as an anode in the central compartment by induction.
4. The brine solution is taken in the two outer compartments and a very dilute NaOH solution is taken in the central compartment.
5. The mercury layer is made to flow from one compartment to another by rocking the cell with the help of an eccentric wheel. On passing electric current, the following reactions take place in the outer and central compartments.
6. In the outer compartment, NaCl undergoes electrolysis. Cl2 gas formed at the anode comes out from the outlet tube while sodium liberated at the cathode combines with mercury to form sodium amalgam.
NaCl → Na++ Cl– ; H2 O → H+ + OH–
- At cathode: Na+ + e →Na; Na + Hg→ Na/Hg
- At anode,: Cl–→ Cl + e; Cl + Cl→ Cl2 ↑
7. In the central compartment, sodium amalgam (Na/Hg) acts as an anode by induction.
⇒ \(\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}^{+}+\mathrm{OH}^{-}, \mathrm{NaOH} \rightarrow \mathrm{Na}^{+}+\mathrm{OH}^{-}\)
- At cathode: \(\mathrm{H}^{+}+e \longrightarrow \mathrm{H} ; \mathrm{H}+\mathrm{H} \longrightarrow \mathrm{H}_2 \uparrow\)
- At anode: \(\mathrm{Na} / \mathrm{Hg} \longrightarrow \mathrm{Na}^{+}+e+\mathrm{Hg}\)
Net reaction: \(2 \mathrm{Na} / \mathrm{Hg}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2\left(\mathrm{Na}^{+}+\mathrm{OH}^{+}\right)+2 \mathrm{Hg}+\mathrm{H}_2 \uparrow\)
The concentration of NaOH in the central compartment gradually increases with the progress of electrolysis and when it becomes 20%, the solution is withdrawn, evaporated and converted into pellets or flakes of NaOH.
Properties of sodium hydroxide:
1. State: It is a white, crystalline hygroscopic solid having a melting point of 318°C.
2. Solubility: It dissolves in water with the evolution of heat, giving a strong alkaline solution. It also dissolves in alcohol.
3. Hygroscopic and corrosive nature:
The crystals of NaOH are deliquescent (hygroscopic). When exposed to air they absorb moisture from air and dissolve in the absorbed water. Moist caustic soda generally absorbs CO2 from air to form sodium carbonate which forms a coating over the surface of the material. As Na2CO3 is non-hygroscopic, wet sodium hydroxide becomes dry again.
⇒ \(2 \mathrm{NaOH}+\mathrm{CO}_2 \rightarrow \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{H}_2 \mathrm{O}\)
It is corrosive. When its concentrated solution comes in contact with the skin it produces a burning sensation. It breaks down the proteins of the skin and because of this property, it is commonly called caustic soda,
4. Reaction with acids, acidic oxides, and amphoteric oxides: Being a strong alkali, it reacts with acids, acidic oxides, and amphoteric oxides to form corresponding salts.
NaOH + HCl→ NaCl +H2O
2NaOH + SO2→ Na2SO3 +H2O
Al2O3 + 2NaOH →2NaAlO2 (Sodium aluminate) + H2O
ZnO + 2NaOH → Na2ZnO2 (Sodium zincate) + H2O
Uses of sodium hydroxide:
It is used
- In the manufacture of soap, paper, artificial silk, dyes, and several chemicals
- In the refining of [etroleum and vegetable oil,
- In the purification of bauxite,
- As a cleaning agent for greasy machines and metal NItoots,
- As a laboratory reagent etc.
4. Sodium chloride (common salt), NaCI
Preparation of sodium chloride:
- The main source of sodium chloride Is seawater which contains 2.7-2,9% of the salt by mass. In tropical countries like India, common salt is generally obtained by the evaporation of seawater.
- Crude sodium chloride obtained by this process contains calcium sulfate (CaSO.), sodium sulfate (Na2SO4), calcium chloride (CaCl2), magnesium chloride (MgCl2), etc as impurities.
- Since MgCI2 and CaCl2 are deliquescent (absorb moisture from the air), impure common salt gets wet in the rainy season.
- For purification, a saturated solution of crude NaCI is prepared and the insoluble impurities are removed by filtration.
- The filtrate is then saturated with hydrogen chloride gas and crystals of pure NaCI separate out due to the common ion effect.
- Chlorides of Ca and Hg being more soluble remain dissolved in the solution. NaCI can also be prepared from rock salt.
Properties of sodium chloride:
- NaCI is a white crystalline solid that melts at 1081K.
- 36 g of NaCI is soluble in 100g of water at 373K. However, solubility does not increase much with an increase in temperature.
Uses of sodium chloride:
- It is used as common salt or table salt for domestic purposes.
- It is used in the manufacture of sodium, caustic soda (NaOH), chlorine, washing soda, sodium peroxide, sodium sulfate, etc.
- It is used in soap industry, in softening hard water, in freezing mixtures, and for regenerating ion exchange resins.
Biological Importance Of Sodium And Potassium
Sodium and potassium ions are the most common cations present in biological fluids. A person weighing 70kg contains about 90g of Na and 170g of K along with 5g of Fe and 0.06 g of Cu.
The daily requirement of Na and K for the human body is about 2 g each.
- The Na+ ions are mainly found outside the cells, in blood plasma, and in the interstitial fluid that surrounds the cells. These ions take part in the transmission of nerve signals, in regulating the flow of water across the cell membranes, and in the transportation of various amino acids and sugars into the cells.
- K+ ions are the most abundant cations in the cell fluids, where they activate a variety of enzymes, and promote the oxidation of glucose into ATP (adenosine triphosphate), and Na+ ions promote the transmission of nerve signals.
- The Na+ and K+ ions differ considerably in concentration on the opposite sides of the cell membrane. In blood plasma, for example, the concentrations of Na+ and K+ ions are 143 million-L-1 and 5 million-L-1 respectively.
- Within the blood cells, however, the concentrations of these ions are 10 millimol-L-1 and 105 millimol-L-1 respectively.
- The activities in a nerve cell depend upon the sodium-potassium ion gradient. These ionic gradients are maintained by an ion transport mechanism that operates the active inclusion of K+ ions and active exclusion of Na+ ions across the cell membrane.
- The transportation of ions requires energy which is obtained by hydrolysis of ATP. The hydrolysis of one ATP molecule to ADP provides enough energy to move three Na+ ions out of the cell two K+ ions and one H+ ion back into the cell.
CBSE Class 11 Chemistry Notes For Chapter 10 S Block Elements Group-2 Elements (Alkaline Earth Metals) Introduction
The outermost shell of these elements contains two electrons and the penultimate shell contains eight electrons except forthe first member beryllium which contains two electrons.0 Since the last electron enters the ns orbital, these are also called s-block elements.
Their outermost electronic configuration may be represented as ns², where n- 2 to 7. Due to their similarity in electronic configuration, they are placed in the same group (Group- 2) of the periodic table and closely resemble each other in physical and chemical properties. Two valence electrons are always involved together giving rise to uniform bivalency of the elements.
Beryllium shows some abnormal properties as its electronic configuration is slightly different from the rest of the members. The main reason is that both the beryllium atom and Be2+ ion are extremely small. Beryllium also shows some similarities with aluminum of group 13. Like alkali metals, the compounds of these metals are also predominantly ionic. The electronic configurations of alkaline earth metals are given in the following table
Electronic configuration of alkaline earth metals

CBSE Class 11 Chemistry S Block Elements Summary
Occurrence Of Alkaline Earth Metals
Due to low ionization enthalpies and high electropositive character, the alkaline earth metals are chemically very reactive and hence, do not occur in the free state but are widely distributed in nature as silicates, carbonates, sulfates, and phosphates.
- Relative abundance of Be, Mg, Ca, Sr, Ba, and Ra in the earth’s crust is 2, 27640, 46600, 384, 390, and 1.3 x 10-6 ppm respectively. 0 Beryllium, the fifty-first most abundant element by mass in the earth’s crust, is found as silicate minerals like beryl (Be3Al2Si6O18) and phenacite (Be2SiO4).
- Magnesium, the sixth most abundant element is found as carbonate, sulphate, and silicate. Its two important minerals are magnesite (MgCO3) and dolomite [MgCO3.CaCO3].
- It is also found in seawater at 0.13% as MgCl2 and MgSO4.
- Calcium, the fifth most abundant element by mass found in the earth’s crust, occurs mainly as CaCO3 in the form of limestone, marble and chalk. Its other important minerals are fluorspar (CaF2), fluorapatite, [3Ca3(PO4)2-CaF3], gypsum (CaSO4-2H2O) anhydride, (CaSO4).
- Strontium and barium are respectively the fifteenth and sixteenth most abundant element. Strontium occurs principally as the mineral celestite (SrSO4) and strontianite (SrCO3) while barium occurs mainly as the mineral barytes (BaSO4).
- Radium is radioactive and extremely scarce. It occurs in very small amounts (1 gin 7 ton) in pitchblende as the decay product of uranium.
General Trends In Atomic And Physical Properties Of Alkaline Earth Metals
The alkaline earth metals show regular trends in their physical and chemical properties with an increase in atomic number. Some important atomic and physical properties of alkaline earth metals are given in the
Atomic and physical properties of alkaline earth metals:

General trends in different atomic and physical properties of alkali metals and their explanations
1. Atomic and ionic radii
The atomic and ionic radii of alkaline earth metals are fairly large but smaller than those of the corresponding alkali metals and these increase on moving down a group.
Atomic and ionic radii Explanation:
The electrons of alkaline earth metals having a higher nuclear charge are more strongly attracted towards the nucleus. On moving down the group, the atomic as well as ionic radii increase. The addition of new shells and the increasing screening effect jointly overcome the effect of increasing nuclear charge down the group
2. Ionization enthalpy
1. The first and second ionization enthalpies of alkaline earth metals are quite low and decrease down the group from Be to Ra
Explanation:
The low ionization enthalpies of alkaline earth metals are due to their smaller nuclear charge and larger atomic size (compared to the other succeeding elements of the same period) which result in weaker forces of attraction between valence electrons (ns2) and nucleus. On moving down the group, atomic size increases and the screening effect of the inner shell electrons also increases
Since the alkali metal atoms are largest in their respective periods, their outermost electrons being far away from the nucleus experience less force of attraction and hence, can be removed easily. These two effects jointly overcome the effect of increasing nuclear charge down the group. Thus, first and second ionization enthalpies decrease down the group.
2. The first ionization enthalpies of alkaline earth metals are higher than those of the corresponding alkali metals but their second ionization enthalpies are lower than those of the corresponding alkali metals.
Explanation:
The alkaline earth metals have higher values of first ionization enthalpy than those of the corresponding alkali metals because they have smaller size and higher nuclear charge which result in stronger forces of attraction between the valence electrons and the nucleus.
The second ionization enthalpy values of alkaline earth metals are much lower than those of the corresponding alkali metals because the loss of the second electron from an alkaline earth metal cation (M+) leads to the attainment of a stable noble gas configuration (ns2np6) while the loss of the second electron from an alkali metal cation (M+) causes loss ofits stable noble gas configuration
Example:


3. Electropositive or metallic character
The alkaline earth metals are highly electropositive and possess high metallic character. However, they are less electropositive than the alkali metals. Their electropositive or metallic character increases on moving down the group.
Electropositive Explanation:
- Due to their relatively low ionization enthalpies, alkaline earth metals have a strong tendency to lose both valence electrons to form dipositive ions. Thus, they exhibit high electropositive or metallic character.
- As their atoms have smaller sizes and higher ionization enthalpies compared to those of the corresponding alkali metals, their tendency to lose valence electrons is less than that of alkali metals. Hence, alkaline earth metals have less electropositive or metallic character as compared to tine alkali metals.
- On moving down the group from Be to Ra, ionization enthalpies decrease due to an increase in atomic radii. Therefore, the tendency to lose electrons increases and so does the electropositive character
4. Hydration enthalpy
Hydration enthalpies of alkaline earth metal ions are much greater than that of the alkali metal ions & decrease down the group from Be2+ to Ba2+
Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
Hydration enthalpy Explanation:
Due to the smaller size of alkaline earth metal ions, their hydration enthalpies are much greater than those of the alkali metal ions. Therefore, the compounds of alkaline earth metals are found to be more extensively hydrated than those of alkali metals. Magnesium chloride and calcium chloride, for example, exist as hexahydrates (MgCl2-6H2O and CaCl2-6H2O) while sodium chloride and potassium chloride do not form such hydrates.
The ionic conductance of hydrated alkaline earth metal ions increases from [Be(H2O)x]2+ to [Ba(HO)2x]2+ due to a decrease in the extent of hydration. The hydration enthalpy of an ion is directly proportional to its charge/radius ratio {q/r). On moving down a group, the radii of the alkaline earth metals increase. As a result, the hydration enthalpies of these metals decrease.
5. Oxidation State
Alkaline earth metals exhibit an oxidation in their compounds. Although the second date of +2 in their compounds. Although the second ionization enthalpy of these elements is nearly double that of the first ionization enthalpy, yet they exist as divalent ions (M2+) in most of their compounds.
NCERT Solutions for S Block Elements Class 11 Chemistry
Oxidation State Explanation:
1. The divalent ions (M2+) of alkaline earth metals have stable noble gas configurations. Thus, M2+ ion is more stable than M+ ion.
M ([Noble gas] ns2) → M2+ [Noble gas] + 2e
2. Due to greater charge and smaller size, the divalent cations lead to the formation of very stable lattices, and hence, a huge amount of energy is released. The high lattice enthalpy easily compensates for the high second ionization enthalpy.
3. Divalent cations for their smaller size get hydrated in water to a greater extent and the energy thus released (hydration enthalpy) is large enough to compensate for the second ionization enthalpy
The ΔHi(3) values of alkaline earth metals are very high because the electron now has to be removed from the stable noble gas configuration. For this reason, the alkaline earth metals do not exhibit an oxidation state of more than +2.
6. Melting & boiling points
Alkaline earth metals have higher melting & boiling points than that of alkali metals. However, on moving down the group, no regular trend is observed.
Melting & boiling points Explanation:
- Due to their smaller size, the atoms of alkaline earth metals form a more close-packed crystal lattice. Moreover, alkaline earth metals have two electrons in their valence shell whereas alkali metals have only one.
- The larger number of valence electrons leads to the formation of stronger metallic bonds.
- No regular trend in melting and boiling point is observed down the group because the atoms adopt different crystal structures.
7. Nature of bonds formed:
Like alkali metals, alkaline earth metals predominantly form ionic compounds. However, these are less ionic than the corresponding alkali metal compounds. Beryllium, the first member of this group, is an exception as its compounds are covalent. Magnesium also tends to form covalent compounds to some extent. On moving down the group, the tendency to form ionic compounds increases.
Nature of bonds formed Explanation:
Alkaline earth metals form ionic compounds because they have low ionization enthalpies. Their compounds, however, are less ionic because their ionization enthalpies are higher than those of the corres¬ ponding alkali metals. Due to its much smaller size and much higher ionization enthalpy, beryllium forms compounds that are predominantly covalent. Down the group, the tendency to form ionic compounds increases because ionization enthalpy decreases.
8. Density and hardness
The alkaline earth metals are denser and harder than the corresponding alkali metals. However, on moving down the group, no regular trend is observed. It initially decreases from Be to Ca and then increases from Ca to Ba.
Density and hardness Explanation:
The extent of cohesive energy determines the density and hardness of metals and this depends on the number of electrons involved in metallic bonding and the size of the atom. In alkali metals, one electron per atom (the valence electron) is involved in metallic bonding while in alkaline earth metals, two electrons per atom (the valence electrons) are involved. Moreover, the atoms of alkaline earth metals are heavier and smaller in size.
Therefore, the extent of cohesive energy is relatively higher in the case of alkaline earth metals & consequently, the atoms in alkaline earth metals are packed more closely in their lattices. Cohesive energy decreases from Be to Ca due to a gradual increase in size while it is found to increase from Ca to Ba due to the formation of different crystal lattices.
9. Conductivity
The Gr-2 metals are good conductors of heat and electricity.
Conductivity Explanation:
Due to the presence of two loosely bound valence electrons (per atom) which can move freely throughout the crystal lattice, the alkaline earth metals are good conductors of heat and electricity.
10. Flame coloration
When the alkaline earth metals and their salts, except beryllium and magnesium, are heated in the flame of a bunsen burner, they impart characteristic color to the flame.
These colors are as follows:
- Ca: Brickred
- Ba: Apple green
- Sr & Ra: Crimson red
Flame coloration Explanation:
- When the alkaline earth metals or their salts are put into a flame, the electrons of their valence shell absorb energy and get excited to higher energy levels.
- When they drop back to the ground state, the absorbed energy is emitted in the form of visible light having characteristic wavelengths.
- Depending upon the wavelength of light emitted, different colors are imparted to the burner flame.
- Due to their smaller size, the valence electrons in Be and Mg are too strongly bound to get excited by the energy available from the flame. Therefore, they do not impart any color to the flame.
Alkaline earth metals (except Be and Mg) can easily be identified by flame test in qualitative analysis. Further, they can be estimated by flame photometry or atomic absorption spectroscopy.
11. Magnetic property
The alkaline earth metals and their salts are diamagnetic.
Magnetic property Explanation:
Since the divalent ions (M2+) of alkaline earth metals have noble gas configurations with no unpaired electrons, their salts are diamagnetic. The metals are also diamagnetic as all the orbitals are filled up with paired electrons.
Chemical Properties Of Alkaline Earth Metals (Group-2 Metals)
Due to their low ionization enthalpies and high electropositive character, alkaline earth metals have a strong tendency to lose their valence electrons. Therefore, they are highly reactive and do not exist in the free state in nature.
1. Reducing nature
The alkaline earth metals are strong reducing agents. However, they are weaker reducing agents than alkali metals. Again, like alkali metals, their reducing strength increases down the group.
Reducing nature Explanation:
The alkaline earth metals except Be, have a fairly strong tendency to lose two valence electrons to form dipositive ions (M→ M2+ 2e) i.e. they possess low ionization enthalpies and hence, they are strong reducing agents.
This is indicated by their high negative values of reduction potentials (E°). Their reducing strength, however, is less than the alkali metals as their atomization enthalpies and ionization enthalpies are relatively higher. Reducing strength increases on moving down the group as their ionization enthalpies decrease & electrode potentials become progressively more negative from Be to Ba.
2. Action of air
- Being fairly reactive, the alkaline earth metals are oxidized by the oxygen of the air and get tarnished due to the formation of a fine layer of oxide on their surface. With increasing atomic numbers, the effect of air on the metals gradually increases.
- Be and Mg being less reactive are not much affected by air. Ca and Sr get easily tarnished in air while Ba readily burns when exposed to air. Hence, Ca, Ba, and Sr are usually stored in paraffin.
3. Reaction with oxygen
Alkaline earth metals burn in oxygen to form oxides. Be, Mg, and Ca form monoxides while Sr and Ba form peroxides when they react with oxygen. This is because larger cation stabilizes a larger anion and hence the tendency to form peroxide increases as the size of the metal ion increases
 (M = Be, Mg or Ca)
(M = Be, Mg or Ca)
 (M = Ba, Sr)
(M = Ba, Sr)
4. Reaction with water
- Alkaline earth metals except beryllium react with water to form the corresponding hydroxides along with the liberation of H2 gas.
- Beryllium having the lowest negative standard electrodepotential (E° of Be2+/Be = -1.97V) among all
The group-2 metals is the least electropositive and hence, do not react with water or steam even at red hot conditions.
Ca, Sr, and Ba have relatively higher negative standard electrode potentials similar to those of the corresponding Gr-1 metals and hence, react even with cold water.
Mg has an intermediate value of E° and does not react with cold water but decomposes in boiling water.
M + 2H2O→M(OH)2 + H2↑ (M = Mg, Ca; Sr or Ba)
Thus, the reactivity of the alkaline earth metals towards water increases on moving down the group. However, they are less reactive towards water as compared to the corresponding alkali metals.
5. Reaction with nitrogen
1. All alkaline earth metals burn in nitrogen to form nitrides of the type M3N2. However, Li forms Li3N.
3M + N2 →M2N2 (M = Be, Mg, Ca, Sr and Ba)
2. The ease of formation of nitrides decreases from Be to Ba. Since N2 molecule is very stable, it requires very high energy to form nitride ions (N3-). This large amount of energy is supplied from the lattice enthalpy evolved when crystalline solids containing ions with high charges (M2+ and N3-) are formed.
Be3N2 is volatile because it is covalent. Other nitrides of this group are not volatile as they are ionic crystalline solids.
6. Reaction with halogens
The alkaline earth metals directly combine with halogens at higher temperatures to form halides having the general formula, MX2

Halides can also be obtained by the action of halogen acids on metals, their oxides, hydroxides, or carbonates
M + 2HX→ MX2 + H2; MO + 2HX→MX2 + H2O
M(OH)2 + 2HX→MX2 + 2H2O
MCO3 + 2HX→MX2 + CO2 + H2O
BeCl2 is, however, conveniently prepared by heating BeO with Cl2 in the presence of charcoal at 1073K

7. Reaction with hydrogen:
All the elements of group-2 except Be, form metal hydrides of the general formula MH2 when heated with hydrogen

Beryllium hydride can be prepared indirectly by reducing beryllium chloride with lithium aluminum hydride.
2BeCl2 + LiAlH4→ 2BeH2 + LiCl + AlCl3
Both beryllium hydride (BeH2) and magnesium hydride (MgH2) are covalent compounds. In these molecules, both Be and Mg have four electrons in their valence shell. Therefore, these molecules are electron deficient. To make up for their electron deficiency, these two compounds exist as polymers, (BeH2)n and (MgH2)n in which each Be or Mg -atom forms four three-centre two-electron (3c-2e) bonds or hydrogen bridge bonds or banana bonds.
The structure of polymeric beryllium hydride is shown below:

CaH2, SrH2, and BaH2 are ionic compounds in which a hydride ion (H–) exists as an anion. Calcium hydride (CaH2 ) which is also called hydrolith is used for the production of H2 by the action of HaO on it.
8. Reaction with carbon
When the alkaline earth metals except for Be, are heated with carbon in an electric furnace or when their oxides are heated with carbon, carbides of the type MC2 are obtained. These carbides are also called acetylides (containing discrete C2 ions) as on hydrolysis they form acetylene.

(M = Mg, Ca, Sr or Ba)
At much higher temperatures ( ~ 1700°C), beryllium reacts with carbon to form Be2C. This carbide is called methanide (containing discrete C4- ion) as on hydrolysis it produces methane. On heating, MgC2 forms Mg2C3, which is called allylide (containing discrete C34- ion) as hydrolysis yields allylene (methyl acetylene).
CaC2 + 2H2O → HC≡ CH + Ca(OH)2
Be2C + 4H2O → 2Be(OH)2 + CH4
Mg2C3+ 4H2O → CH3C ≡ CH + 2Mg(OH)2
When calcium carbide (CaC2), an important chemical intermediate, is heated in an electric furnace with atmospheric nitrogen at 1375K, it produces calcium cyanamide (CaNCN).

The mixture of CaNCN and carbon is called nitrolim. It is used as a slow-acting nitrogen fertilizer as it undergoes very slow hydrolysis and evolves NH3 gas for a long period.
CaC2 + 3H2O → CaCO3 + 2NH3
9. Reaction with acids
The alkaline earth metals react with dilute acids to form the corresponding salt with the liberation of H2 gas.
M + H2SO4 → MSO4 + H2↑ (M = Be, Mg, Ca, Sr or Ba)
Beryllium is the only group-2 metal which reacts with alkali to form H2 and beryllate salt.
Be + 2NaOH + 2H2O → Na2 [Be(OH)4] (Sodium beryllate)+ H2 ↑
This is observed due to the diagonal relationship between aluminum and beryllium.
10. Solutions in liquid ammonia
Like alkali metals, alkaline earth metals dissolve in ammonia to give deep blue-colored solutions containing ammoniated cations and ammoniated electrons.
M + (x+ 2y)NH3 → [M(NH3)x]2+ + 2[e(NH3)y ]–
Evaporation of ammonia from these solutions leads to the formation of hexammoniates M(NH3)6 which slowly decompose to yield the corresponding metal amides, M(NH2)2 and H2

11. Tendency to form complexes
The group-2 elements tend to form stable complexes and it is found to be greater than that of alkali metals because their ions have smaller size and higher charge. The tendency to form complexes decreases down the group and this is due to the decrease in ion-dipole interaction with increasing size of the metal ion. Be and Mg have the maximum tendency to form complexes.
Examples of two stable complexes of Be and Mg are \(\left[\mathrm{BeF}_4\right]^{2-}\&\left[\mathrm{Mg}\left(\mathrm{NH}_3\right)_6\right]^{2+}\) respectively
- Complexation of Ca2+ by EDTA and polyphosphates plays an important role in the removal of the metal in water softening.
- In chlorophyll, the complex formed by the combination of Mg and the tetrapyrrole system (porphyrin) is very crucial in photosynthesis.
12. Extraction of alkaline earth metals
Like alkali metals, alkaline earth metals are also very reactive and strong reducing agents. So they cannot be extracted by ordinary chemical reduction methods. These metals also cannot be prepared by electrolysis of aqueous solutions of their salts because in that case, hydrogen is discharged at the cathode instead of the metal which has a much higher discharge potential. However, electrolysis can be carried out using a Hg-cathode, but in that case, recovery of the metal from amalgam becomes difficult. These metals are best isolated by electrolysis of their fused salts, usually chlorides.
S Block Elements Class 11 Important Topics
General Characteristics Of The Compounds Of Alkaline Earth Metals
Compounds of group-2 elements are predominantly ionic but are less ionic than the corresponding compounds of group 1 elements and this is due to their increased nuclear charge and smaller size. The general characteristics of some of the compounds of alkaline earth metals are discussed below.
1. Oxides of alkaline earth metals
1. Crystal structure:
Except for BeO (covalent solid), the oxides of the remaining alkaline earth metals are crystalline ionic solids and possess a rock-salt (NaCl) structure with coordination number 6. BeO though covalent, is an extremely hard solid because of its polymeric nature. BeO possesses a covalent lattice with coordination number 4. Both BeO and MgO have several properties that make them useful as refractory materials (for lining furnaces).
These properties are:
- They have high melting points (BeO-2500°C and MgO – 2800°C),
- They have very low vapor pressures,
- They are good conductors of heat, O they are chemically inert and
- They can act as electrical insulators.
2. Stability:
Due to much higher lattice enthalpies, the oxides are very stable towards heat. The lattice enthalpies decrease with an increase in the size of the metal ion.

3. Basic character:
Beryllium oxide, BeO reacts with both acids and alkalis, i.e., it is amphoteric while the oxides of other group-2 metals are basic.
BeO + 2HCl→BeCl2 + H2O
BeO + 2NaOH → Na2 BeO2 (Sodium beryllate) + H2O
The basic strength of the oxides increases on moving down the group.
BeO (Amphoteric) < MgO (Weakly basic) < CaO (Basic ) < SrO, BaO( Strongly basic)
4. Reaction with water:
All these oxides except BeO and MgO, react with water to form sparingly soluble hydroxides. These reactions are exothermic.
MO + H2O→M(OH)2 + heat, M = Ca, Sr or Ba
2. Hydroxides
1. Basic character:
All the alkaline earth metal hydroxides are basic except Be(OH)2 which is amphotericin nature. Their basic strength increases on moving from Be(OH)2 to Ba(OH)2

The alkaline earth metal hydroxides are, however, less basic than the alkali metal hydroxides.
Basic character Explanation:
Due to low ionization enthalpies of the alkaline earth metals, the M — O bond present in their hydroxides is weak and breaks up easily to give OH– ions. For this reason, their hydroxides exhibit basic character. On moving down the group, the tendency of the M — OH bond to break heterolytically increases because ionisation enthalpies decrease and consequently, the basic character of the hydroxides increases.
Due to larger ionic sizes and lower ionization enthalpies of alkali metals, the M — OH bonds in their hydroxides are still weaker than those in alkaline earth metal hydroxides. Thus, the alkali metal hydroxides are more basic than the alkaline earth metal hydroxides.
2. Solubility in water:
Hydroxides of alkaline earth metals are less soluble in water than the hydroxides of alkali metals. Again, the solubility of these hydroxides increases markedly on moving down the group

Solubility in water Explanation:
On moving down the group, both the lattice enthalpy and the hydration enthalpy decrease with increasing ionic size. However, the lattice enthalpy decreases more rapidly than the hydration enthalpy, and consequently, their solubility increases down the group.
3. Thermal stability:
The alkaline earth metal hydroxides decompose on heating to give the metal oxide and water.

Thermal stability Explanation:
Thermal stability of these hydroxides increases down the group as the polarising power of the M2+ ion and the lattice enthalpy of the oxide formed decreases with increasing ionic size down the group.
3. Halides
- Due to the high polarising power of the Be2+ ion, beryllium halides have a covalent nature having low melting points.
- All other alkaline earth metal halides are ionic and their ionic character increases as the size of the metal ion (M2+) increases down the group. These ionic halides are non-volatile solids having high melting points.
- Due to its covalent nature, beryllium halides are sparingly soluble in water but readily soluble in organic solvents. The halides of other group-2 alkaline earth metals are readily soluble in water.
- Except for BeCl2, all other anhydrous halides of the alkaline earth metals are hygroscopic in nature and form hydrates.
- For example: MgCl2-6H2O, CaCl2-6H2O, SrCl2-2H2O and BaCl2-2H2O
- The tendency to form hydrate decreases down the group. Thus, anhydrous calcium chloride is used as a dehydrating agent in the laboratory.
- The dehydration of the hydrated chlorides, bromides, and iodides of Ca, Sr, and Ba can be achieved by heating. However, the corresponding hydrated halides of Be and Mg on heating suffer hydrolysis.
- BeF2 is highly soluble in water due to the much higher hydration enthalpy of the very small Be2+ ion.
- All other fluorides (MgF2 > CaF2, SrF2, and BaF2 ) are almost insoluble in water because their lattice enthalpies are higher than their hydration enthalpies.
- Except for BeCl2 and MgCl2, all other alkaline earth metal chlorides impart characteristic color to a flame.
- For example: CaCl2: is brick red, SrCl2: Is crimson red, BaCl2: Is grassy green, etc.
Structure of BeCl2 In the solid state, beryllium chloride has a polymeric chain structure with chlorine bridges as given below:

Which are bonded by two covalent bonds while the other two by coordinate bonds. The Be -atoms in (BeCl2)n sp³ -hybridized.
In the vapor phase, BeCl2 exists as a chlorine-bridged dimer which dissociates into linear triatomic monomer at about 1200K. In the dimer, Be is sp² -hybridized while in the monomeric is sp² -hybridized

CaF2 an industrially important compound, is the main source of both F2 and HF
CaF2 + H2SO4→ 2HF + CaSO4
![]()
- CaF2 is also used for making prisms and cell windows for spectrophotometers, an important instrument used in the spectroscopic analysis of compounds.
- In cold countries, CaCl2 is Used for treating ice on roads, because 30% eutectic mixture of CaCl2 +H2O freezes at – 55%C.
4. Salts of Oxoacids
Trends in the properties of some salts of group-2 elements are discussed below
1. Carbonates:
Solubility in water:
The carbonates of the alkaline earth metals are practically insoluble in water. Their solubilities decrease on moving down the group. BeCO3 is sparingly soluble in water while BaCO3 is insoluble in water.
Solubility in water Explanation:
On moving down the group, lattice enthalpy of carbonates remains almost unchanged” (the size of the metal ion is much smaller compared to CO32- ion) but hydration enthalpies of cations (M2+) decrease. Consequently, the solubilities of carbonates decrease down the group.
The extremely low solubility of alkaline earth metal carbonates in water is very important in the precipitation of Ba2+, Sr2+, and Ca2+ ions as their carbonates, in Gr-IV qualitative analysis of basic radicals.
Thermal stability: Carbonates of gr.-2 metals decompose on heating to give metal oxide and carbon dioxide

Thermal stability of the carbonates increases down the group with increasing cationic size

Thermal stability Explanation:
The trend can be explained in terms of the stability of the monomeric is sp –hybridized. of the resulting metal oxides. With increasing stability of Cl the metal oxide, the carbonate becomes more unstable BeCl2 [monomer] towards heat. The stability of metal oxides decreases down the group due to a decrease in lattice enthalpy with increasing cationic (M2+) size. Hence, the stability of the carbonates towards heat increases down the group.
2. Bicarbonates:
The bicarbonates of alkaline earth metals do not exist in the solid state but are found in solutions. When such solutions are heated, bicarbonates decompose to form carbonates with the evolution of CO2

3. Sulfates
1. Solubilityin water:
The sulfates of alkaline earth metals are relatively less soluble in water than the corresponding sulfates of alkali metals. Further, their solubilities decrease down the group. For example, BeSO4 and MgSO4 are more soluble in water, CaSO4 is less soluble in water and SrSO4, BaSO4, and RaSO4 are practically insoluble in water.
Solubilityin water Explanation:
On moving down the group, the hydration enthalpies decrease with increasing cationic size but the lattice enthalpy remains almost unchanged because the anion, SO4 is much larger as compared to the metal ions (M2+). For this reason, the solubilities of sulfates decrease down the group.
2. Thermal stability: The alkaline earth metal sulfates white solids which dissociate on heating to give the metal oxides and sulfur trioxide.

The thermal stability of the sulfates increases down the group due to an increase in ionic character. This is due to a decrease in the polarising power of the metal ions with increasing ionic size. It becomes evident from the dissociation temperatures of the sulfates given below

4. Nitrates
Nitrates of alkaline earth metals are prepared by heating corresponding metal carbonate with dilute HNO3
MCO3 + 2HNO3→ M(NO3) + H2 O + CO2 (M = Be, Mg, Ca, Sr or Ba)
Magnesium nitrate crystallizes as Mg(NO3)2. 6H2O whereas barium nitrate crystallizes as an anhydrous salt. This again shows that the tendency to form hydrates decreases with increasing ionic size and decreasing hydration enthalpy as we move down the group. Upon heating, all nitrates decompose to give the corresponding oxides with the evolution of NO2 and O2.

The nitrates of all these metals are soluble in water. Beryllium is unusual for the fact that it forms a basic nitrate in addition to the normal salt.
1.

2.

Anomalous Behaviour Of Be And Similarities Between Be And Al
Beryllium, the first member of group 2, shows some anomalous behavior, i.e.,it differs from the rest of the members of its family.
Reasons for anomalous behavior of beryllium:
- The extremely small size of the Be atom and Be2+ ion,
- Much higher polarising power of Be2+ ion,
- Higher ionization enthalpy and electronegativity as compared to the other members, absence of vacant d -d-orbitals in its valence shell.
- Again, beryllium resembles its diagonally placed element aluminum, the second typical element of group 13 and period 3, in several properties
Difference between beryllium and other alkaline earth metals


Reasons for the similarities between beryllium and aluminum:
- They have approximately the same polarising power. The polarising power of Be2+ is 0.064, while that of Al3+is 0.060.0
- The standard electrode potentials of Be and A1 are much closer i.e. Be2+/Be = -1.85V and Al3++/Al = -1.66V.0
- The electronegativity of both the elements i.e., beryllium and aluminum are the same (1.5in the Pauling scale).
Similarities between beryllium and aluminium:


Class 11 Chemistry Chapter 10 S Block Elements Overview
Uses Of Alkaline Earth Metals


- and different milk products.



























































































































































































 What happens ether?
What happens ether?




































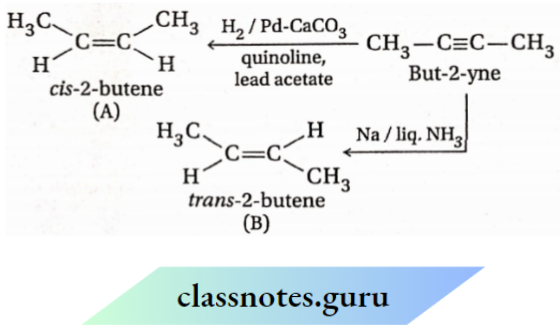



















 formed.
formed.















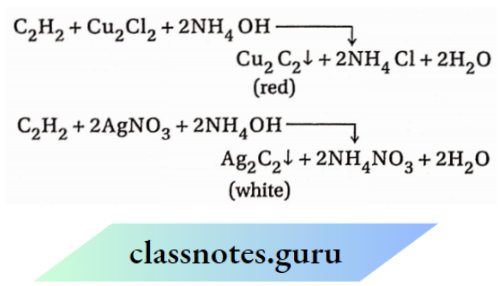






























 A and B are the two geometrical isomers. Identify them.
A and B are the two geometrical isomers. Identify them.



































 Cyclooctatetraene has a non-planar structure and there are 8π -electrons in it. So, cyclooctatetraene is a non-aromatic compound.
Cyclooctatetraene has a non-planar structure and there are 8π -electrons in it. So, cyclooctatetraene is a non-aromatic compound.








































































 show?
show?