Urinary Tract Anti Infective Agents Introduction
Urinary tract infection (UTI) is defined as significant bacteriuria in the presence of a constellation of symptoms such as dysuria (painful urination), increased urinary frequency and urgency, suprapubic discomfort, and costovertebral angle tenderness.
- It is a common cause of infections, particularly among young, sexually active women; an estimated 1 in 3 women will develop a urinary tract infection before the age of 24 years.
- Infection may involve either only the lower urinary tract or both the upper and lower tracts.
- The term cystitis is used to describe the syndrome involving dysuria, and suprapubic tenderness with urinary frequency and urgency.
- These symptoms may also be related to lower tract inflammation without bacterial infection and can be caused by urethritis (for example gonorrheal or chlamydial urethritis).
- Acute pyelonephritis refers to the syndrome of cystitis accompanied by significant bacteriuria and acute infection in the kidney it is characterized by clinical symptoms such as flank pain, fever, dysuria, urinary urgency, and frequency.
Classification Of Drugs
Fluoroquinolones:
- The fluoroquinolones are a family of synthetic, broad-spectrum antibacterial agents with bactericidal activity. The parent of the group is nalidixic acid, discovered in 1962 by Lescher and colleagues.
- The first fluoroquinolones were widely used because they were the only orally administered agents available for treating serious infections caused by gram-negative organisms, including Pseudomonas species.
- The newer fluoroquinolones have a wider clinical use and a broader spectrum of antibacterial activity including gram-positive and gram-negative aerobic and anaerobic organisms.
- Some of the newer fluoroquinolones have an important role in the treatment of community-acquired pneumonia and intra-abdominal infections.

Urinary Tract Anti Infective Agents Mechanism Of Action
- The mechanism of action of quinolones is through the inhibition of bacterial gyrase, an enzyme involved in DNA replication, recombination, and repair.
- By interfering with gyrase, quinolones arrest bacterial cell growth.
- The affinity of quinolones to metal ions seems to be an important prerequisite of their antibacterial activity: probably, quinolones bind to the DNA-gyrase complex via a magnesium ion.
Read and Learn More Medicinal Chemistry III Notes
Chemical Structures Of Some Commonly Used Fluoroquinolones

Structure-Activity Relationship
Position – 1:
- Earlier studies indicated that substitution at the N-l position is important for Anti-bacterial activity.
- QSAR analysis of a set of N-l allyl and alkyl derivatives suggested an optimum STERIMOL length of 0.42 nm, corresponding approximately to an ethyl group.
Position – 3:
- Position 3 and 4, having a link between the carboxylic acid and the keto groups are generally considered necessary for binding quinolones to DNA gyrase.
- Classical studies have produced no active quinolone with a significant modification of the C-3 carboxylic acid group, except groups that are converted in vivo to the carboxylic acid group.
Position – 4:
Position – 4 has not been extensively explored and the replacement of the 4- keto group with other groups has generally produced inactive or weakly active compounds.
Position – 5:
- Compounds with small substituents such as nitro, amino, halo, and alkyl groups have been synthesized. Among them, the C-5 amino group enhances absorption and tissue distribution, for example Sparfloxacin.
- The incidence of phototoxicity of Sparfloxacin is the lowest of the fluoroquinolones, because of the presence of the 5amino group, which counteracts the effect of the 8- 8-fluoro substituent.
Position – 6:
- Of various C-6 substituents, H, Cl, Br, F, Cl12, S- CM3, CO CH2, CN, NO2, etc the addition of a fluorine atom resulted in a dramatic increase in anti-bacterial potency.
- The Fluoro group at C-6 seems to improve both the DNA gyrase complex binding (2 to 17 folds) and cell penetration (1 to 70 folds) of the corresponding derivatives with no substitution at C-6.
Position – 7:
- C-7 piperazinyl group in addition to the C-6 fluorine substituent has anti-bacterial potency superior to that of earlier classical quinolones against both gram-positive and gram-negative bacteria.
- In general, quinolones with small or linear C-7 substituents I, Cl, Cl, NH2-CH2-O h-
NH2, NH- CH3, and NH-NH2) possess moderate to weak anti-bacterial activities. - Various substitutions tried at the C-7 position are –
- substituted piperazinyl.
- substituted pyrrolidinyl
- substituted morpholinyl
- In general, the substitution of methyl at the C-4 position of the piperazinyl group enhances gram-positive anti-bacterial activity with a slight decrease in gram-negative activity.
Position – 8:
- C-8 fluoro or chloro derivatives are more active in vivo, owing to better oral absorption.
- Oxygen substituent at the C-8 position, where the substituent is part of the ring system has been shown to have better in vivo efficacy.
- C-8 methoxy or ethoxy group appears to increase the spectrum of activity.
- C-8 methoxy (gratifloxacin) has been shown to contribute significant activity against anaerobes.
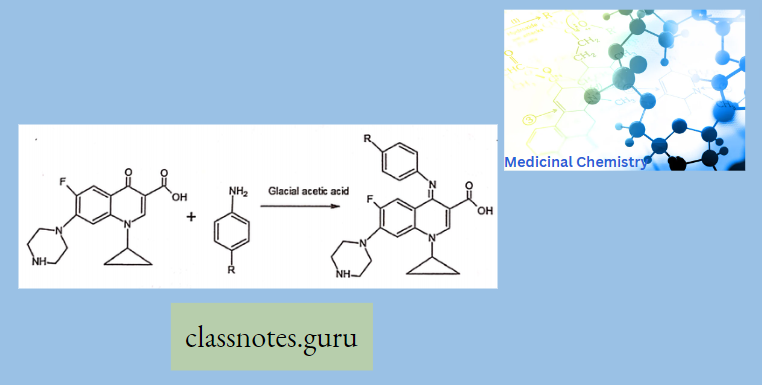
- Synthesis OF Ciprofloxacin
- By -cyclopropyl-6-fluoro-4-oxo-7-(pi pertain-l-yl)-l, 4-dihydroquinoline -3-carboxylic add
Urinary Tract Anti Infective Agents Multiple Choice Question And Answers
Question 1. Ciprofloxacin is…
- Fluoroquinolones
- Trioxane
- Guanidine Derivative
- None
Answer: 1. Fluoroquinolones
Question 2. Fluoroquinolone act on…
- DNA gyrase
- Arabinogalactan
- Cox
- All
Fluoroquinolones
Answer: 1. DNA gyrase
Question 3. Nalidixic Acid Act on…
- Glycolic Acid
- DNA gyrase
- Cox
- All
Answer: 2. DNA gyrase
Urinary Tract Anti Infective Agents Short Question And Answers
Question 1. Write the mode of action of Fluoroquinolones.
Answer:
The Mode Of Action Of Fluoroquinolones: The mechanism of action of quinolones is through the inhibition of bacterial gyrase, an enzyme involved in DNA replication, recombination, and repair. By interfering with gyrase, quinolones arrest bacterial cell growth.
Question 2. Write the mode of action of pyrazinamide.
Answer:
The Mode Of Action Of Pyrazinamide: PZA enters the cell wall of M. tuberculosis viva passive diffusion and it is converted to pyrazinoic acid (Active metabolite) by the pyrazinamidase enzyme. Then later it inhibits mycobacterial fatty acid synthase-I enzyme and disrupts mycolic add synthesis needed for mycobacterium cell wall synthesis.
Question 3. Write the adverse effect of Cycloserine.
Answer:
The Adverse Effects of Cycloserine: Peripheral Neuritis, Tremors, Psychotic, and Behavioral changes
Question 4. Write the adverse effect of isoniazid.
Answer:
The Adverse Effect Of Isoniazid: Peripheral neuritis-co administration of Pyridoxine (Vit B6) with INH prevents the symptoms of peripheral neuritis. GIT disturbance (Constipation, Loss of appetite) Hepatotoxicity
Question 5. Write the mode of action of Rifampicin.
Answer:
The Mode Of Action of Rifampicin: It strongly binds to the p subunit of bacterial ‘DNA dependent RNA polymerase’ enzyme. Thereby inhibits the RNA synthesis of bacteria. Mammalian RNA polymerase does not bind to rifampicin.
