Physical Properties Of Dental Materials Important Notes
1. Tarnish
- Tarnish is the surface discoloration
- Occurs due to
- Formation of calculus, plaque on the surface of the metal
- Formation of oxides, sulfides, chlorides
- Tarnish is the forerunner of corrosion
2. Corrosion
- Corrosion is the actual deterioration of the metal by a reaction with its environment
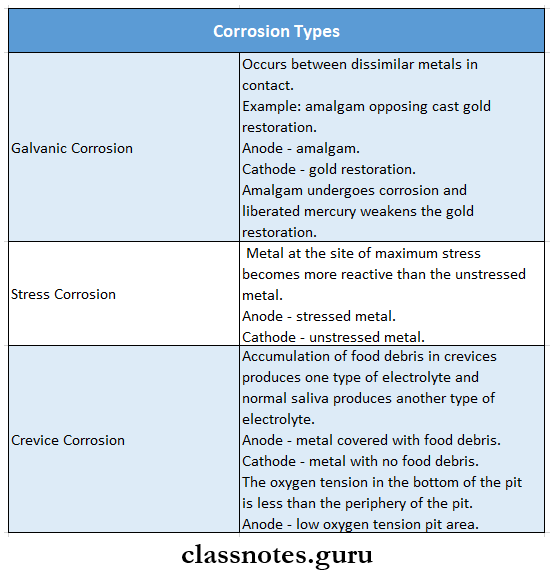
- Corrosion Types
- Dry Or chemical corrosion
- In it, the metal reacts to form oxides, chlorides, and sulfides in the absence of electrolytes
- Wet Or electrochemical corrosion
- It requires the presence of water or other fluid as an electrolyte
- It has 3 types-crevice corrosion, stress, corrosion, and galvanic corrosion
- Dry Or chemical corrosion
3. Passivation
- Chromium, aluminum, and titanium form strong adherent oxide films on their surface to protect from corrosion
- This is called passivation
Read And Learn More: Dental Materials Question and Answers
4. Creep
- Creep is defined as time-dependent plastic deformation or strain of material under static load
- Creep Types
- Static creep
- Dynamic creep
5. Flow
- Flow is deformation under a small static load
- Flow describes the behavior of amorphous materials such as waxes
6. Metamerism
- Objects that appear to be color-matched under one type of light may appear very different under another light source
- This phenomenon is known as metamerism
- Different light sources used in dental procedures are
- Daylight
- Incandescent lamps
- Fluorescent lamps
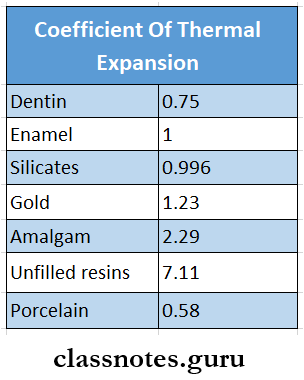
7. Coefficient Of Thermal Expansion
The linear coefficient of thermal expansion of some materials concerning tooth enamel are:
Physical Properties Of Dental Materials
Physical Properties Of Dental Materials Long Essays
Question 1. What are Tarnish and Corrosion? What are the causes for it? How to avoid it?
Answer:
1. Tarnish:
Tarnish Definition:
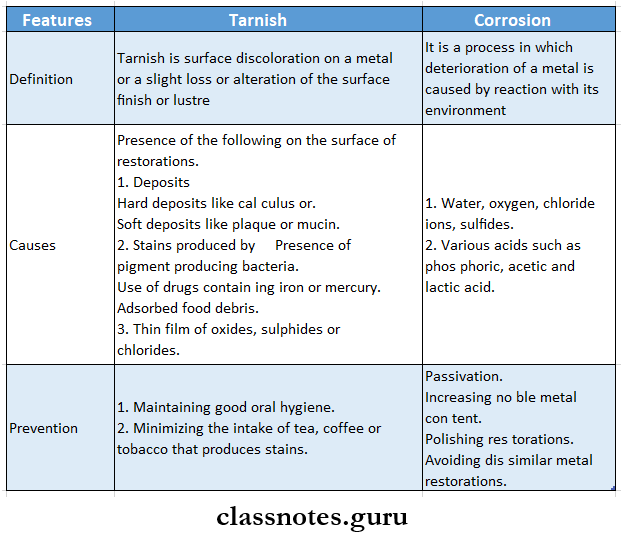
- Tarnish is surface discoloration on a metal or a slight loss or alteration of the surface finish or luster
- It is often the forerunner of corrosion
Tarnish Causes: In the oral cavity
The presence of the following on the surface of restorations
- Deposits
- Hard deposits like calculus or
- Soft deposits like plaque or mucin
- Stains produced by
- Presence of pigment-producing bacteria
- Use of drugs containing iron or mercury
- Adsorbed food debris
- A thin film of oxides, sulfides, or chlorides.
Protection Against Tarnish: It can be protected by the following measures
- Maintaining good oral hygiene
- Minimizing the intake of tea, coffee, or tobacco that produces stains
2. Corrosion:
Corrosion Definition: It is a process in which the deterioration of a metal is caused by a reaction with its environment
Corrosion Causes: Corrosion occurs due to
- Water, oxygen, chloride ions, sulfides
- Various acids such as phosphoric, acetic, and lactic acid
Corrosion Types:
1. Aqueous or Electrolytic or wet corrosion
Its subtypes are
- Galvanic corrosion
- Heterogeneous composition corrosion
- Stress corrosion
- Concentration cell corrosion
2. Dry or chemical corrosion
Protection against corrosion:
It can be protected by the following measures
1. Passivation:
- The process of formation of a strong oxide layer on its surface by metal is called Passivation
- This strong film protects the metal from corrosion
- Examples: Chromium, titanium, and aluminum are passive metals
2. Increasing noble metal content
- Gold, platinum, and palladium are examples of noble metals
- It is suggested that dental alloys must contain at least 50% of these metals
- As these metals are EMF-positive they resist corrosion better than any other metal
3. Polishing
- Well-polished restorations remove surface roughness
- This reduces the chances of concentration cell corrosion
4. Avoiding dissimilar metal restorations
- Dissimilar metals cause Galvanic corrosion
- Thus it should be avoided
Mechanical Properties Of Dental Materials
Physical Properties Of Dental Materials Short Essays
Question 1. Types of corrosion.
Answer:
Types Of Corrosion
1. Dry Corrosion Of Chemical Corrosion:
Corrosion is a non-aqueous corrosion in which the metal reacts to form oxides and sulfides in the absence of electrolytes.
Corrosion Example:
- Formation of Ag2S in dental restorations containing silver
- Oxidation of alloy particles in dental amalgam.
2. Electrolytic or EIectrochemical Or Wet corrosion:
- It requires the presence of electrolytes
- This electrolyte supplies the ions needed at the cathode and carries away the corrosion products at the anode
- The anode undergoes an oxidation reaction with the production of free electrons
- The reduction reaction occurs at the cathode by accepting electrons from the anode
- The production of electrons should be balanced by the consumption of electrons
- During a reaction, metal with the lower electrode potential becomes the anode
- Its subtypes are
- Galvanic Corrosion
- It occurs due to adjacent dissimilar metal restorations
- Example: Presence of amalgam occlusal restoration with opposing gold inlay
- Between these two dissimilar metals, saliva acts as an electrolyte producing short-circuit
- As a result, the patient experiences pain
- It can be minimized by painting varnish on the surface of the amalgam restoration.
- Heterogeneous Composition Corrosion
- It occurs within the structure of the restoration
- Examples: Eutectic alloys and peritectic alloys
- Impurities in any alloy enhance corrosion
- Stress Corrosion
- A metal is called to be stressed when it undergoes cold working
- If stressed and unstressed metals are contacted the stressed metals get corroded
- The stressed metal acts as an anode while unstressed metal is a Cathode
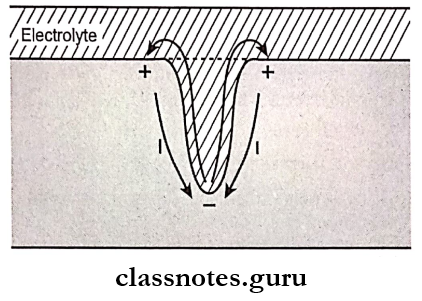
- Concentration Cell Corrosion Or Crevice Corrosion.
- It occurs due to variations in the composition of the given electrolyte
Types Of Corrosion Example:
- Differences in the composition of saliva
- The presence of food debris in between the teeth alters the composition of the saliva
- Due to this corrosion occurs under the layer of food debris
- It can be prevented by maintaining good oral hygiene
- Differences in oxygen concentration
- Due to the presence of pits, the oxygen concentration of the restorations differ
- The region at the bottom of the pit has a lower oxygen concentration
- This region behaves as an anode while the alloy surface around the pit becomes a cathode and corrosion results
Physical Properties Of Dental Materials Short Question And Answers
Question 1. Difference between tarnish and corrosion.
Answer:
Difference Between Tarnish And Corrosion
Properties Of Dental Composites
Question 2. Creep and flow.
Answer:
1. Creep Definition:
Creep is defined as time-dependent plastic deformation or strain of material under static load or constant stress near its melting point
Creep Types:
- Static creep: It is a time-dependent deformation produced in a completely set solid subjected to constant stress.
- Dynamic creep: Produced when the applied stress is fluctuating
Creep May Cause
- Deformation of dental restorations like an amalgam
- Improper fit of fixed partial dentures
Creep Flow: It is used to describe the rheology of amorphous substances like waxes
Question 3. Hue, value, and chroma
Answer:
Hue, Value, And Chroma: Hue, value, and chroma are the three dimensions of the color
- Hue
- It describes the dominant color of an object
- Example: red, blue, or green
- Value
- It is the relative lightness or darkness of the color
- It is independent of hue
- Chroma
- It represents the degree of saturation of a particular color
- It is always associated with value and hue
- The higher the chroma, the more intense the color
Hue, Value, And Chroma Measurement:
- The color is determined by the Munsell system
- It consists of a cylinder that shows the three dimensions of color
- Hue- Changes occur in a circumferential direction
- Value- Increases towards the top and decreases towards the bottom
- Chroma- It increases along the radius from an axis
Corrosion Resistance In Dental Materials
Question 4. Metamerism
Answer:
Metamerism Objects that appear to be color-matched under one type of light may appear very different under another light source.
- This phenomenon is known as Metamerism
- To overcome metamerism, it is recommended that the color-matching procedures should be carried out under two or more different light sources out of which one should be daylight
- The light sources used should be the same in the clinic as well as in the laboratory
- The different sources used in dental procedures are Daylight, incandescent lamps, and fluorescent lamps
Question 5. Coefficient of thermal expansion.
Answer:
Coefficient Of Thermal Expansion Definition: The change in length per unit of the original length of a material when its temperature is raised by lk is known as the coefficient of thermal expansion.
Coefficient of thermal expansion Significance:
- It is important in dental applications to produce cast restorations that fit and maintain the seal at a restoration margin.
- It Influences the procedures Involving wax patterns, casting metals, and crowns, placing amalgam and composite resin restorations, and preparing metal-ceramic crowns and bridges.
Question 6. Thermal conductivity.
Answer:
Thermal Conductivity Synonym: Coefficient of thermal conductivity
Thermal Conductivity Definition:
- It is the quantity of heat in calories per second that passes through a specimen 1 cm thick having a cross-sectional area of 1 cm2 when the temperature difference between the surfaces perpendicular to the heat flow of the specimen is 1 degree K.
- In general thermal conductivities increase in the following order:
- polymers< ceramics < metals
Thermal Conductivity Significance:
- Materials having high thermal conductivity are called conductors and those with low conductivity are insulators.
- The higher the thermal conductivity, the greater the ability of the substance to transmit thermal energy
- Increased thermal conductivity induces greater pulpal sensitivity
Thermal Conductivity Value:
Thermal conductivity is measured as watt per meter per second per degree Kelvin.
Corrosion Resistance In Dental Materials
Question 7. Electrolytic corrosion
Answer:
Electrolytic Corrosion
- It requires the presence of electrolytes
- This electrolyte supplies the ions needed at the cathode and carries away the corrosion products at the anode
- The anode undergoes an oxidation reaction with the production of free electrons
- The reduction reaction occurs at the cathode by accepting electrons from the anode
- The production of electrons should be balanced by the consumption of electrons
- During a reaction, metal with the lower electrode potential becomes the anode
Its Subtypes Are:
- Galvanic corrosion
- Stress corrosion
- Concentration cell corrosion
- Heterogeneous surface composition corrosion
