Chapter 4 The Structure Of The Atom
As you know, everything is ultimately made of atoms. It was earlier thought that atoms are indivisible but now we know that they are made up of subatomic particles—electrons, protons and neutrons. This idea has brought about a revolution in science.
How The Idea Of Atoms Emerged
The Views of Kanad
Way back in the sixth century BC, the Indian philosopher Kanad came up with the following idea.
Read And Lean More Class 8 Chemistry
- Not continuous, and
- Made up of tiny particles, named paramanus. .
(In Sanskrit, param means final or ultimate, and anu means particle.)
Kanad further said that two or more paramanus combine to form bigger particles.
NCERT Class 8 Chemistry Chapter 4 Structure of the Atom
The Views of Democritus and Leucippus
In the fifth century BC, the Greek philosophers Democritus and Leukiposs came up with a similar idea. They thought that on dividing a piece of a substance, one would ultimately get a particle that could not be divided further. They gave the name atomos (in Greek, atomos means indivisible) to these ultimate particles
Dalton’s Theory
The theories of Kanad as well as of Democritus and Leukiposs remained forgotten for more than two thousand years. But when experimental chemistry developed, it became necessary to explain observed facts. In 1803, English chemist John Dalton put forward his atomic theory,
Which can be summarised as follows:
- Elements are made up of very small particles of matter, called atoms (derived from the Greek word atomos).
- Atoms are indivisible.
- The atoms of an element have the same weight.
- The atoms of different elements have different weights.
- It is the atoms of elements that take part in a chemical reaction.
- The atoms of an element combine in a simple numerical ratio with those of other elements (s) to form a compound.
An atom is defined as the smallest part of an element that takes part in a chemical reaction
The Subatomic Particles
In the late nineteenth century, however, it was proved that atoms are divisible. And it was later found that atoms are made up of subatomic (or fundamental) particles— electrons, protons and neutrons. 35
The Electron
Under ordinary conditions, gases are bad conductors of electricity. But a gas becomes a good conductor of electricity if
- The pressure of the gas is very low (say, 10 mm of mercury or lower), and
- The voltage applied is very high (say, 10,000 V).
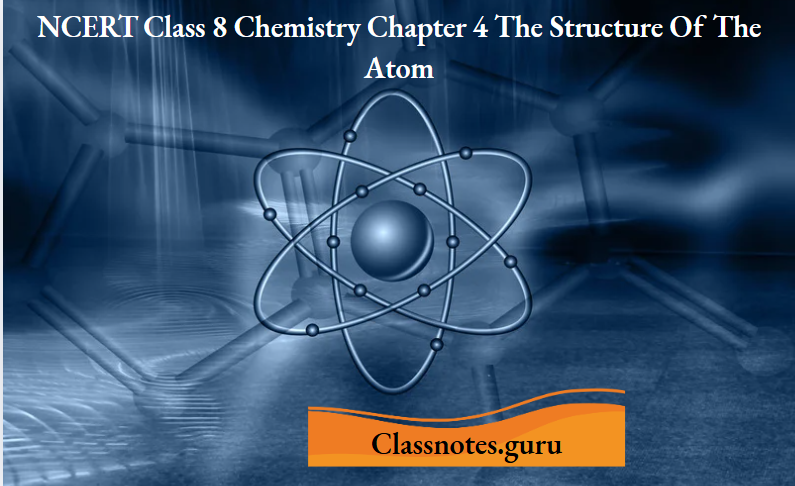
These conditions are achieved in what is called a discharge tube.
Cathode rays:
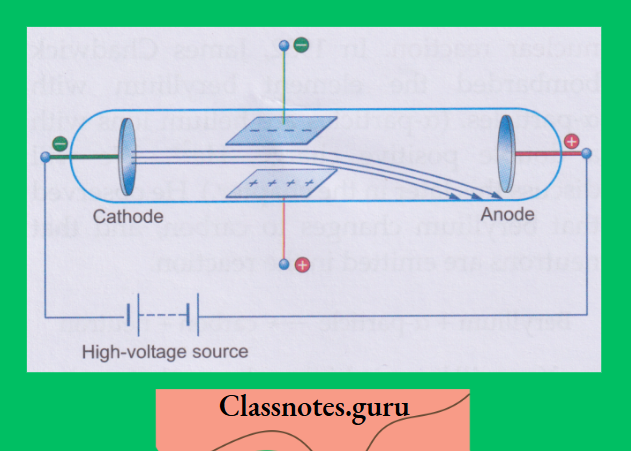
A discharge tube is a long glass tube at the two ends of which are sealed two metal plates.
- These plates can be connected to a high-voltage source and are called electrodes.
- The electrode connected to the negative terminal of the source is called the cathode, and the one connected to the positive terminal is called the anode.
- There is also a side tube which can be connected to an exhaust pump, used for lowering the pressure of the gas inside the discharge tube.
- When a high voltage is applied across the terminals, and the pressure inside the tube is 0.01-0.001 mm of mercury, the end of the tube opposite the cathode starts glowing.
- This phenomenon is called fluorescence.
Investigations have shown that some invisible rays, starting from the cathode, fall on the opposite wall of the tube, causing fluorescence. These rays were named cathode rays.
The characteristics of cathode rays:
Sir J J Thomson and others found that cathode rays have the following characteristics.
Atomic Structure NCERT Notes
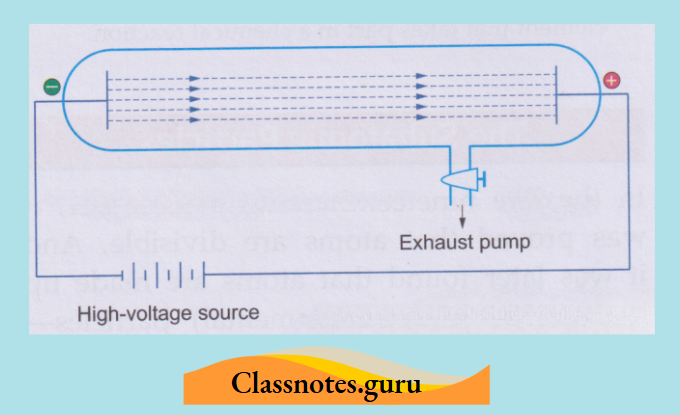
1. They originate at the cathode and travel in straight lines:
When an object is placed in the path of cathode rays, a shadow of the object falls on the wall opposite the cathode. A shadow can be formed only when the rays travel in straight lines.
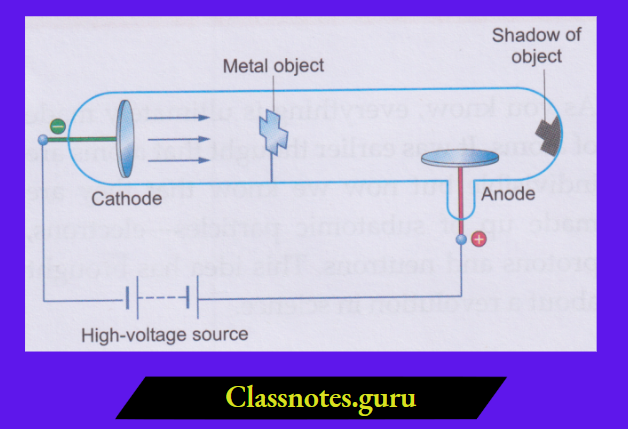
2. Cathode rays are a stream of particles:
A light paddle wheel, placed in the path of the cathode rays, rotates. This shows that some particles strike the plates of the wheel
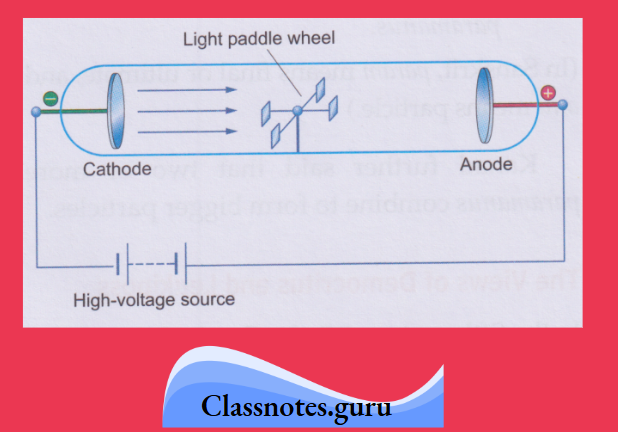
3. The particles constituting cathode rays are negatively charged:
This is proved by the fact that the cathode rays bend towards the positive plate in an electric field.
4. The particles constituting cathode rays are O O all alike. They do not change with the gas, the electrodes and the kind of glass used for making the tube
Thus, Sir J J Thomson concluded that the particles constituting cathode rays are a universal constituent of all atoms. These particles were named electrons in 1897
Relative charge and mass:
The charge on an electron is taken as the unit of negative charge. So an electron is said to have a charge of -1 unit. The mass of an electron is about 1/1840th of a hydrogen atom, and so it is treated as negligible.
The Proton
An atom is electrically neutral. But the electrons present in it are negatively charged particles. Hence, the atom must also contain some positively charged particles so that the overall charge on it becomes zero. These particles should be found in the discharge tube itself, when cathode rays are formed.
NCERT Solutions for The Structure of the Atom
Anode rays:
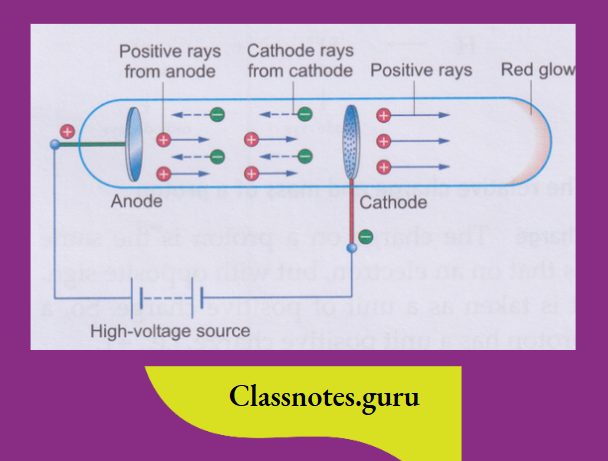
Goldstein repeated the cathode-ray experiment using a perforated cathode.
- He observed that there was a red glow on the wall opposite the anode.
- So, some rays must have travelled in the direction opposite to that of the cathode rays, i.e., from the anode towards the cathode.
- These rays were called anode rays or canal rays (as they moved through the perforations, or canals, in the cathode).
It was found that these rays contained positively charged particles, and so J J Thomson called them positive rays
The characteristics of anode rays
The characteristics of anode rays were found by carrying out experiments similar to those with cathode rays.
The following features distinguish anode rays from cathode rays:
- Anode rays are a stream of positively charged particles (because they bend towards the negative plate in an electric field).
- The particles constituting anode rays differ with the gas used in the discharge tube.
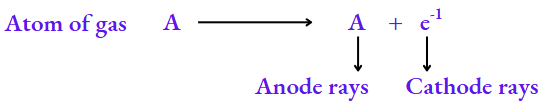
What constitutes anode rays?
The electrons constituting the cathode rays must come from the atoms of the gas inside the discharge tube. Electrons are negatively charged particles, so the atoms must be left with an equivalent amount of positive charge. It is these positively charged particles that constitute the anode rays.
The Structure of the Atom Class 8 NCERT Notes
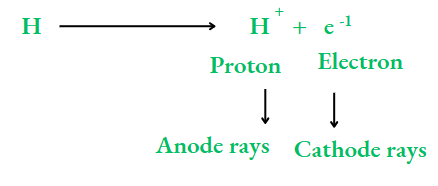
As an electron has a negligible mass, the particle A+ will have the same mass as A. So, the particles constituting anode rays will differ from gas to gas. When hydrogen, the lightest element, is taken in the discharge tube, the particles constituting the anode rays are called protons
The relative charge and mass of a proton
Charge:
The charge on a proton is the same as that on an electron, but with opposite sign. It is taken as a unit of positive charge. So, a proton has a unit positive charge, i.e., +1. The mass of a proton is the same as that of a hydrogen atom, i.e., 1 u (u stands for unified mass, which is the unit of atomic mass). A proton is about 1840 times heavier than an electron
The Neutron
The Discovery of the Nucleus Till now, we have learnt of only one particle that can account for the mass of an atom —the proton.
- The electron has negligible mass. But, except in the case of hydrogen, it was found that the mass of an atom is greater than that of the protons in it.
- Further, the unaccounted mass (i.e., the actual mass of the atom minus the mass of the protons) is either equal to or a multiple of the mass of a proton.
This suggested that an atom must contain one more kind of particle, which should have
- The same mass as a proton, but
- No electrical charge on it.
These particles were named neutrons as they should be electrically neutral. Experimentally, however, the neutron was observed much later in what is called a nuclear reaction. In 1932, James Chadwick bombarded the element beryllium with α-particles.
(α-particles are helium ions with a double positive charge, He2+. We will discuss this later in the chapter.) He observed that beryllium changes to carbon and that neutrons are emitted in the reaction.
Beryllium + α – particle → carbon + neutron
You will learn in higher classes that nuclear reactions are very different from chemical reactions. In a nuclear reaction, an atom can change altogether whereas in a chemical reaction, it will only rearrange itself but will not change.
Charge and mass of the subatomic particles:

The Discovery of the Nucleus
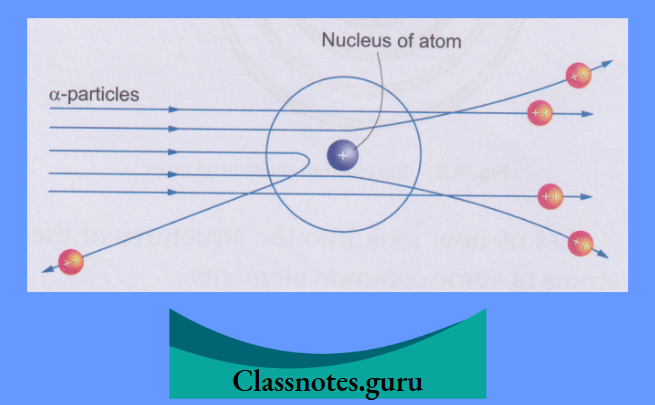
The concept of the nucleus was given by Ernest Rutherford in 1911. The idea was based on the results of his famous experiment, known as the α-particle scattering experiment.
Rutherford’s α-Particle Scattering Experiment
Rutherford bombarded a thin gold foil with α-particles. Alpha particles are emitted by radioactive substances like radium and polonium. (You will learn about radioactivity in higher classes.)
His observations and conclusions are described below:
- Most of the α-particles went straight through the foil. This is explained by the fact that they were not attracted to or repelled by any particle. In other words, the atom is mostly empty.
- Some of these particles deviated slightly from their path. This showed that they were repelled to a small extent by a positive charge.
- Very few of the particles, the ones at the centre, almost retraced their path. This meant they were strongly repelled by a small positively charged body at the centre of the atom. This positively charged body is called the nucleus.
- Since the electron has negligible mass, the mass of the atom is concentrated in the nucleus.
- Rutherford also theorised that electrons revolve around the nucleus at large distances from it.
The Atom and its Structure Class 8
Thus emerged the nuclear model of the atom from Rutherford’s α-particle scattering experiment
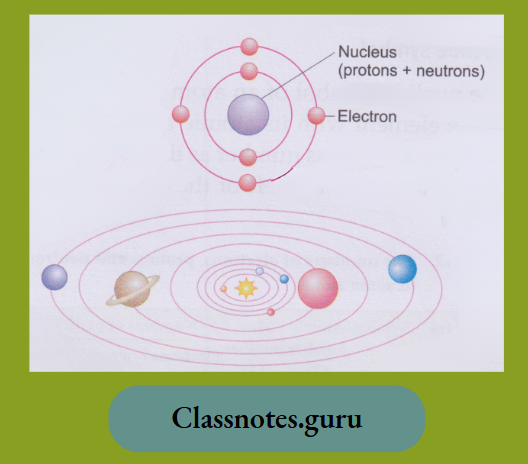
How Are the Subatomic Particles Placed in the Atom?
As we have just said, the mass of an atom is concentrated in its nucleus.
- The mass of an electron is negligible, but that of a proton or a neutron is lu.
- So, protons and neutrons must reside in the nucleus. Remember that the nucleus of a hydrogen atom contains only a proton and no neutrons.
- The nucleus of an atom is positively charged due to the presence of proton(s) in it.
In 1913, Niels Bohr presented the atomic model. According to it, electrons revolve around the nucleus in their orbits, just as planets do in the solar system.
Number Of Subatomic Particles In An Atom
Atomic Number and Mass Number
To know the numbers of subatomic particles in an atom, one needs to know the atomic or proton number (Z) and the mass number (A) of the atom.
- The atomic number, or the proton number, of an element is the number of protons present in the nucleus of an atom of the element.
- The sum of the numbers of protons and neutrons in an atom is known as the mass number of the atom.
- As an atom is electrically neutral, the number of electrons must be equal to the number of protons. So, in an atom,
The number of electrons = Z,
The number of protons = Z, and
The number of neutrons
= mass number- number of protons
= mass number- atomic number
= A-Z
Atomic Models NCERT Notes
Nuclide symbol
The nuclide symbol of an atom is the symbol of the element with its atomic number as the subscript and mass number as the superscript, which are set to the left of the symbol of the element
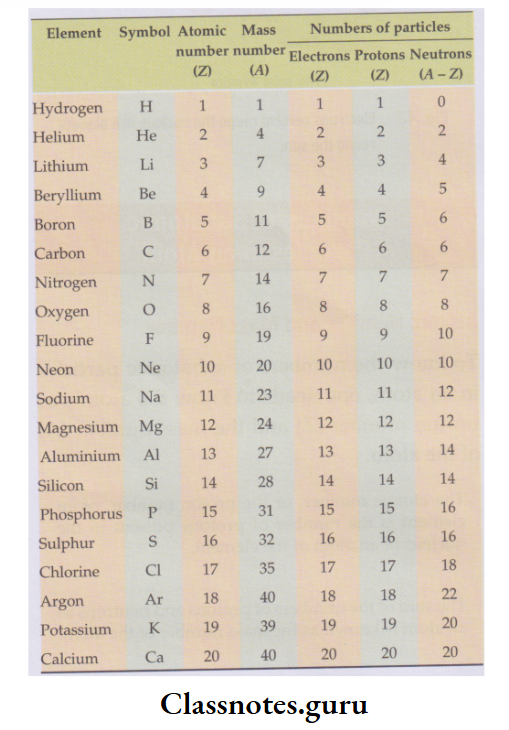
The numbers of electrons, protons and neutrons in some atoms:
The nuclide symbol is expressed as X.
For example:
- The nuclide symbol 17 CI represents a chlorine atom, whose atomic number is 17 and mass number is 35.
- You can immediately guess that there are 17 electrons, 17 protons and 35- 17 (= 18) neutrons in the atom
- The number of fundamental particles in atoms with atomic numbers 1 to 20 is given in the table
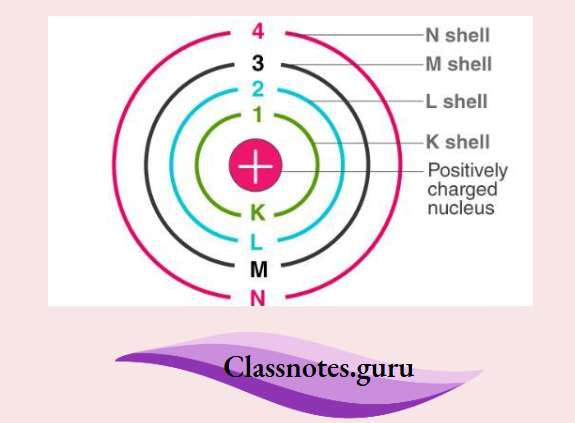
How Electrons Are Arranged
According to the Bohr model, the electrons revolve around the nucleus in shells, called K, L, M, N, … shells. They are named in this order, starting from the innermost shell. The first shell is called the K shell, the second is called the L shell, and so on. The numbers of electrons in these shells follow a set of rules. You will learn about the arrangement of electrons in detail in higher classes.
Let us now look into the structures of the atoms of some common elements.
Electron, Proton, Neutron Class 8 Chemistry
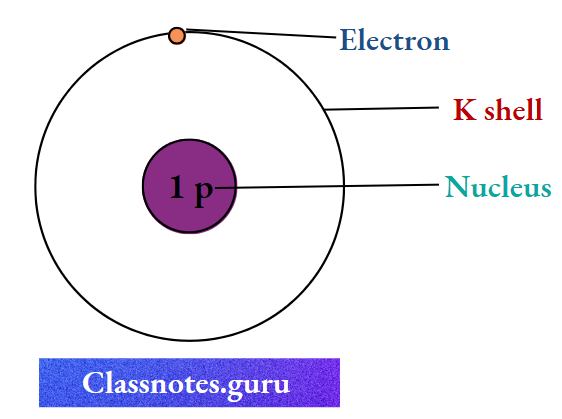
1. Hydrogen (1 H 1)
The number of electrons
Nucleus
The number of protons
= Z = 1
The number of neutrons = A- Z
= 1-1
= 0
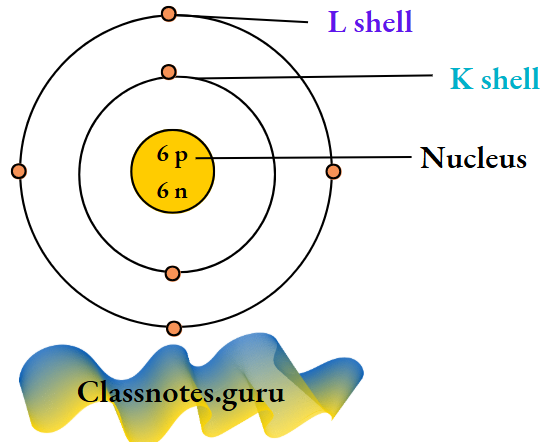
2. Carbon (12 C 6)
The number of electrons = Z = 6.
The number of protons = Z = 6.
The number of neutrons = A-Z
=12 – 6
= 6
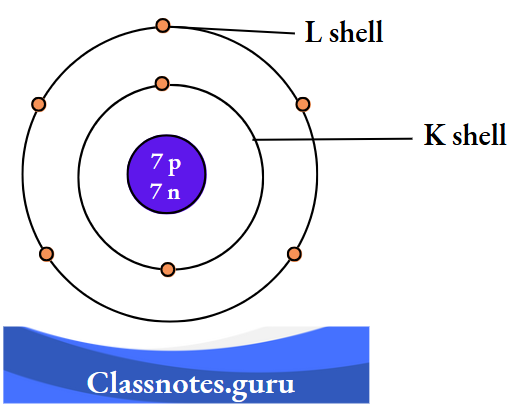
3. Nitrogen (12 N 7)
The number of electrons = Z = 7.
The number of protons = Z = 7.
The number of neutrons = A- Z
= 14-7
= 7.
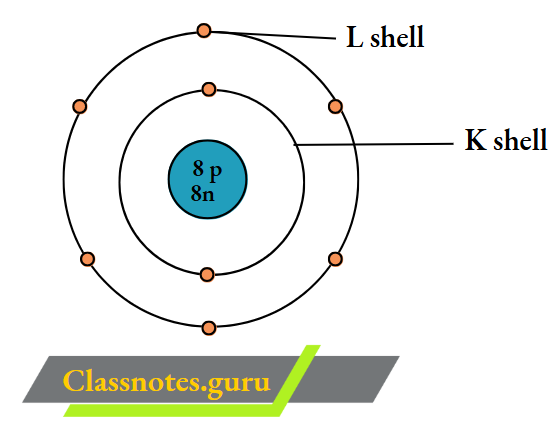
4. Oxygen (16 O 8)
The number of electrons = Z = 8.
The number of protons = Z = 8.
The number of neutrons = A- Z
= 16 – 8
= 8.
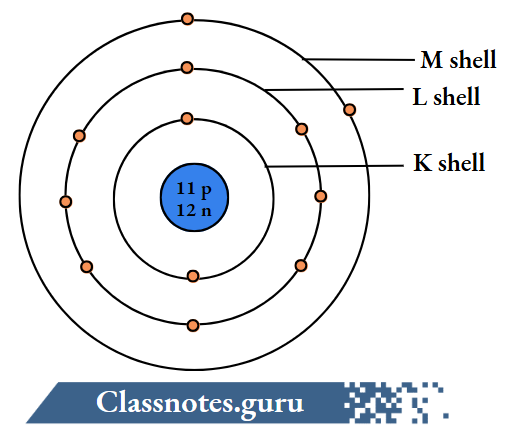
5. Sodium (23Na11)
The number of electrons = Z = 11.
The number of protons = Z = 11.
The number of neutrons = A-Z
= 23-11
= 12.
Atomic Number and Mass Number Class 8
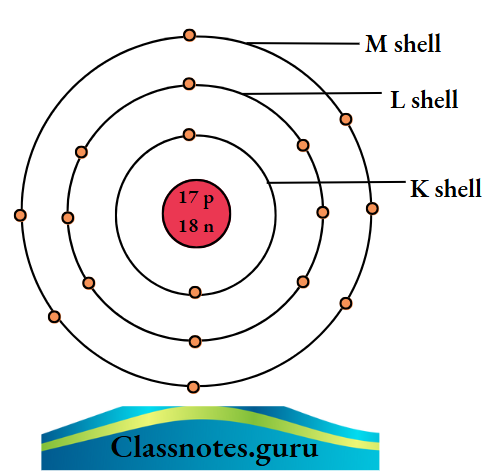
6. Chlorine (35Cl17)
The number of electrons = Z = 17.
The number of protons = Z = 17.
The number of neutrons = A- Z
= 35 – 17
= 18.
Ions
An atom is electrically neutral because the charge of the protons is balanced by that of the electrons. But what happens when an atom loses or gains an electron? If an atom loses an electron, the number of protons exceeds that of electrons. So the atom gets positively charged.
For example:
- A sodium atom has 11 protons and 11 electrons. On losing an electron, it would have 10 electrons and 11 protons.
- The greater number of protons causes the sodium atom to have a positive charge. Therefore, we say that a Na+ ion is formed.
- On the other hand, if an atom gains an electron, the number of electrons exceeds that of protons.
So, the atom becomes negatively charged.
NCERT Class 8 Atomic Structure Concepts
For example:
A chlorine atom contains 17 protons and 17 electrons. If it gains an electron, there are 18 electrons as against 17 protons.
- So, the chlorine atom gets a negative charge on it, i.e., a Cl– ion is formed.
- And what if the electron lost by sodium is gained by chlorine? The ions Na+ and Cl– will be formed, which, being oppositely charged, will remain together. In other words, the compound NaCl will be formed.
- Thus, it appears that electrons play a great role in the formation of compounds. You will learn about all this in higher classes.
