Chapter 1 Matter Notes
You know that matter is made up of tiny particles called molecules. Molecules are held together by a force of attraction called an intermolecular force. There is also a space between the particles called the intermolecular space.
Matter And Its Properties
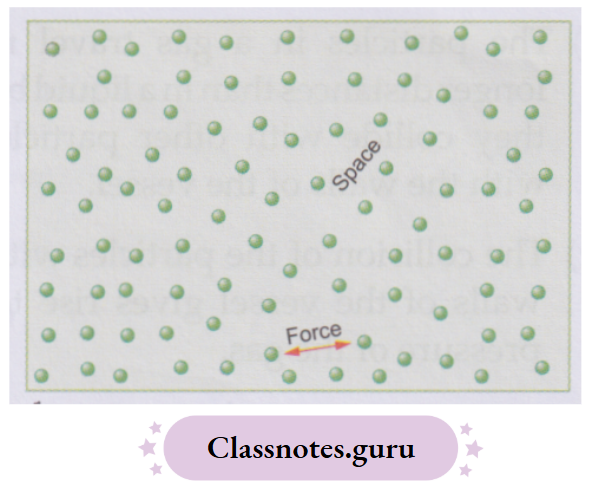
The force of attraction and the space between the particles (i.e., molecules) differ from substance to substance. But for any substance,
Read And Lean More Class 8 Chemistry
- The stronger the intermolecular force, the smaller is the intermolecular space, and
- The weaker the intermolecular force, the larger is the intermolecular space
States Of Matter
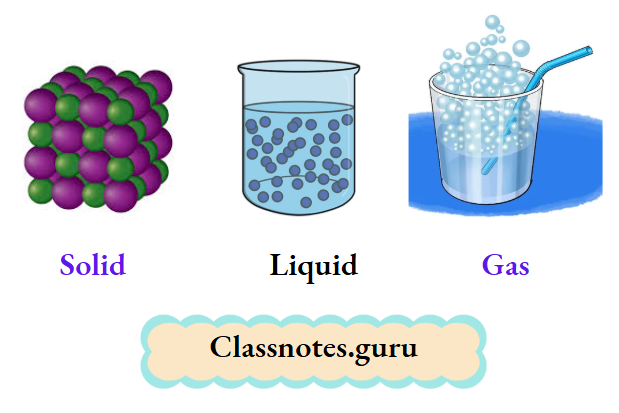
You have learnt earlier that matter exists in three states
- Solid
- Liquid
- Gaseous
For example:
NCERT Solutions For Matter Class 8
Water exists in three states— ice (solid), water (liquid), and water vapour (gaseous). Here we must understand that the kind of matter, i.e., the molecules constituting ice, water, or water vapour, is the same, i.e., water (H2O).
What Causes the Difference in States
For any substance, the molecules making up the substance do not change in the three states.
- Then why is it that the substance exists in three states? It is, in fact, the force and the space between the molecules that determine the state of matter.
- The intermolecular force is the strongest in the solid state, weaker in the liquid state, and weakest in the gaseous state.
And so, the intermolecular space is the smallest in the solid state, larger in the liquid state, and the largest in the gaseous state
Thus, the molecules are most tightly held in solids, loosely held in liquids, and almost independent of each other in gases. This explains the general behaviour of solids, liquids, and gases.
Interconversion of States of Matter
As you know,
- On heating, ice turns into water and water into steam, and
- On cooling, steam turns into water and water into ice.
NCERT Class 8 Chemistry Chapter 1 Matter
Such a change in state may be brought about in other substances too, without a change in the composition
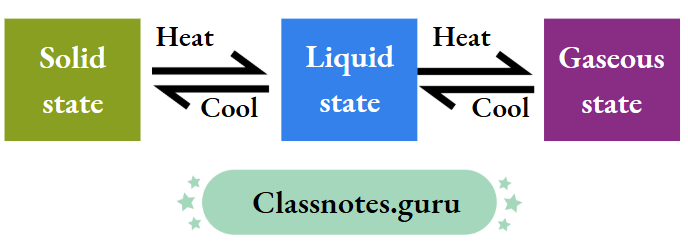
This is called the interconversion of states of matter.
- The constant temperature at which a solid turns into the corresponding liquid is called the melting point of the solid.
- The constant temperature at which a liquid freezes, i.e., turns into the corresponding solid, is called the freezing point of the liquid.
- Ordinarily, the freezing point of a liquid is the same as the melting point of the corresponding solid.
For example:
- Water freezes as well as ice melts at 0°C.
- Also, the constant temperature at which a liquid boils is called the boiling point of the liquid.
For example:
- The boiling point of water is 100°C.
- It is the temperature at which steam will condense.
States Of Matter NCERT Notes
The Kinetic Theory of Matter
The kinetic theory of matter explains the behaviour of the three states of matter.
It is summarised below.
- All matter is made up of very tiny particles called molecules.
- In the solid state, the particles are rigidly held in positions, about which they can only vibrate.
- In the liquid state, the particles are in continuous motion, but are not completely separated from each other. While in motion, they collide among themselves.
- In the gaseous state, the particles are in continuous random motion, almost independent of each other.
- The particles in a gas travel much longer distances than in a liquid before they collide with other particles or with the walls of the vessel.
- The collision of the particles with the walls of the vessel gives rise to the pressure of the gas
- The particles possess some energy due to their motion. This energy is called kinetic energy (KE).
- The KE possessed by the particles is dependent on temperature. The higher the temperature, the higher the KE, and the lower the temperature, the lower the KE
How Kinetic Theory Explains the Interconversion of States of Matter
1. Solid-liquid interconversion
Solid to liquid:
- The particles constituting a solid only vibrate about their mean positions.
- As a solid is heated, the KE of the particles increases. With rising temperature, the particles vibrate more and more vigorously till they move away from their fixed positions at a particular temperature, called the melting point of the solid.
- Thus, a solid becomes a liquid.
Liquid to solid:
- As a liquid is cooled, the KE of the particles decreases.
- The particles move shorter and shorter distances as the temperature is lowered.
- At the freezing point, the translational motion (i.e., from one point to another) of the particles ceases, and the particles get rigidly fixed. This is how a liquid changes into a solid.
- However, the particles in a solid continue to vibrate about their mean positions
Types Of Matter Class 8
2. Liquid-gas (vapour) interconversion
Liquid to gas:
The particles in a liquid are in continuous motion, during which they collide among themselves.
- If they collide strongly, some of the particles may overcome the attractive force and escape.
- A liquid evaporates in this manner. As the temperature is raised, the KE of the particles increases, and the particles collide more strongly. This leads to faster evaporation of the liquid.
- At the boiling point of the liquid, the KE of the particles becomes so great that all the particles tend to escape.
- Thus, at the boiling point, the entire liquid may turn into vapour.
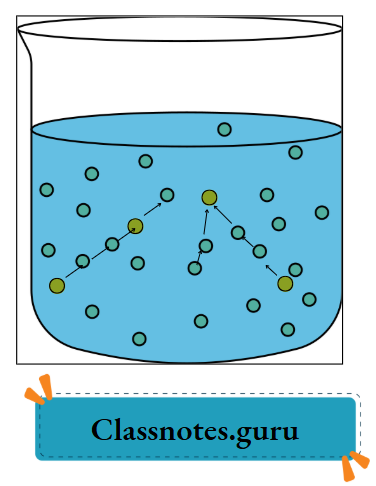
Gas (vapour) to liquid:
In the gaseous state, the particles move very fast, independently of each other. As the temperature is lowered, the KE of the particles is also lowered. When low-energy gaseous particles collide with each other, they may form bigger lumps or clusters, and the gas may condense into a liquid.
Solid Liquid Gas NCERT Notes
The Conditions of Change of State Differ from Substance to Substance
A liquid freezing at 0°C and boiling at 100 °C is water.
- One boiling at 78°C is ethanol.
- The melting and boiling points are characteristic of a substance, and you can identify the substance by determining these points.
- In other words, the conditions of change of state differ from substance to substance.
- This is because the intermolecular force and the intermolecular space differ from one substance to another
Conservation Of Mass
You know that only a rearrangement of atoms or molecules takes place when a change occurs. And as the atoms or molecules of a substance have fixed masses, the total mass of the substances involved in a change should remain the same.
Law of Conservation of Mass
Lavoisier, in the late eighteenth century, gave a law, called the law of conservation of mass, which can be stated as follows.
- Matter can neither be created nor destroyed, but can be changed from one form to another, and the total mass of the substances before and after the change remains the same.
- The law of conservation of mass is true for physical as well as chemical changes.
- In a nutshell, the total mass of the substances before and after the change is the same.
Conservation of Mass Physical changes:
It is easily understood that there is no change in the mass of a substance when it undergoes a physical change.
Properties Of Matter Class 8
For example:
The mass of an electric bulb does not change after it remains lit for some time.
- Similarly, a given mass of ice, on melting, gives the same mass of water.
- And a given mass of water, on boiling, gives the same mass of water vapour.
Conservation of Mass Chemical changes:
According to the law of conservation of mass, in a chemical change, the sum of the masses of the reactants is the same as that of the products.
We must look at the total mass of the substances before and after the reaction.
Mass of the reactants = Mass of the products
Matter Science Notes
Let us consider the burning of a piece of paper. During the process, it appears that there is a loss of mass.
- But that is not the case.
- While burning, the paper takes up oxygen from the air and forms some ash, plus carbon dioxide and water vapour.
- So, we have to see whether the mass of paper plus oxygen taken from the air is equal to that of the ash plus carbon dioxide and water vapour formed.
- A detailed experiment shows that the two masses are equal.
Mass of paper + oxygen = Mass of ash + carbon dioxide + water vapour
You can verify the law with the help of a simpler experiment, as described below.
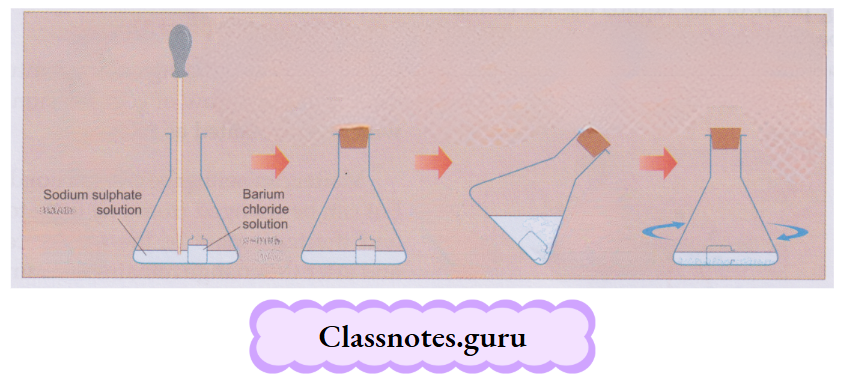
Experiment:
Put a small tube or bottle containing a solution of barium chloride into a conical flask.
NCERT Chemistry Matter Chapter
- Place some sodium sulphate solution in the flask with the help of a dropper, carefully, ensuring that the two substances do not come in contact with each other.
- Close the mouth of the flask with a cork and weigh the flask. Tilt the flask and swirl it slowly.
- A white precipitate is formed. Leave the flask for some time and weigh it again.
- You will find that there is no change in the weight of the flask.