Direct Filling Gold Important Notes
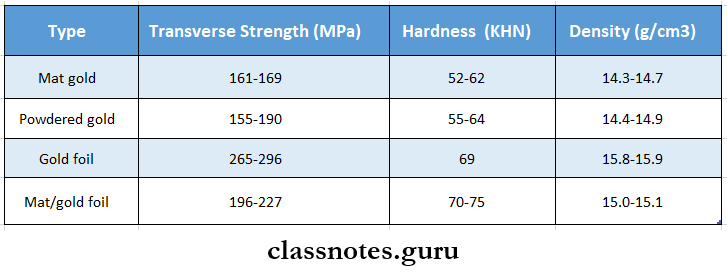
1. Direct Filling Gold Gold foil
- It is manufactured by beating pure gold into thin sheets
- Size: 4*4 inches
- Surface impurities and foil treated with ammonia make the gold foil noncohesive
- It is used for the external surface veneering of the restoration
2. Direct Filling Gold Mat gold
- It is used as a bulk filler
- Available as strips or cakes
3. Direct Filling Gold Mat foil
- Mat gold wrapped in gold foil
- Used for veneering the external surface of the restoration
4. Direct Filling Gold Electroalloy
- Electrolytic precipitates alloyed with 0.1% calcium
- Calcium increases strength and hardness
Read And Learn More: Dental Materials Question and Answers
5. Direct Filling Gold Powdered gold
It is chemically precipitated gold powder with an average particle size of 15 pm
6. Direct Filling Gold Condensation
- It is a process of cold working
- It increases hardness, strength
- Results in fibrous grain structure
- The force of condensation is inversely proportional to the square of the diameter of the nib
- Forces of condensation must be
- 45° to the cavity walls and floor
- 90° to the previous increment
- Steeping is the process of condensation where each condenser overlaps the previous compaction
Direct Filling Gold Long Essays
Question 1. In what forms is gold used in dentistry? Describe in detail the various types of direct-filling gold available. Briefly mention the degassing technique.
Answer:
Various Types of Direct Filling Gold Available are
- Foil
- Sheet
- Cohesive
- Noncohesive a Ropes
- Cylinders
- Laminated foil
- Platinized foil
- Electrolytic precipitate
- Mat gold
- Mat foil
- Gold-calcium alloy – Granulated or powdered gold
Degassing:
Degassing Synonyms: Desorbing, annealing
Degassing Description:
- Except noncohesive golds, the direct filling gold is available 1 cohesive forms
- During storage and packaging, they are exposed to gases from the atmosphere
- Adsorbed gases prevent gold from fusing
- Thus it is necessary to heat the foil or pellet immediately before it is carried into a prepared cavity
- This process is called annealing, degassing, or desorbing
- It can be done by one of the two methods
- In bulk on a tray by gas flame or electricity
- Piece by piece in a well-adjusted alcohol flame
- Electric annealing
- Annealer is maintained at a temperature between 340° C and 370°C
- The time required varies from 5-20 min
- Problems associated with it are
- Adhesion of pellets
- Air currents that affect heating uniformity
- Heating an excessive amount of gold
- Excessive sintering
- Greater exposure to contamination
- Size selection is limited
- Flame desorption

- Fuel for the flame may be alcohol or gas but alcohol is preferred
- Degassing Advantages:
- Selection of piece of appropriate size
- Desorption of only those pieces used
- Reduced exposure to contamination
- Less danger of overwintering
- Underheating leads to
- Does not adequately remove impurities
- Incomplete cohesion
- The remaining impurities and carbon are deposited by the flam
- Causes pitting and flaking of the surface
- Overheating leads to
- Excessive sintering
- Contamination from the tray, instrument, or flame
- Incomplete cohesion
- Embrittleness of the portion being heated
- Poor compaction
Direct Filling Gold Restorations
Question 2. Explain Direct-filling gold’s physical properties, manipulation, and advantages.
Answer:
Direct Filling Gold’s Physical Properties:
- Direct Filling Gold is the noblest of all dental metals
- Direct Filling Gold’s rarely tarnish or corrode
- Direct Filling Gold’s is inactive chemically
- Direct Filling Gold’s is not affected by air, heat, moisture, or most solvent
- Direct Filling Gold’s is the most ductile and malleable
- Strength
- Greater strength is in the most dense area and the weakest part is the porous area
- Hardness
- Pure gold is extremely soft but after cold working its hardness increases
- Density-19.3 g/cm3
- Biocompatibility
- The pulpal response is minimal if compacted well
Direct Filling Gold’s Manipulation:
- Direct Filling Gold’s involves three processes
- Desorbing
- With the exception of noncohesive golds, the direct filling gold is available 1 cohesive forms
- During storage and packaging, they are exposed to gases from the atmosphere
- Adsorbed gases prevent gold from fusing
- Thus it is necessary to heat the foil or pellet immediately before it is carried into a prepared cavity
- This process is called annealing, degassing, or desorbing
- It can be done by one of the two methods
- In bulk on a tray by gas flame or electricity
- Piece by piece in a well-adjusted alcohol flame
- Compaction

- Finishing
- A small amount of excess material is provided to ensure proper contour
- Excess material is trimmed away with sharp gold knives and files
- The surface is burnished with a ball burnisher to strain and harden the surface
- The final polish is achieved with Soflex disks
Direct Filling Gold’s Advantages:
- Direct Filling Gold’s is the noblest of all dental metals
- Direct Filling Gold’s rarely tarnish or corrode
- Direct Filling Gold’s is the most ductile and malleable
- Direct Filling Gold’s is biocompatible
Direct Filling Gold Short Essays
Question 1. Cohesive and noncohesive gold
Answer:
Cohesive Gold:
- For cold welding, gold should have a clean surface free from impurities
- Gold attracts gases to its surface and any adsorbed gas film prevents the intimate atomic contact required for cold welding
- This prevents the cohesion of individual increments of gold during their compaction
- Therefore manufacturers supply the foil essentially free of surface contaminants
- This type of gold is called cohesive gold
Non Cohesive Gold
- Most gold sheets are provided with absorbed protective gas film such as ammonia
- This substance minimizes the adsorption of other less volatile substances
- It prevents premature cohesion of sheets or segments of sheets that may come into contact
- The ammonia-treated foil is called noncohesive foil
- This volatile film is readily removed by heating to restore cohesive character
- Other agents used are
- Iron salts
- Acidic gas
- Use:
- To build up the bulk of the direct gold restoration
Gold Restorations In Dentistry
Question 2. Types of gold alloy
Answer:
Types Of Gold Alloy
- Various Types of Direct Filling Gold Available are
- Foil
- Sheet
- Cohesive
- Non-cohesive
- Ropes
- Cylinders
- Laminated foil
- Platinized foil
- Electrolytic precipitate
- Mat gold
- Mat foil
- Gold-calcium alloy
- Granulated or powdered gold
Question 3. Mat gold
Answer:
Mat gold
- Mat gold is an electrolytically precipitated crystalline form that is sandwiched between sheets of gold foil and formed into strips which can be cut by the dentist into the desired size
- This form is often preferred for its ease in building up the internal bulk of the restoration because it can be more easily compacted within and adapted to the retentive portions of the prepared cavity
- Because it is loosely packed, it is friable and contains numerous void spaces between particles
- Therefore it is generally not recommended for the external surface of the restoration
- The loosely packed crystalline form of the mat powder with its large surface area does not permit easy welding into a solid mass
- Therefore there is a greater tendency for voids that may be seen as pits to form if mat gold is used on the surface of the restoration
Advantages Of Gold Fillings
Direct Filling Gold Short Question And Answers
Question 1. Cohesive and noncohesive gold
Answer:
Cohesive And Noncohesive Gold
- Manufacturers supply the foil essentially free of surface contaminants
- This type of gold is called cohesive gold
- Most gold sheets are provided with absorbed protective gas film such as ammonia
- This substance minimizes the adsorption of other less volatile substances
- It prevents premature cohesion of sheets or segments of sheets that may come into contact
- This ammonia-treated foil is called noncohesive foil
Question 2. Gold foil
Answer:
Gold Foil
Gold Foil is the oldest of all products
Gold Foil Manufacturer:
A cast ingot of 15 mm thickness is beaten to a submit- microscopic thickness of 15-25 pm
Gold Foil Supplied As:
- Sheets
- Pellets
- Cylinders
- Ropes
- Partially condensed
- Laminates
Gold Foil Numbering:
It reflects the weight of the standard sheet as well as the thickness
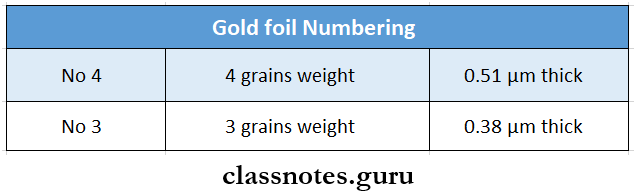
Gold foil Uses:
- A number of sheets are placed over one another to form laminated gold foil
- No.3 is used in the electrolytic and powder products
Properties Of Gold As A Dental Material
Question 3. Degassing
Answer.
Degassing
- With the exception of noncohesive golds, the direct filling gold is available 1 cohesive forms
- During storage and packaging, they are exposed to gases from the atmosphere
- Adsorbed gases prevent gold from fusing
- Thus it is necessary to heat the foil or pellet immediately before it is carried into a prepared cavity
- This process is called annealing, degassing, or desorbing
- It can be done by one of the two methods
- In bulk on a tray by gas flame or electricity
- Piece by piece in a well-adjusted alcohol flame
Question 4. Mat gold, electrolytic gold
Answer:
Mat gold, electrolytic gold
- Mat gold, electrolytic gold is a form of direct-filling gold formed by electrolytic precipitation
- Mat gold, electrolytic gold is crystalline
- Mat gold, electrolytic gold is formed in strips
- These strips are cut by the dentist into the desired size
- Mat gold is preferred because it is easy to build up the internal bulk of the restoration
Direct Vs Indirect Gold Restorations
Question 5. Karat and fineness
Answer:
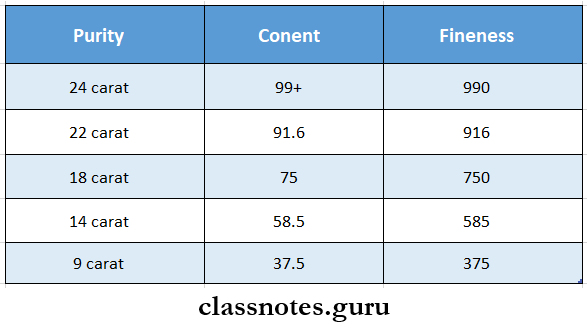
Karat And Fineness
- The purity of gold is expressed in carats often abbreviated as ct or K
- Pure gold has a purity of 24 carats
- An alternative method of expressing purity is fineness
- This expresses the purity in parts per 1000
- Relationship between gold content carat and fineness
- Gold is too soft to be used for jewelry
- So it may be alloyed with other metals such as silver, copper, zinc, or silicon
- This produces purities of less than 24 carats
