Class 11 Chemistry Hydrocarbons Aliphatic Hydrocarbons Alkanes
Open-chain saturated hydrocarbons are referred to as alkanes. At ordinary temperature and pressure, they generally do not show any affinity towards most of the reagents such as acids, bases, oxidizing, and reducing agents and because of this inertness, they are called paraffin (Latin: param = litde, affinis= affinity).
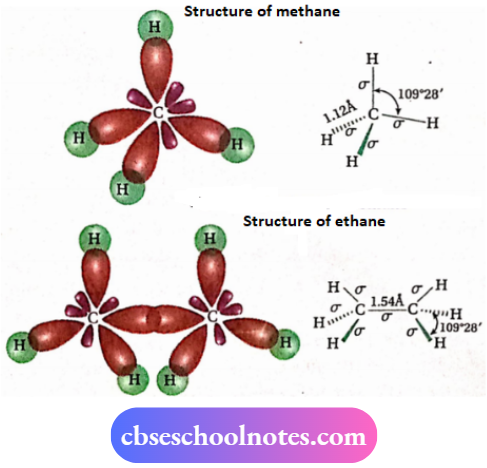
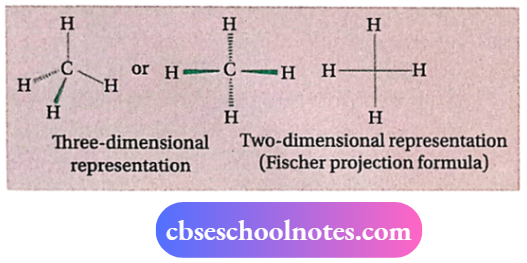
⇒ Each C-atom present in an alkane molecule is sp³ -hybridized. Four σ -bonds formed by each sp³ -hybridized carbon are directed towards the comers of a regular tetrahedron.
Read and Learn More CBSE Class 11 Chemistry Notes
⇒ Thus, alkanes have a tetrahedral structure around each carbon atom. The molecular formula of alkanes is CnH2n + 2 [where n = 1, 2, ].
⇒ Their general formula is RH (R: alkyl group).
Aliphatic Hydrocarbons Alkanes Class 11 Notes
1. Nomenclature of alkanes
The nomenclature of alkanes according to the IUPAC system has been thoroughly Here, only the trivial names of the isomers of butane and pentane and the IUPAC names of some higher alkanes are mentioned
2. Structure of alkanes
Alkanes contain only carbon-carbon and carbon-hydrogen single bonds. They have the following structural
characteristics:
- Each C-atom is sp³ -hybridized. Four sp³ -hybrid orbitals are directed towards the comers of a regular tetrahedron. The carbon atom lies at the center of the tetrahedron.
- All C—C and C—H bonds are strong sigma bonds. Each C —C cr -bond is formed as a result of the axial overlapping of two sp³ orbitals, one from each carbon atom, and each C—H bond is formed by the axial overlapping of one sp³ orbital of carbon with the s -orbital of hydrogen.
- C—C and C—H bond lengths are 1.54A & 1.12A respectively. lv] All bond angles in alkanes (C —C —C, C —C —H, and H—C—H) have a value of 109°28′. Thus, alkanes possess tetrahedral structure
Alkanes in Organic Chemistry Class 11 Notes
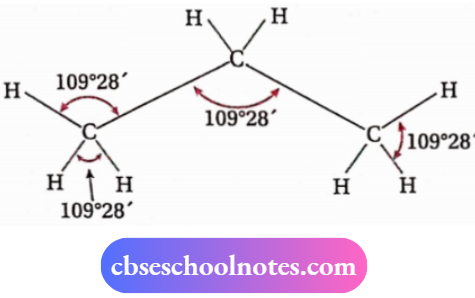
- Carbon atoms in an alkane molecule having three or more carbon atoms do not lie along a straight line. Instead, they form a zig-zag pattern. This is because each carbon atom is sp³ -hybridized and naturally the C—C— C bond angle is 109°28′ instead of 180°. It becomes clear from the structure of propane
- C—C and C—H bond dissociation enthalpies are 83kcal -mol-1 and 99 kcal-mol-1 respectively
3. Structural isomerism in alkanes
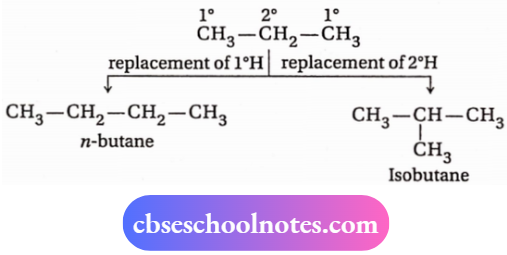
Alkanes (except methane, ethane, and propane) exhibit chain isomerism, a type of structural isomerism. This type of isomerism arises due to the difference like the carbon chain or the skeleton of the carbon atoms.
Example:
1. The two chain isomers having molecular formula (C4H10) are n-butane and isobutane. If a 1° or 2° H atom of a propane molecule is replaced by a methyl group, then these two isomers are formed.
NCERT Class 11 Chemistry Alkanes Notes
Example:
2. Three chain isomers of molecular formula C5H12 are n -pentane (CH3CH2CH2CH2CH3), isopentane [(CH3)2CHCH2CH3] and neopentane [(CH3)4C].
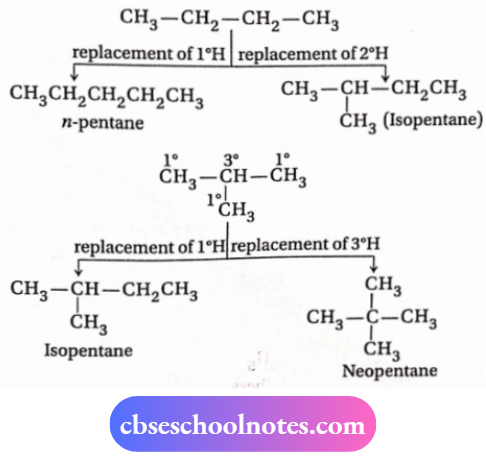
3. These isomers are formed on the replacement of different H -atoms of n-butane and isobutane by methyl group
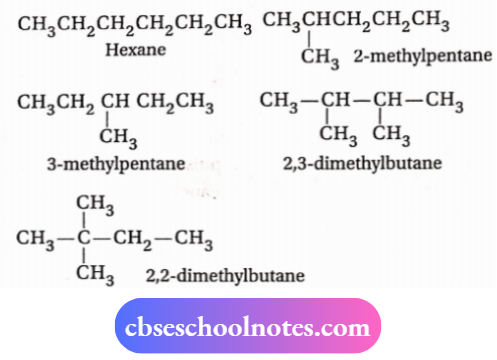
4 . Five chain isomers have molecular formula C6H14 and these by obtained by replacement of different types of H-atoms of n-pentane, isopentane, and neopentane by a methyl group.
These are as follows:
Alkanes Properties and Reactions Class 11 Notes
4. Conformational isomerism in alkanes
Conformational isomerism definition:
Electronic distribution of the sigma molecular orbital of a C—C bond is cylindrically symmetrical around the internuclear axis and as this is not disturbed due to rotation about its axis, free rotation about the C—C single bond is possible. An infinite number of spatial arrangements of atoms that result through rotation about a single bond are called conformations or conformational isomers or rotational isomers or simply conformers or rotamers and the phenomenon is called conformational isomerism.
The difference in potential energy between the most stable conformation and the conformation under consideration is called the conformational energy of the given conformation.It is to be noted that the rotation around a C—C single bond is not completely free.
It is hindered by a very small energy barrier of 1-20kl-mol-1due to very weak repulsive interaction between the electron clouds of different σ -bonds. Such repulsive interaction is called torsional strain.
Conformations are three-dimensional.
These are generally represented in paper by three projection formulae:
Flying wedge formula, sawhorse projection formula and Newman projection formula.
Conformations of ethane:
A molecule of ethane (CH3—CH3) contains a carbon-carbon single bond (σ -bond) and each carbon atom is attached to three hydrogen atoms. The two —CH3 groups can rotate freely around the C—C bond axis.
Rotation of one carbon atom keeping the other fixed results into an infinite number of spatial arrangements of hydrogen atoms attached to the rotating carbon atom concerning the hydrogen atoms attached to a fixed carbon atom.
- These are called conformational isomers or conformations or conformers.
- Thus, there are an infinite number of conformations of ethane. However, there are two extreme cases. The conformation in which the hydrogen atoms attached to two carbons are as close together as possible.
- In which the dihedral angle between the two nearest C —H bonds of two — CH3 groups is zero, is called the eclipsed conformation.
- The conformation in which the hydrogen atoms are as far apart as possible, i.e., the dihedral angle between two C —H bonds is 60° is called the staggered conformation.
- The eclipsed conformation suffers from maximum torsional strain whereas in staggered conformation this strain is minimal.
- So, the eclipsed conformation is much less stable than the staggered conformation.
- Any other intermediate conformation i.e., the conformation in which the dihedral angle is between 0-60°, is called the skew conformation.
Aliphatic Hydrocarbons Alkanes Definition and Examples Class 11
Its stability is in between the two extreme conformations. Therefore, the order of stability of these three conformations is:
Staggered > skew > eclipsed.
It is to be noted that in all these conformations, the bond angles and the bond lengths remain the same.
Saturated hydrocarbons containing more than two carbon atoms have different conformations. However, as there is only one carbon atom in methane, it does not exist in the above-mentioned conformations. The eclipsed and the staggered conformations of ethane can be represented by the flying wedge formula, sawhorse projection formula and
Newman projection formula is as follows:
1. Flying wedge formula:
In this representation, the two bonds attached to a carbon atom are shown in the plane of the paper and of the other two, one is shown above the plane and another below the plane. The bonds that are in the plane are shown by normal lines (—) but the bond
Above the plane is shown by a solid wedge ( —) bond below the plane is shown by a hashed wedge
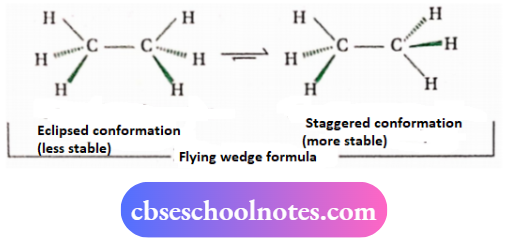
2. Sawhorse projection formula:
In this projection, the molecule is viewed along the molecular axis. It is then projected on paper by drawing the central C —C bond as a somewhat elongated line. The upper end of the line is slightly tilted towards the righthand side. The front carbon is shown at the lower end of the line, whereas the rear carbon is shown at the upper end.
Each carbon has three lines attached to it corresponding to three H -atoms. The lines are inclined at a 120° angle to each other.
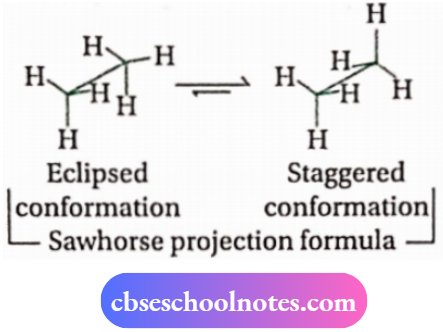
3. Newman projection formula:
In this projection, the molecule is viewed along the C —C bond. The C-atom nearer to the eye of the viewer (i.e., the front carbon) is represented by a point and the three H-atoms attached to the front C-atom are shown by the three lines drawn at an angle of 120° to each other. The C-atom situated farther from the eye of the viewer (i.e., the rear carbon) is represented by a circle, and the three hydrogen atoms attached to it are represented by three shorter lines drawn at an angle of 120° to each other.
Eclipsed and staggered conformations of ethane in I H H terms of the
Newman projection formula (along with dihedral angles, ) are shown below:
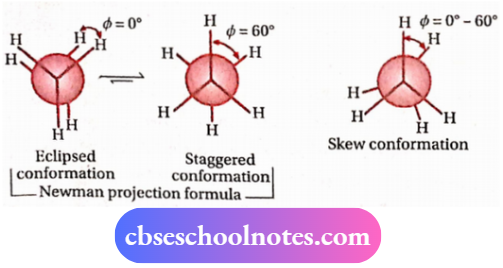
CBSE Class 11 Chemistry Alkanes Chemical Reactions
The energy barrier between two extreme conformations is tiny and so, the rotation of two —CH3 groups takes place extremely rapidly. Due to this, it is not possible to separate the conformations of ethane. However, at any moment, the majority of ethane molecules exist in the staggered conformation of minimum energy {i.e., maximum stability)
The eclipsed conformation is least stable because hydrogens and bonding pairs of electrons eclipsed C —H bonds involving adjacent C-atoms are very close to each other causing maximum repulsion. The staggered conformation is most stable because the hydrogens and bonding pairs of electrons of each pair of C —H bonds involving adjacent C-atoms are at a maximum distance. This causes minimum electronic as well as steric repulsion
The potential energy of the molecule is minimal for staggered conformation. It increases with rotation and reaches a maximum at eclipsed conformation. Experimentally, it has been found that the staggered conformation of ethane is 2.8 kcal-mol-1 more stable than the eclipsed conformation. (Eeclipsed eclipsed – Estaggered = 2.8 kcal-mol-1 ).
Therefore, rotation about C—C bond is not completely free. However, this energy barrier is not large enough to prevent rotation at room temperature as collisions between the molecules supply sufficient kinetic energy to overcome this energy barrier
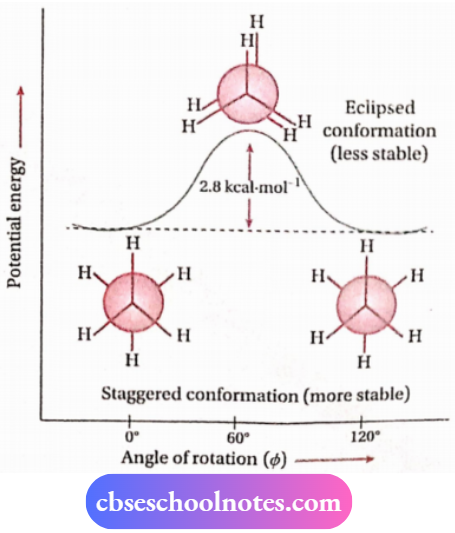
Dihedral angle:
The dihedral angle (Φ) is the angle between the X—C—C and the C—C—Y plane of X—C—C—Y unit in a molecule. In ethane, it is the
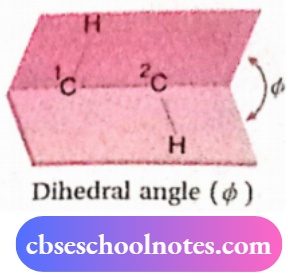
The angle between the H—1C— 2C plane and 1C—2C—H plane, 1 2 i.e., it is the angle between the 2C—H bond and the C—H bond in the Newman projection formula. It is also called the angle of torsion.
Conformations of propane (1CH3– 2CH2–3CH3):
In propane molecules, both C1—C2 & C2—C3 bonds are equivalent. An infinite number of conformations of propane can be obtained as a result of rotation about the C1—C2 (or C2—C3) bond. The two extreme conformations are the eclipsed conformation (I) and the staggered conformation
The staggered conformation is more stable than the eclipsed conformation by 3.4 kcal-mol-1
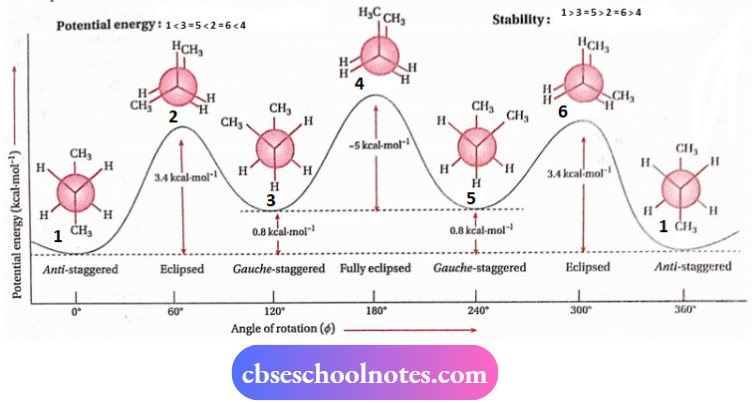
Conformations of n-butane (CH3-CH2-CH2-CH3):
n-butane contains two kinds of C —C bonds. So, conformations likely to be generated depend on that particular C —C bond around which C-atoms are made to rotate
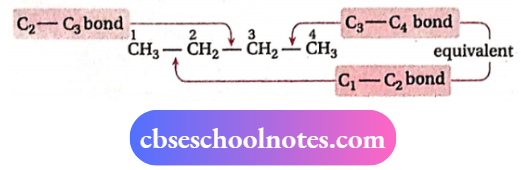
1. Rotation about the C1—C2 bond:
Keeping C1 fixed, when C2 is rotated around the C1—C2 bond axis, infinite numbers of conformations are obtained. Among these, twoprincipal conformations are eclipsed (I) and staggered (II) conformations.
Their order of stability is:
Staggered > eclipsed, i.e., molecules of n-butane spend most of their time in staggered conformation (II).

2. Rotation about the C2-C3 bond:
An infinite number of conformations are possible if C3 is made to rotate around C2 — C3 bond axis, keeping C2 fixed.
Alkanes Preparation and Properties Class 11
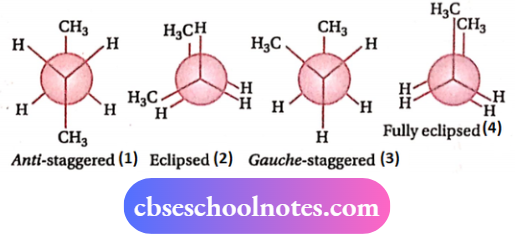
Among these, the four chief conformations are:
- Anti-staggered (1)
- Gauchestaggered (3)
- Eclipsed (2)
- Fully eclipsed (4).
In anti-staggered conformation, the two —CH3 groups exist anti to each other, i.e., they are oriented at an angle of 180° (Φ = 180°). In the gauche-staggered conformation, the two —CH3 groups make an angle of 60° with each other (Φ = 60°). In the eclipsed conformation, the two pairs of —CH3 and H and one pair of H -atoms are in direct opposition, while in the fully eclipsed conformation, the two pairs of H-atoms and one pair of CH3 groups are in direct opposition.
The order of their stability:
1 >3 > 2 > 4, i.e., the molecules of n-butane pass most of their time in anti-staggered conformation (I).
Their Newman projection formulae are shown below:
Alkanes and Their Types in Organic Chemistry Class 11
The most stable and least stable conformations of n-butane are anti-staggered and fully eclipsed conformations respectively. The angular distance between two similar bonds in the anti-staggered conformation is maximum (180°). Thus, repulsion between electrons of such a bond pair is minimal.
Again, two —CH3 groups are located farthest from each other so, no steric hindrance or steric strain acts between them. On the other hand, the angular distance between two similar bonds in the fully eclipsed conformation is minimum (0°). Thus, the repulsion between electrons of each bond pair is maximum.
Again, two —CH3 groups are in direct opposition and hence there occurs severe steric strain involving these two CH3 groups. For this reason, anti-staggered conformation is the most stable while fully eclipsed conformation is the least stable conformation of n-butane
The potential energy changes during rotation about the C2—C3 bond of n-butane is shown in the following diagram