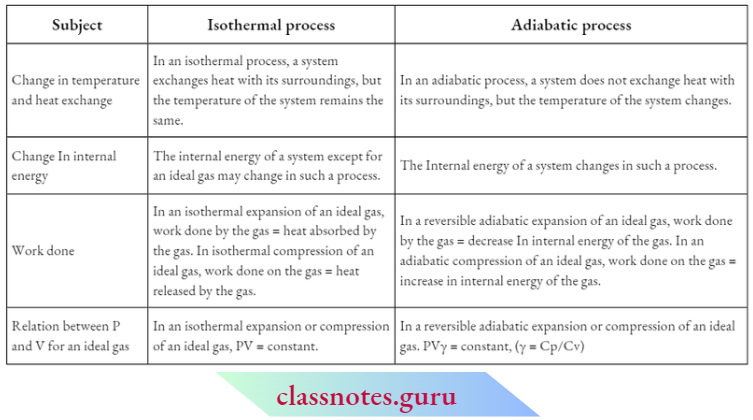
Thermodynamic Process
Thermodynamic Process Definition: A system is said to undergo a thermodynamic process when it goes from one equilibrium state to another.
Path Of A process: The sequence of states through which a system passes when it undergoes a process is called the path of the process.
A system can undergo a change from an equilibrium state to another through a variety of processes or paths. In most of these processes, one or more of the properties of the system are held constant. Some of the common thermodynamic processes encountered in chemical thermodynamics arc are discussed below.
Read and Learn More CBSE Class 11 Chemistry Notes
Cyclic process
Cyclic Process Definition: A system is said to have undergone a cyclic process if it returns to Its initial state after a series of successive.
Cyclic Process Explanation: Let us consider a process, in which the initial state of a gaseous system is A (Pv Tx). The system returns to its initial state after undergoing consecutive processes IB, BC, and CA. Since the system returns to its initial state through three successive operations, it is a cyclic process.
Cyclic Process Example: The following change indicates a cyclic process because 1 mol of water (system) returns to its initial state again through successive changes
Thermodynamic Processes and Their Types Class 11 Chemistry Notes
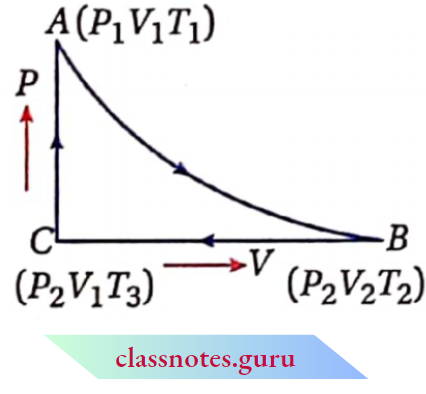
The changes in state functions are zero in a cyclic process. The value of a state function depends upon the present state of the system. Since the initial and the final states of the system are the same in a cyclic process, the rallies of the state functions in these two states are also the same. So die change in state function (AP, AV, A7′, A77.A/7, etc.) becomes zero for a cyclic process.
Isothermal Process
Isothermal Process Definition: If the temperature of a thermodynamic system remains constant throughout a process, then the process Is said to be an Isothermal process.
At the time of conducting this process, the system Is kept lit contact with Constant temperature heat (f.u, thermostat) with a high heat capacity. Such a heat hath Is capable of gaining or losing heat without changing Its temperature.
Condition(s) For The Isothermal Process: During an Isothermal process, the temperature of the system remains constant. So we can write, 7′[system] = constant and dV[system] or AT [system] = 0.
Condition(s) For The Isothermal Process Example: The boiling of a liquid at its boiling point Is an isothermal process. This Is because the temperature of the liquid remains constant until the entire liquid converts to vapor. Thus, the boiling of water at 100°C and l atm is an isothermal process
The temperature of the system remains fixed in the isothermal process. U does not mean that heat is not absorbed or liberated by the system during this process.
CBSE Class 11 Thermodynamic Processes and Their Types Notes
Isobaric Process
Isobaric Process Definition: A process in which the pressure of the system remains fixed at each step of the process is called an isobaric process.
Condition(s) During Constant For This And Isobaric Process [System] Process: remains APAs [system]theconstant,pressure= Pof. [system]the system=
Condition(s) During Constant For This And Isobaric Process [System] Process Example: The vaporization of any liquid in an open container occurs under atmospheric pressure. If the atmospheric pressure remains fixed, then the process of vaporization is said to be an isobaric process.
Isochoric Process
Isochoric Process Definition: A process in Which The Volume Of The System remains constant throughout the process is called an isochoric process
Condition(s) For An Isochoric Process: The volume of the system remains constant during this process. Hence, V[system] = constant and dV[system] or ΔV [system] =0.
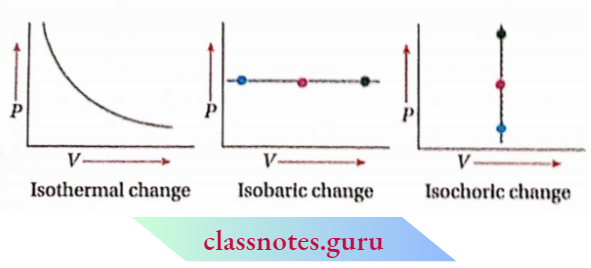
Condition(s) For An Isochoric Process Example: The combustion of a substance In a bomb calorimeter. For a closed system consisting of an ideal gas. the plots of p vs V for O Isothermal Isobarle and Isochoric changes are given below.
Class 11 Chemistry Thermodynamic Processes Types Explanation
Adiabatic Process
Adiabatic Process Definition: A process in which no exchange of heat takes place between the system and Its surroundings at any stage of the process is called an adiabatic process. This process requires the system to be covered with a perfect thermal insulator. But in reality, no such material is available and hence the process never becomes one hundred percent adiabatic.
Condition(s) For The Adiabatic Process: No heat is exchanged between a system and its surroundings In an adiabatic process. So, for such type of process q – 0; where q = heat absorbed or released by the system.
Condition(s) For The Adiabatic Process Example: The sudden expansion or compression of a gas is considered to be an adiabatic process because when a gas (system) undergoes such a process, it does not get a chance to exchange heat with its surroundings.
As a result, the sudden compression of a gas results in an increase in the temperature of the gas, while its sudden expansion leads to a decrease in temperature. For example, when the valve of a bicycle or car tire is removed, the air coming out of the tire undergoes very fast expansion and gets cold.
The temperature of a system does not remain constant in an adiabatic process (except in the case of an adiabatic expansion of an Ideal gas against zero pressure).
For a process involving more than one step, the algebraic sum of heat absorbed or released in different steps may be zero qx + q2 + q3 + … = o, but it does not mean that the process is an adiabatic one.
Thermodynamic Processes Types Notes Class 11 Chemistry
Reversible Process
Reversible Process Definition: A process It salt to be reversible If It Is carried out Infinitesimally slowly so that In each step the thermodynamic equilibrium of the system Is maintained, and any Infinitesimal change In conditions can reverse the process to restore both the system and Its surroundings lo their Initial states.
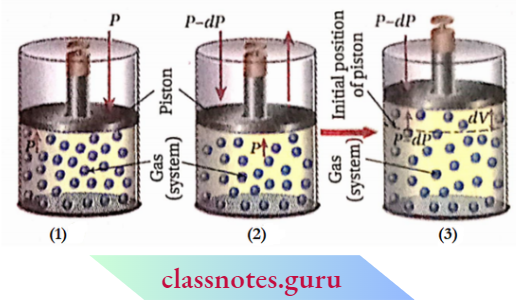
Reversible Process Explanation: Lot ns consider that gas (system) Is enclosed lit a cylinder lilted with a weightless and frictionless piston and the cylinder Is kept In a constant temperature hath (surroundings). So, any change occurring In the lire system would be Isothermal.
Since the temperature of the system and surroundings tiro the same, the system will remain In thermal equilibrium. Let the applied external pressure acting on the piston Is the same as that of the gas. So, the system will remain In mechanical equilibrium. Hence, In this condition, the system (the gas) Is In a state of thermodynamic equilibrium and the properties of the system remain unchanged.
CBSE Class 11 Thermodynamic Processes NCERT Notes
Now, if the external pressure is decreased by an infinitesimal amount of dP, the volume of the gas will increase very slowly by an infinitesimal amount until the pressure of the gas becomes equal to that of the external pressure. Let the infinitesimal increase of volume = dV.
If the external pressure is further decreased by an Infinitesimal amount of dP, the volume of the gas will also increase by an infinitesimal amount of dV. In this way, if the gas is allowed to expand very slowly in an infinite number of steps by decreasing an infinitesimal amount (dP) of the external pressure at each step, then the expansion of the gas will reversibly take place.
In a step, if the external pressure is increased by an infinitesimal amount (dP), then the volume of the gas will decrease by an infinitesimal amount dV. Hence, by decreasing or increasing the external pressure by an infinitesimal amount, the direction of the process can be reversed.
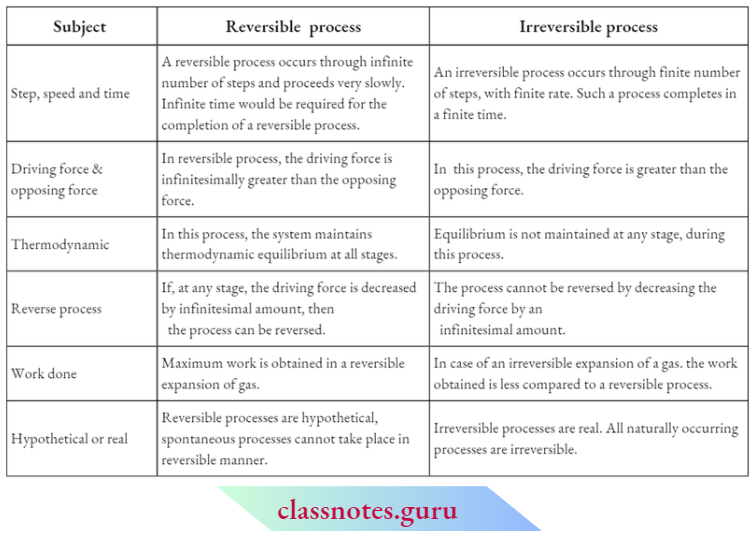
Characteristics Of A Reversible Process:
The driving force of a reversible process is infinitesimally greater than the opposing force and by increasing or decreasing the driving force by an infinitesimal amount, the direction of the process can be changed.
In this process, the system remains in thermodynamic equilibrium at every intermediate step. This process is extremely slow. From the theoretical point of view, any reversible process requires infinite time for its completion.
After the completion of a reversible process, if the system is made to return to its initial state by traversing the forward sequence of steps in the reverse order, then both the system and its surroundings are restored to their initial states.
The work done by a system in a reversible process is always the maximum. The reversible process is extremely slow and infinite time is required to complete the process.
A true reversible process is a hypothetical concept. In practice, no process can be carried out reversibly. All processes occurring in nature (i.e., real processes) are irreversible. Nevertheless, the concept of reversibility has immense importance in thermodynamics
Thermodynamic Processes and Their Types NCERT Solutions
Examples Of Some Reversible pProcesses:
The vaporization of a liquid at a particular temperature in a closed container can be considered a reversible process. Let us consider a certain amount of water is in equilibrium with its vapor at T K temperature in a closed container. Here water and water vapour together constitute a system.
If the temperature of the system is increased by an infinitesimal amount of dT, then a very small amount of water willvaporise, and a new equilibrium will be established. Consequently, the vapor pressure of water will be increased by an infinitesimal amount of DP.
If the temperature of the system is decreased by an infinitesimal amount of dT, the same amount of water vapor will condense to establish equilibrium. As a result, the vapor pressure of water is also decreased by an infinitesimal amount of DP.
So, by increasing or decreasing the driving force (i.e., by increasing or decreasing temperature) the direction of the process can reversed. Thus, the vaporization of a liquid at a particular temperature in a closed vessel approximates a reversible process.
The reaction occurring in a galvanic cell is reversible. If an external potential applied between the two electrodes is slightly less in magnitude but opposite in sign than the electromotive force (EMF) of the cell, then the direction of flow of the current and the cell reaction remains unaltered.
But, if the externally applied potential slightly exceeds the EMF of the cell in magnitude, then the direction of the cell reaction and the direction of the current are found to be reversed. Therefore, by slightly increasing or decreasing the external potential (for EMF of the cell), the direction of the cell reaction can be changed. Thus, the reaction occurring in a galvanic cell approximates a reversible process.
Irreversible Process
Process which occurs at a finite rate finite changes in properties of the system, and at any stage during the process, the system cannot get a chance to remain in thermodynamic equilibrium is called an irreversible process
All Natural Processes Are Irreversible
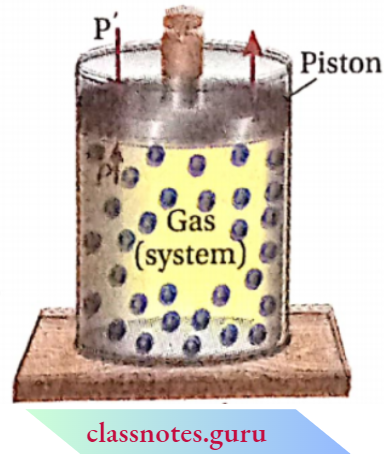
All Natural Processes Are Irreversible Explanation: Let us consider that a certain amount of gas is enclosed in a cylinder fitted with a weightless and frictionless piston and the cylinder is kept in a constant temperature heat bath (thermostat). Therefore, the temperature of the system (gas) becomes equal to that of the 5(system) surroundings (thermostat).
Let the external pressure applied on the piston (P) and Urnmibk the pressure of the gas be the same. Now, if the external pressure is suddenly reduced to P’, then the gas will expand at a finite rate and this will be irreversible because during expansion the system does not maintain thermodynamic equilibrium.
Characteristics Of An Irreversible Process
- In an irreversible process, there is an appreciable difference between the driving force and the opposing force (actually, the driving force is greater than the opposing force). As a result, such a process takes place at a finite rate, although sometimes a very slow process may also be irreversible.
- In an irreversible process, the system does not remain in thermodynamic equilibrium at any stage during the process.
- If an irreversible process is reversed and the system is made to go back to its initial state, then the work done in the backward direction will not be the same as that in the forward direction.
CBSE Class 11 Chemistry Types of Thermodynamic Processes
After The Completion Of An Irreversible Process, Although The System Can Be Brought Back To Its Initial State, Its Surroundings Cannot Be.
- Irreversible processes complete in a finite time
- The flow of heat from an object of higher temperature to an object of lower temperature.
- The downward flow of water from a mountain.
- The expansion of a gas against zero pressure.
- The formation of curd from milk
NCERT Class 11 Chemistry Notes Thermodynamic Processes and Types