Quantum Number
Quantum Number Definition: A set of four numbers that provide complete information about any electron in an atom are known as quantum numbers.
Quantum Number Classification: The four quantum numbers are
- Principal Quantum Number (N)
- Azimuthal Or Subsidiary quantum number (l)
- Magnetic quantum number (m or m1)
- Spin quantum number (5 or mg ).
- To specify an electron in an atom, the following four quantum numbers should be mentioned.
- Principal quantum number [n]
Quantum Number Origin:
- From Bohr’s postulates, it is known that each electronic orbit surrounding the nucleus in an atom represents an energy level.
- The average energy of the electrons revolving in a particular orbit is fixed. So, these orbits are called principal energy levels or principal quantum levels.
- Depending on their distance from the nucleus, these orbits or principal energy levels are designated by the numbers 1,2,3, 4… etc. These numbers 1,2,3,4… etc. are called principal quantum numbers.
Quantum Number Designation: The principal quantum number is denoted by the letter ‘n ’. For AT-shell n = 1, for L -shell n = 2, for Mshell n = 3 and so on.
Read and Learn More CBSE Class 11 Chemistry Notes
Information Oobtained:
- The higher the value of n, the greater the distance of the orbit from the nucleus, and hence, the greater the size ofthe orbit. Thus, r1<r2<r3< r4< …
- The higher the value of ‘ n,’ the greater will be the electronic energy associated with the orbit.
- Thus, El<E2<E3<E4<………..
- A maximum number of electrons that can be accommodated in a principal quantum level n is given by the formula 2n2.
- Limitations of 2β2 The maximum number of electrons in any orbit can never be more than 32 even if the value of n exceeds 4.
- The outermost electronic shell does not contain more than 8 electrons.
- The penultimate shell (i,e., the shell just preceding the outermost shell) does not contain more than 18 electrons.
Quantum Numbers Class 11 CBSE Notes
Azimuthal Or Subsidiary Quantum Number
Azimuthal Or Subsidiary Quantum Number Origin: A spectrograph with high resolving [/]power has revealed that each bright line in the spectrum of atomic hydrogen consists of some closely spaced finer lines.
This fact suggests that each orbit or energy level in an atom is composed of subshells. Electrons occupying these subshells within the same -shell, exhibit slight differences in energy.
In order to explain the formation of the fine structure of spectral lines, Sommerfeld proposed the existence of elliptical orbits, besides Bohr’s circular orbits.
To specify the shape of the elliptical orbit, another supplementary quantum number is necessary.
This supplementary quantum number which indicates the captivity of the electronic orbit is called azimuthal or subsidiary quantum number denoted by the letter.
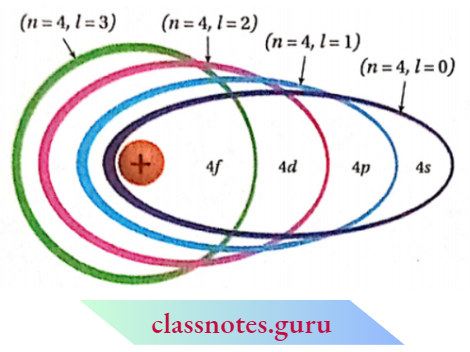
If the principal quantum number of any orbit is n, then the total number of subshells incorporated in that orbit will also be n.
Magnitude:
- As per quantum mechanical calculations, the angular momentum of a moving electron in an elliptical path is given by, L = Jl(l+ 1) X.
- This is often called orbital angular momentum.
- The value of l determines the shape of the path. So, with the help of the principal quantum number and azimuthal quantum number, a precise idea about the size and shape of the electronic path can be obtained.
- If n stands for the principal quantum number of an electronic orbit, the values of l will be from to (n- 1) i.e., with respect to the value of principal quantum number n, the azimuthal quantum number / may assume n number of different values including zero, e.g., for n = 4, 1=0, 1, 2 and 3.
- To indicate the subshells within a shell, spectroscopic symbols are used instead ofthe numbers 0, 1, 2, 3 etc.
- The symbols s, p, d,f, etc., (spectroscopic coinage) are merely the first letters ofthe words sharp, principal, diffuse, and fundamental, used extensively in spectral analysis.
- To express the position of an electron in the atom, the principal quantum number should be written first followed by the symbol of the azimuthal quantum number to its right side, e.g., the subshells included in K, L, M, and N-shells are represented as

- The ratio of the major axis to the minor axis of an elliptical path is given by = (/ + 1)/n .
- An elliptical path for which l = (rc- 1), becomes circular e.g., in the case of 4-orbit if 1 = 3, then that orbit becomes circular. The greater the difference between the values of l and n, the larger the ellipticity of that path.
- The penetrating power and screening effect of an elliptical orbit increases on increasing the ellipticity of the orbit.
- So the penetrating and screening powers of different subshells within the same shell follow the sequence: s> p> d>f.
- Due to the difference in the internal energies of the subshells [s, p, d, f, etc.), the electrons moving in those subshells also possess different energies. Energy associated with the subshells in a particular orbit increases in the following order: s <p<d<f.
Class 11 Chemistry Quantum Numbers Notes
Magnetic Quantum Number (m Or mt)
Magnetic Quantum Number Origin:
- Zeeman in 1896 observed that each fine line in atomic spectra splits further into finer lines in the presence of the highly powerful magnetic field.
- In the absence of a magnetic field, such finer splitting i.e., hyperfine splitting disappears. This phenomenon is called the Zeeman effect. To explain the Zeeman effect, a third type of quantum number, known as a magnetic quantum number was introduced.
Magnetic Quantum Number Discussion:
- Due to the angular motion of electrons around the nucleus, a magnetic field is produced, which interacts with the external magnetic field.
- As a result subshells of definite energy split into three-dimensional spatial regions called orbitals.
- Magnetic quantum number (MI) signifies the orientation of the orbitals in space in which the electron exists.
- The value of m depends on the azimuthal quantum number l.
- For a certain value of l, m has an o total of (2Z +1 ) different values. These values may be any whole number starting from -Z to +1 (including zero).
- For s- subshell, l = 1 and m – 1. This subshell, l = 0 and m – 0. This orbital (i.e., s-orbital). Z = 1 denotes p -subshell consisting of three orbitals which are directed along three axes.
- These are marked as px, py, and pz orbitals which have the respective values of m = -1, 0, and + 1 . Similarly, d and /-subshells contain 5 and 7 orbitals respectively.
- The negative values of the magnetic quantum number signify that these orbitals are inclined in the direction opposite to the magnetic field and the positive values indicate that these orbitals are inclined in the direction of the magnetic field.
- shows the different directions of the d -d-subshell (Z = 2) in the magnetic field.
Orientation Of Different Orbitals Of Zv-Shell (N = 4] Under The Influence Of Magnetic Field.
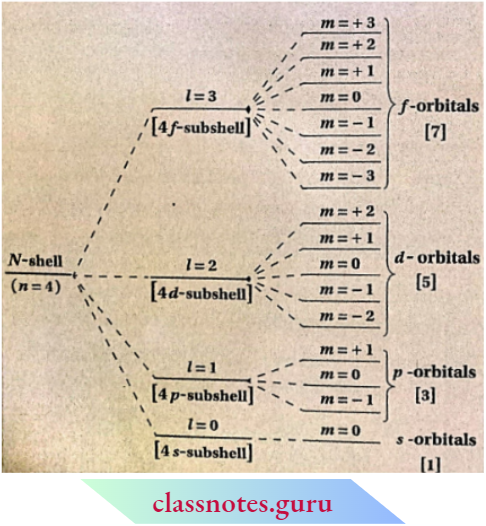
Values Of Magnetic Quantum Number (m] For Different Values Of Azimuthal Quantum Number [l]
Spin Quantum Number [s or ms]
Uhlenbeck and Goudsmit introduced a fourth quantum number called the spin quantum number.
This is because the other three quantum numbers were not able to give sufficient explanation to the hyperfine structure of the atomic spectra.
% Just like the earth, an electron while moving around the nucleus also spins about its own axis either in a clockwise or in an anti-clockwise direction,
Each type of spin can give rise to characteristic spectral lines with the formation of a hyperfine spectrum in the spectral series.
The spin quantum number denoted by the symbol ‘s’ expresses two opposite types of spinning motions of each electron.
The spin quantum number ‘s’ can have only two values, \(+\frac{1}{2} \text { and }-\frac{1}{2}\) The positive and negative signs represent two opposite directions of spinning motion of any spinning motion of any spinning motion of electron are very often represented by two arrows pointing in opposite directions,| and.
Q A spinning electron behaves like a tiny magnet with a definite magnetic moment. The angular mentum associated with the spinning electron is given by the mathematical expression.
Quantum Numbers in Chemistry Class 11 CBSE
Spin Quantum number (s) signifies the mode of Electron Spin (Clockwise or Anti-clockwise).
Shapes Of Orbitals From Wave Function
It has been stated earlier that the three-dimensional space around the nucleus in which the probability of finding an electron is maximum is called an orbital.
In order to obtain a clear idea about the shapes of orbitals, we will first discuss the variation of—
- The radial part of the wave function,
- Square of the radial wave function, and
- Radial distribution function with an increase in distance from the nucleus.
CBSE Class 11 Quantum Numbers Chemistry Notes
Variation Of Radial Part Of Wave Function With Distance From The Nucleus
Schrodinger wave equation for the electron in a one-electron atom (H-atom) can be solved to get different expressions for wave function \((\psi)\) for different orbitals.
The orbital wave function for an electron in an atom has no physical meaning. It is simply a mathematical function of the coordinates of the electron.
However, for different orbitals the plots of the radial part of the corresponding wave functions as a function of r (distance from the nucleus) are different. depicts such plots for Is, 2s, 2p and 3s orbitals.
For is -orbital, the radial part of the wave function [ψ(r) or R] decreases sharply with increasing distance, r, from the dying nucleus.
For 2s -orbital ψ (r) or R, decreases sharply in the beginning, becomes zero at a particular distance, and then becomes negative as r increases.
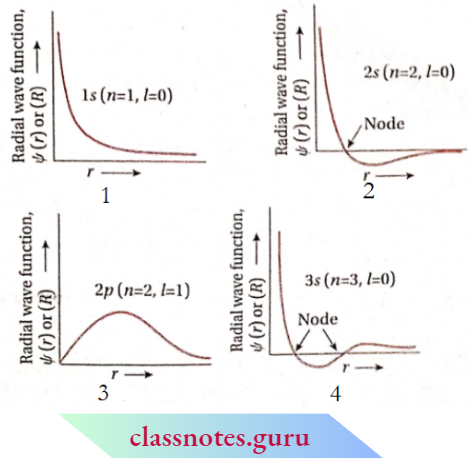
FM 3s-orbilal \(\psi\). decreases sharply in the Beginning with an Increase In r, becomes zero At A Particcular Distance, And Then Becomes negative. On Further Increases In \(r, \psi(r)\) again becomes zero and finally becomes positive.
For 2p -orbital if \(\psi(r)\) rises from zero to a maximum, then decreases with increasing distance (r) from the nucleus. On further increase in distance, ifr(r) approaches almost zero.
For 3p -orbital,\(\psi(r)\) rises from zero and attains a maximum value. On further increase in ψ(r) begins to decrease and becomes zero at a particular distance. Then it becomes negative with a further increase in r.
Quantum Numbers Class 11 NCERT Notes
Characteristic Features Observed In The Plots Of R Vs Ψ(r):
- The radial part of the wave functions for 2s, 3s, 3p, etc. orbitals can be positive or negative depending upon the distance (r) from the nucleus. These are not related to the positive and negative charges.
- For each orbital, the radial part of the wave function Ψ(r) approaches zero as r→∞.
- For 2s, 3s, and 3p -orbitals, one common feature for the variation of wave function Ψ(r)) with distance is that Ψ(r) becomes zero at a finite distance from the nucleus. However, for different orbitals if Ψ(r) becomes zero at different distances Ψ(r).
- The distance Ψ(r) at which becomes zero is called a nodal point radial node or simply node. At the nodal point, the radial wave function if Ψ(r) changes its sign from positive to negative or vice versa.
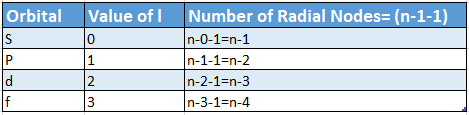
- For different orbitals, the number of radial nodes =(n-1-1).
- This indicates that the number of radial nodes is determined by the values of the principal quantum number ( n) and azimuthal quantum number (Z) of the orbital under consideration.
There is no relation between the positive and negative values of the wave function with the positive and negative charges.
Radial Probability Density [Ψ²(R) Or R²] Graphs Variation Of The Square Of Radical Wave Function With Distance From The Nucleus
The square of the radial wave function, Ψ²(r) or R2 for an orbital gives the radial density.
According to the German physicist, Max Bom, the radial density, Ψ²(r) at a point gives the probability density of the electron at that point along a particular radial line.
The variation of Ψ²(r) as a function of r for different orbitals is given in the figure. The nature of these curves is different for different orbitals.
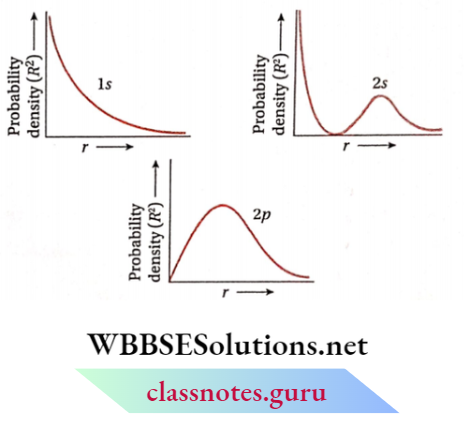
For Is -orbital, probability density is maximum near the nucleus (r≈0) and decreases sharply as we move from it.
For 2s -orbital the probability density is maximum near the nucleus (r≈0).
With increasing distance, Ψ2(r) first decreases sharply to zero and starts increasing again. After reaching a small maxima it decreases again and approaches zero as the value of r increases further.
The intermediate region (a spherical shell) where this probability density reduces to zero is called the nodal surface or simply node.
In general ns -orbital has (n- 1) nodes. Thus, the number of nodes for 2s -orbital is one, two for 3s and so on, i.e., the number of nodes increases with an increase of principal quantum number n.
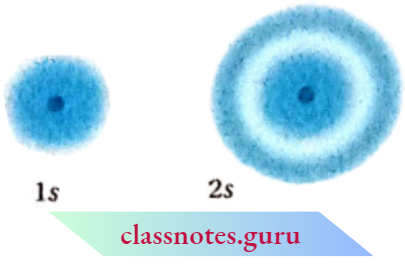
The probability density variation for Is and 2s orbitals can be visualized in terms of charge cloud diagrams. In these diagrams, the density of the dots in a region represents the electron probability density in that region.
For 2p- Orbital Probability Density Is zero at r = 0. However, with increasing distance, it begins to increase and reaches a maximum and then decreases gradually as the distance (r) from the nucleus increases and ultimately approaches zero.
From similar plots of various orbitals, it has been found that all orbitals except s, have zero electron density at r = 0.
Radial probability distribution curve: Variation of radial distribution function (RDF) with distance from the nucleus (r)
The plot of Ψ²(r) versus r gives the probability density for the electron around the nucleus. However, in order to determine the total probability in an infinitesimally small region, we have to multiply probability density if Ψ2(r) by the volume of the region i.e., probability = Ψ²(r) x dv [where dv = volume of the region].
Since the atoms have spherical symmetry, it is more useful to discuss the probability of finding the electron in a spherical shell between the spheres of radii r and (r + dr).
The volume of such a shell of extremely small thickness, dr, is 4nr2dr. So we have, Probability = R2 x 4rrr2dr = 47tr2Ψ²(r)(r)dr [since R = Ψ²(r))].
This gives the probability of finding the electron at a particular distance (r) from the nucleus. This is called radial distribution function (RDF).
Radial distribution function (RDF) = 4πr2ψ2(r)dr
Quantum Numbers in Chemistry Class 11 Explanation
Important information obtained from the plots of RDF vs r:
NCERT Class 11 Chemistry Quantum Numbers Theory
- For all orbitals, the probability is zero at the nucleus.
- If the point r = 0 is neglected, then it can be seen that,
- The number of radial nodes for any orbital -n-l- 1,
- The number of maxima (peak) for any orbital =(n-l- 1) +1 = (n-/). The peak in any curve gives the distance from the nucleus to that point where the probability of finding the electron is maximum. This is called the radius of maximum probability.
- All the s -orbitals, except the first one (Is), have a shell-like structure, rather like an onion, or a hailstone, consisting of concentric layers of electron density. Similarly, all but the first p -p-orbital (2p) and the first dorbital (3d) have shell-like structures.
- The first s -s-orbital (Is), first p -p-p-orbital (2p) and first orbital (3d) have two important characteristics—
- they do not contain radial nodes and contain only one maxima.
- Examination of the plots for Is, 2s, and 3s -orbitals shows that the most probable distance of maximum probability density increases markedly as the principal quantum number increases.
- Furthermore, by comparing the plots for 2s and 2p, or 3s, 3p, and 3d -orbitals it is seen that the most probable radius decreases slightly as the azimuthal quantum number increases.
