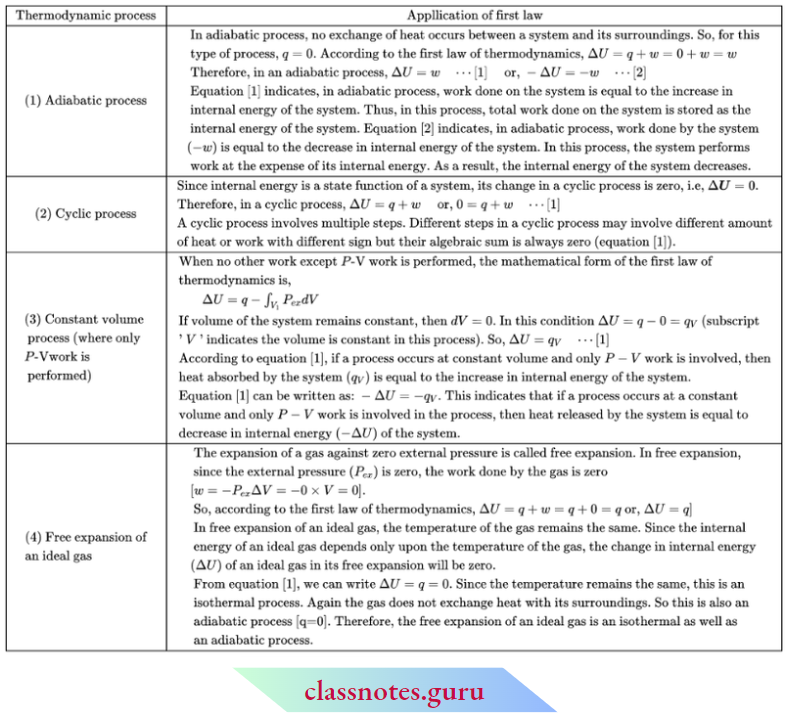
The First Law Of Thermodynamics
The first law of thermodynamics is an extension of the law of conservation of energy. The law of conservation of energy states that energy can neither be created nor destroyed. It can only be converted from one form to another in an equivalent amount.
The First Law Of Thermodynamics Can Be Stated In Different Ways: The total energy of the universe is always constant. or, Energy can neither be created nor be destroyed. It can only be transformed from one form to another in an equivalent quantity.
Whenever a definite quantity of one form of energy disappears, an exactly equivalent amount of another form of energy appears. or, The energy of an isolated system is always constant.
Justification Of The First Law Of Thermodynamics
There is no theoretical proof of the first law of thermodynamics. Human experience of the macroscopic behavior of a large number of systems is indeed the basis of the law. The law, when applied to various fields, is valid.
NCERT Class 11 Chemistry Notes on First Law of Thermodynamics
Read and Learn More CBSE Class 11 Chemistry Notes
Equivalence Of Work And Heat: From different experiments it has been observed that heat and work are always equivalent. Irrespective of the method by which work is performed, a definite quantity of heat is obtained in exchange for a definite quantity of work. Scientist Joule proved that 1 calorie of heat is produced in exchange for 4.1843 J of work.
So 4.184J of work is equivalent to 1 cal of heat. Therefore, creation or destruction of energy is not possible. When one form of energy disappears exactly an equivalent amount of another form ofenergy is obtained.
Perpetual Motion Machine: The impossibility of constructing a perpetual motion machine of the first kind supports the first law A perpetual motion Is a type that can work In a cyclic process and produce work without consuming any energy or producing more work by consuming lost energy.
All efforts In making such machines are In vain. Hence, it can be concluded that energy can neither be created nor be destroyed, but one form of energy can be converted Into an equivalent quantity of another form of energy.
First Law of Thermodynamics Class 11 Chemistry Notes
The Total Energy Of A System Is Constant: Since an Isolated system does not Interact with Its surroundings, it cannot exchange either matter or energy with Its surroundings. This is why the energy of the system always remains constant.
The Mathematical Form Of The First Law Of Thermodynamics
A closed system changes its state if it exchanges heat or work or both with its surroundings. Consequently, the internal energy of the system also changes. Suppose, a closed system has an internal energy of Ux. Let q amount of heat be added to the system.
This increases the internal energy of the system to a value of ( Ux + q). Now, if an amount of work w is done on the system, then the internal energy of the system will further increase and will become (Ul+q + w). Suppose, the internal energy of the system at the final state is U2. So, U2 = Ux + q + w or, U2– t1 = q + w
∴ AU=q+w
[AU = U2-U1 = the change in internal energy of the system] vi i.e., the change in internal energy of a closed system = heat absorbed by the system + work done on the system. The equation is the mathematical formulation of the first law of thermodynamics. Important points regarding the equation, AU = q + wi]
Class 11 Chemistry First Law of Thermodynamics Notes
Unit of AU, q, or w: In this equation, AU, q, and w must be expressed in the same unit such as joule or calorie.
IUPAC sign convention of q and w: w = positive: work is done on the system. w = negative: work is done by the system. q = positive: heat is absorbed by the system. q = negative: heat is lost by the system
Illustration: In a process, If a system absorbs 140 1 of heat and performs 80 J of work, then q = +140 J and w = -80 I.
In this case the change in internal energy, AU = q + w – 140- 80 = 60 J.0 In a process, if a system rejects 2001 heat and performs 60 J of work, then q = – 200 I and w = – 60 J. In this case, the change in internal energy, AU = q+w = -200-60 = -260 J.
Although q and w are not state functions, the sum of these two quantities does not depend upon the path. This Is because the sum of q and w is equal to AU, which is path-independent.
The mathematical expression of the first law of thermodynamics for an infinitesimal change: According to the first law of thermodynamics, for a closed system, AU = q + w; where q = heat absorbed by the system, w = work done on the system, A U = change in internal energy.
CBSE Class 11 First Law of Thermodynamics Notes
In Case Of An Infinitesimal Change, The Above Equation Can Be Written As: dU = 6q + 5w where 5q = infinitesimal amount of heat absorbed by the system, 8w = infinitesimal amount of work done on the system, dU = infinitesimal change in the internal energy. Equation [1] represents the mathematical form of the first law of thermodynamics for infinitesimal changes.
The Mathematical Form Of The First Law Of Thermodynamics In Case Of A Process That Involves Only P-Work: According to the first law, AU = q+w. In this equation, w represents the sum of all types of work (mechanical, electrical, magnetic work, etc.). If only P-Vwork is performed, then \(w=-\int_{V_1}^{V_2} P_{e x} d V \quad\left[P_{e x}=\text { external pressure on the system }\right]\)
V1 and V2 are the initial and final volumes of the system respectively. When only P-V work is performed, the mathematical form ofthe first law of thermodynamics is
⇒ \(\Delta U=q-\int_{V_1}^{V_2} P_{e x} d V\)
If the volume of the system increases, then V2 > V1 (expansion). In this case, work is done by the system of the surroundings against the external pressure (Pex).
If the volume of the system is decreased by the external pressure (Pa) then V2<VX (compression). In this case, work is done on the system by the surroundings.
If the die volume of the system increases from V1 to V2 against a constant external pressure (PfX). then AU = q-Pex{Vs-V{)=q-PexV <Va>
If the volume of the system is decreased From V1 to V2 bv a constant external pressure (Ppv), then A U = q-P1V2-V2)=q-PexAV(V2< V2) isolated system and the first law of thermodynamics: According to the first law, AU = q + w. An isolated system does not interact with its surroundings. So such a system does not exchange heat (q) or work (w) with its surroundings and hence, q = 0 and w = 0. Therefore, the internal energy of an isolated system remains constant (AC/ = 0)
First Law of Thermodynamics NCERT Notes Class 11