NCERT Solutions For Class 8 Chemistry
- Chapter 1 Matter Notes
- Chapter 2 Physical And Chemical Changes Notes
- Chapter 3 Elements, Compounds And Mixtures Notes
- Chapter 4 The Structure Of The Atom

Life would not have been possible on Earth without water. Water is the most abundant compound on our planet, covering three-fourths of the Earth’s surface. It constitutes about seven tenths of our bodies and more than nine tenths of fruits and vegetables.
Water can dissolve many substances —solids, liquids and gases alike. It controls the temperature of the atmosphere and also our bodies.
Read And Lean More Class 8 Chemistry
We need water for:
NCERT Solutions for Water Class 8

The sources of water are surface water bodies like ponds, rivers, lakes, seas and oceans, and groundwater. Both surface water and groundwater are replenished by the rain. In this chapter, we will discuss the properties of water as a solvent and also the types of natural water (i.e., hard and soft water).
Water is called a universal solvent as it dissolves a larger number of substances than any other.
It is this property of water that helps a river dissolve many minerals from the soil and finally discharge them into the sea. Thus, a sea or an ocean is a vast store of minerals and other soluble substances.
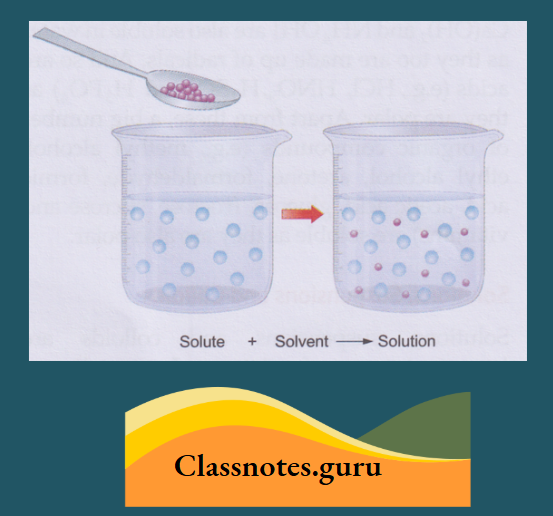
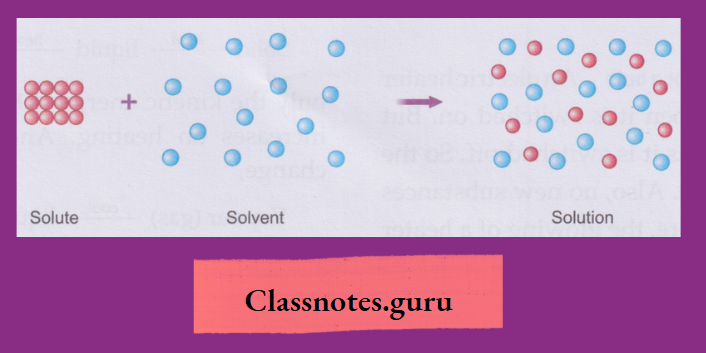
How a Solute Dissolves in Water
A solute, when mixed with water or any other solvent, separates into molecules. And these molecules hide themselves in the intermolecular space of water or the solvent and appear to have disappeared.
As the solute molecules occupy the intermolecular space of the solvent, the volume of the solvent does not change when a solute dissolves in it.
Water is Superior to Other Solvents
Water is superior to other solvents as it helps the solute fragment into molecules or radicals. A water molecule is electrically neutral.
Example: HCl, HNO3, H2SO4 and H3PO4) as they are polar.
Apart from these, a big number of organic compounds
Example: Methyl alcohol, ethyl alcohol, acetone, formaldehyde, formic acid, acetic acid, glucose, fructose, sucrose and vitamins) They are soluble as they are also polar
Solutions, Suspensions and Colloids
Solutions, suspensions and colloids are important types of mixtures. Let us discuss them now
Solutions:
A solution has the important characteristics of:
The size of the solute particles must be very small—1 nm (i.e., 10-9 m or a millionth of a millimetre) or less in diameter.

The terms solute and solvent are used in respect of a solution. They are replaced by the terms dispersed phase and dispersion (or continuous) medium, respectively, for a suspension as well as a colloid.
Suspensions
A suspension is a heterogeneous mixture of one or more dispersed phases in a dispersion medium
Muddy water is a common example of a suspension. Here, soil is the dispersed phase and water, the dispersion medium. Similarly, chalk (CaCO3) or gypsum (CaSO4.2H2O; blackboard chalk), when stirred in water, gives a suspension.
NCERT Class 8 Chemistry Chapter 8 Water
For a suspension, it is not necessary that the dispersed phase be a solid and the dispersion medium a liquid. The suspensions of

The size of a dispersed particle in a suspension is much larger than that of a solute in a solution. It is 10-6 m (i.e., a millionth of a metre) or more in diameter.
A suspension is not transparent. And the dispersed particles slowly settle down because, being large, they are heavy too. You must have seen that the soil settles down from muddy water in a glass.
Colloids
A colloid is a homogeneous mixture of one or more dispersed phases in a dispersion medium. Milk is the most common example of a colloid —butterfat globules dispersed in water. Jam, jelly, whipped cream and gelatin are also common examples of a colloid.

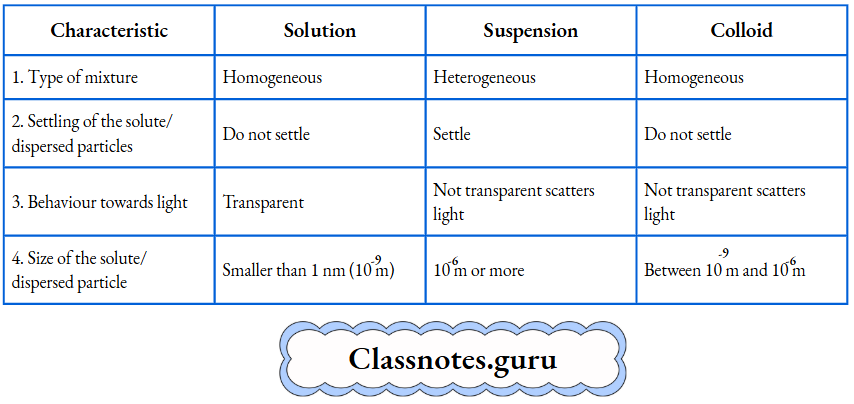
Colloids are not transparent. And the dispersed particles do not settle down. The size of a dispersed particle is in between those of a solute in a solution and a dispersed particle in a suspension, i.e., between 10-9 m and 10-6m (or greater than 1 nm and smaller than 1000 nm). The characteristics of a solution,s uspension and colloid are given in Table
Characteristics of a solution, suspension and colloid:
Solutions
For preparing a solution, you generally add the solute in instalments (or bit by bit) to the solvent, stirring the mixture all the time. A stage comes when the mixture refuses to take in more solute. And whatever solute you add afterwards remains undissolved and settles down at the bottom.
This usually happens with those salts (as solutes) which crystallise as hydrates,
Example: Copper(II) sulphate, which crystallises as CuSO4.5H2O and iron (II) sulphate, which does so as FeSO4.7H2O. Depending on the solute content of the solution,
Water Class 8 NCERT Notes
We find that there are three kinds of solutions:
These are:
Activity:
Take about 50 mL of water in a beaker and dissolve some blue vitriol crystals (CuSO4.5H2O) in it. Use a glass rod for stirring the mixture. Again, add a bit of the crystals and stir.
Nothing separates, and that solution is supersaturated. Transfer the solution to a china dish, evaporate a bit of it and leave it for a few hours (preferably overnight). Beautiful crystals of blue vitriol are obtained.
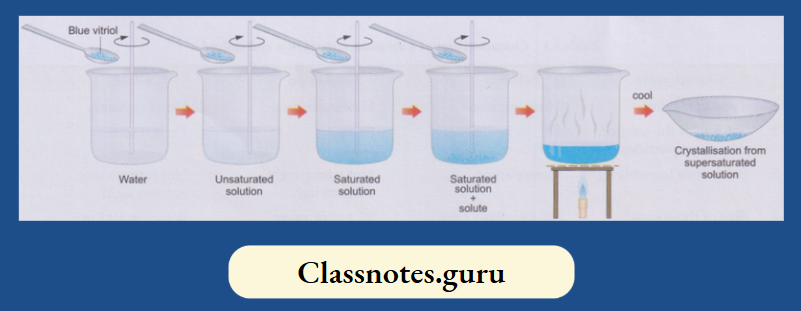
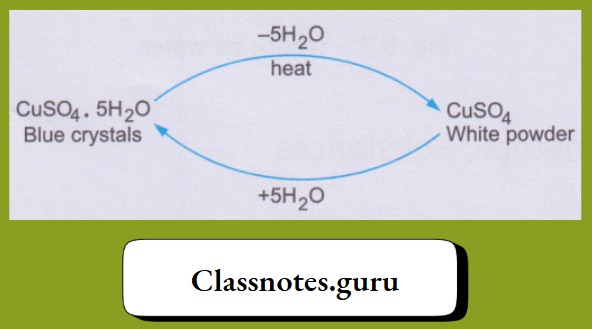
It has been found that whenever copper(II) sulphate is crystallised from an aqueous solution, the crystals have the formula CuSO4.5H2O.
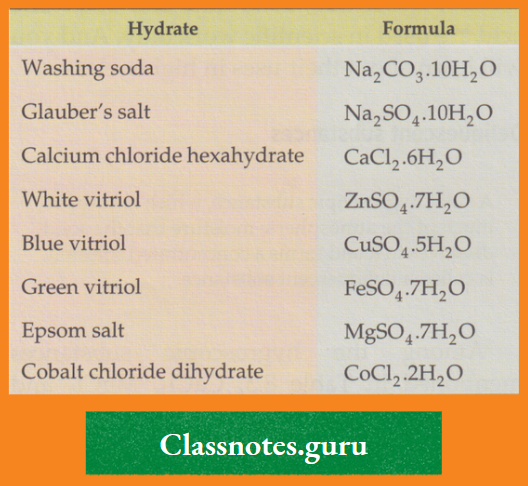
Some examples are mentioned in the Table
Some common hydrates:
The Loss of Water of Crystallisation on Heating
A hydrate, on being heated, loses its water of crystallisation. And it has been observed that it loses its crystalline structure too. You can find this for yourself by doing the following activity
Activity:
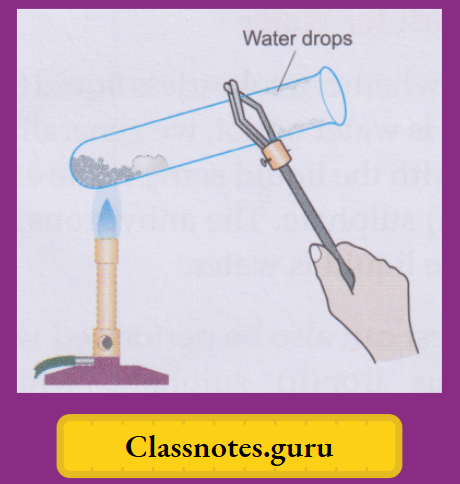
Take a few crystals of blue vitriol in a dry test tube and heat gently. You will observe that
Cool the white powdery substance and moisten it with a drop of water. The solid turns blue again.
Water and its Properties Class 8
What happens during these changes can be summarised as follows.
1. The blue copper(II) sulphate pentahydrate, on being heated, loses the water molecules and changes to the white anhydrous (meaning without water) copper(II) sulphate. And, on treatment with water, the anhydrous salt changes back to the hydrated salt.
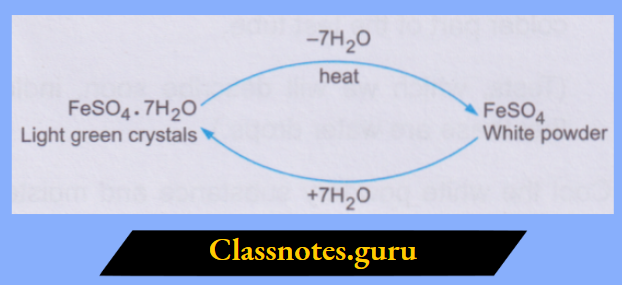
2. The crystalline structure of the hydrated salt is lost when it loses the water molecules. Thus, the water of crystallisation is a part of the crystalline structure. You can repeat the activity with the crystals of green vitriol, i.e., FeSO4.7H2O. By losing the water of crystallisation on being heated, the light green crystals change to a white, powdery solid (the anhydrous salt).
How to Test for Water
To know whether a colourless liquid (in bulk or in drops) is water or not, we generally bring in contact with the liquid some white anhydrous copper(II) sulphate. The anhydrous salt turns blue if the liquid is water. The test can also be performed with white anhydrous iron(II) sulphate, which turns green on treatment with water.
The above-mentioned anhydrous salts can be prepared by slowly heating the hydrated salts.

Hygroscopic Substances
A substance that absorbs moisture from the atmosphere is called a hygroscopic substance.
Some examples are given in the Table
Importance of Water Class 8
Some common hygroscopic substances:
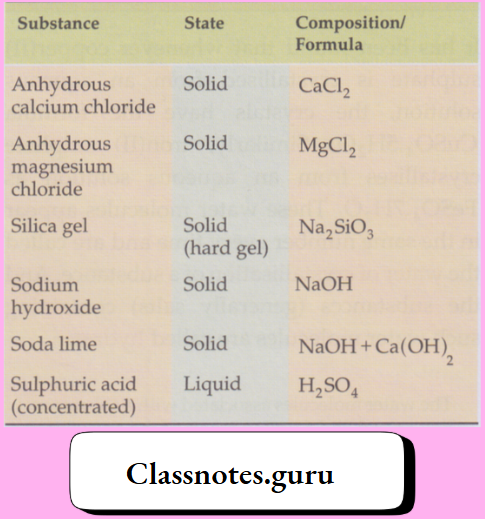
They are generally used as drying agents. You might have seen a small cloth bag, containing a solid, inside a box of medicine, thermoflask or a camera. The bag contains silica gel—a common drying agent—which keeps the air inside the box dry. Other drying agents like calcium chloride, sodium hydroxide, soda lime and sulphuric acid are used in scientific work only. And you will learn about their uses in higher classes.
Deliquescent substances
A solid hygroscopic substance, which absorbs so much of the atmospheric moisture that the solid dissolves in it and forms a concentrated solution, is called a deliquescent substance.
Many metals and metal oxides react with water. To understand these reactions, we need to have an idea about the activity series. Metals along with hydrogen, have been arranged according to their activity in this series. The series consisting of some common metals is given here.
The Action of Metals on Water
Whenever a metal reacts with water, it does so with a view to displacing hydrogen from water. Obviously, only those metals can displace hydrogen from water which are more active than hydrogen, i.e., higher than hydrogen in the activity series. We can also understand that the more active the metal (i.e., the higher the metal in the activity series), the more vigorous is its reaction with water
We will discuss here the action of potassium (K), sodium (Na), calcium (Ca), magnesium (Mg) and iron (Fe) on water. We should remember that though tin (Sn) and lead (Pb) are higher than hydrogen in the activity series, they do not act on water
Water Cycle Class 8 Chemistry
Action of potassium and sodium on water:
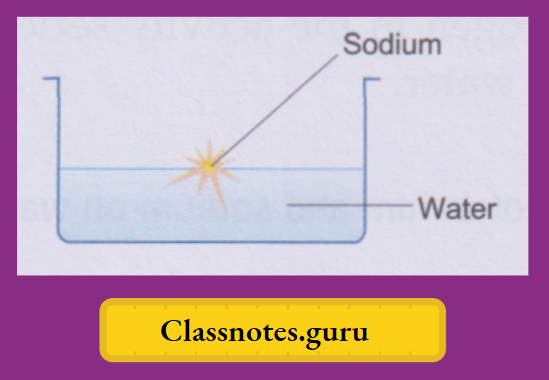
Among the common metals, potassium and sodium are the most active ones. They are soft and get quickly affected by the moisture (and also oxygen) of the air and are, therefore, preserved in kerosene. A small piece of the metal is cut with a knife, dried by pressing between the folds of a filter paper and dropped into a trough of water. We make the following observations about the two metals
Sodium:
The metal soon changes into a silvery white globule that does not sink but darts around on the surface. A hissing sound is constantly heard. And a yellow spark flies intermittently with a ‘pop’.
The resulting solution turns red litmus blue, and so it is alkaline. We infer that sodium reacts vigorously with water to form sodium hydroxide and liberate hydrogen. At the same time, the reaction is highly exothermic, and so the metal melts to form a globule.
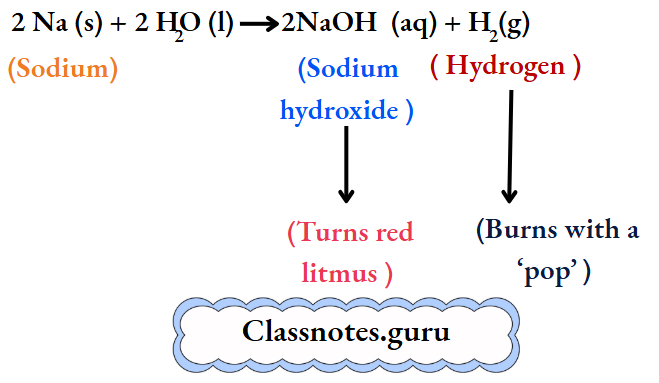
The hydrogen burns with a ‘pop’. And yellow sparks are produced by small particles of sodium. (Sodium imparts a yellow colour to a flame. Throw some common salt, i.e., sodium chloride, into the flame of a kitchen stove, and watch the colour imparted to the flame. It is yellow. Also, doesn’t a sodium vapour lamp have a yellow light?)
Potassium:
Potassium also reacts vigorously and exothermically with water to form potassium hydroxide and liberate hydrogen.
2K(s)(potassium)+ 2HO(l) → 2KOH(aq)(Potassium hydroxide) + H2(g)
The action of calcium on the water ribbon
Calcium is heavier than water and a piece of the metal sinks in it. The evolution ofhydrogen starts briskly but slows down soon as the lime produced forms a coating on the metal. Calcium hydroxide (slaked lime) is much less soluble than sodium hydroxide or potassium hydroxide and makes the solution turbid. The solution is alkaline, turning red litmus blue.
Ca(s)(calcium) + 2HOH(l) → Ca(OH)2 (aq) (calcium hydroxide (alkaline) + H2(g)
The action of magnesium on water
Magnesium, being less active than calcium, displaces hydrogen from water very slowly at room temperature. However, the reaction is fast with steam.
⇒ \(\mathrm{Mg}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{MgO}+\mathrm{H}_2\)
When magnesium powder is mixed with water, the evolution of hydrogen starts slowly and stops soon because the MgO forms a coating over the metal particles. But you can verify for yourself how fast the reaction with steam is
Activity:
Boil some water in a conical flask to replace the air inside with water vapour. Continue boiling and introduce a burning piece of magnesium ribbon into the mouth of the conical flask.
The particles of magnesium oxide falling into the water make it alkaline —the solution or the mixture turns red litmus blue.
Water Purification Class 8 Chemistry
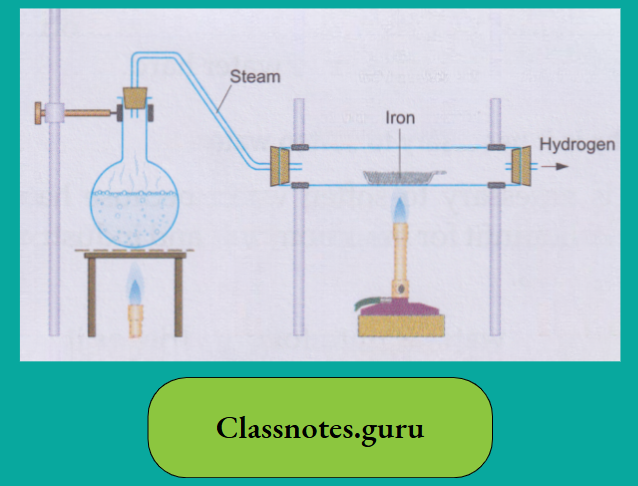
The action of iron on water
Though iron is above hydrogen in the activity series, it is much less active than magnesium. Iron displaces hydrogen from water only when steam is passed over the red-hot metal. A black oxide triiron tetroxide(Fe3O4), also called ferrosoferric oxide, is formed.
3 Fe (iron)+ 4H2O(steam) → Fe3O4 (Triiron tetroxide) (black) + 4H2 (hydrogen)
(Triiron tetroxide is considered a mixed oxide of iron(II) and iron(III), i.e., FeO. Fe2O3.)
The Action of Water on Metal Oxides
The oxide of the highly active metal, like K, Na and Ca, reacts vigorously and exothermically with water to form the hydroxide of the metal.
K2O + H2O→ 2KOH
Na2O + H2O→ 2NaOH
CaO + H2O → Ca(OH)2
You know that these hydroxides are alkalis.
However, as we move down the activity series, the reactivity of the metal oxide with water sharply decreases. For example, MgO reacts with water to form Mg (OH)2 to a small extent and Al2O3, ZnO and Fe2O3, etc., to a still smaller extent.
You may have noticed that the water of some places forms lather easily with soap whereas that of other places does not lather easily.
What Makes Water Hard?
The presence of soluble salts (like hydrogen-carbonates, sulphates or chlorides) of calcium and magnesium in a sample of water makes it hard
Soap contains sodium salts of fatty acids. (Fatty acids are organic acids containing a large number of carbon atoms.) These salts produce lather with water. However, the calcium and magnesium salts of these fatty acids are insoluble. So, when a soap is treated with hard water, the calcium and magnesium salts of the fatty acids precipitate in the form of a scum. As a result, the soap is consumed, but no lather is produced
2Na (Ft) ( sodium salt of the fatty acid) + Ca (HCO3)2(calcium hydrogencarbonate) → 2 NaHCO3 (sodium hydrogencarbonate) + Ca(Ft)2 ↓ (calcium salt of the fatty acid)
Precipitation over clothes leaves dirty stains, and that over your body irritates the skin.
The hardness of water increases with the amount of dissolved calcium and magnesium salts. But remember that dissolved sodium or potassium salts
Example: NaCl, K2SO4, etc.) do not make water hard. This is because the sodium and potassium salts of fatty acids do not precipitate. And water containing sodium and potassium salts does lather with soap.
Temporarily and Permanently Hard Water
The hardness of some water samples can be removed by boiling, but not of all. On this basis, hard water is classified into two types.
Temporary hardness is caused by the dissolved hydrogencarbonates of calcium and magnesium carbonates. Permanent hardness is caused by the dissolved sulphates and chlorides of calcium and magnesium.
Softening of Water
If the hardness of water is removed, soft water is produced, and the process is called softening of water.
Physical and Chemical Properties of Water Class 8
The following methods are used to soften water:
1. Boiling:
Temporarily hard water can be softened by boiling it. When such water is heated, the hydrogencarbonates of calcium and magnesium are decomposed to the carbonates. Being insoluble, the carbonates precipitate out
Ca(HCO3)2 (calcium hydrogencarbonate)→ CaCO3 + CO2↑ + H2O
2. Treating with washing soda. Permanent hardness of water is removed by treating with washing soda (Na2CO3.10H2O). A solution of washing soda is added to the water, and the carbonates of calcium and magnesium are precipitated
CaSO4(calcium sulphate) + Na2CO3 (sodium carbonate) → CaCO3 ↓ (calcium carbonate) + Na2SO4 (sodium sulphate) (in solution)
CaCl2(calcium chloride)+ Na2CO3 (sodium carbonate) → CaCO3 (calcium carbonate) + 2 NaCl (sodium chloride) (in solution)
The sodium sulphate and sodium chloride formed will not make the water hard
Water Resources and Conservation Class 8
Why is it necessary to soften water?
It is necessary to soften water because hard water is unfit for most domestic and industrial purposes.
As you know, everything is ultimately made of atoms. It was earlier thought that atoms are indivisible but now we know that they are made up of subatomic particles—electrons, protons and neutrons. This idea has brought about a revolution in science.
The Views of Kanad
Way back in the sixth century BC, the Indian philosopher Kanad came up with the following idea.
Read And Lean More Class 8 Chemistry
(In Sanskrit, param means final or ultimate, and anu means particle.)
Kanad further said that two or more paramanus combine to form bigger particles.
NCERT Class 8 Chemistry Chapter 4 Structure of the Atom
The Views of Democritus and Leucippus
In the fifth century BC, the Greek philosophers Democritus and Leukiposs came up with a similar idea. They thought that on dividing a piece of a substance, one would ultimately get a particle that could not be divided further. They gave the name atomos (in Greek, atomos means indivisible) to these ultimate particles
Dalton’s Theory
The theories of Kanad as well as of Democritus and Leukiposs remained forgotten for more than two thousand years. But when experimental chemistry developed, it became necessary to explain observed facts. In 1803, English chemist John Dalton put forward his atomic theory,
Which can be summarised as follows:
An atom is defined as the smallest part of an element that takes part in a chemical reaction
In the late nineteenth century, however, it was proved that atoms are divisible. And it was later found that atoms are made up of subatomic (or fundamental) particles— electrons, protons and neutrons. 35
The Electron
Under ordinary conditions, gases are bad conductors of electricity. But a gas becomes a good conductor of electricity if
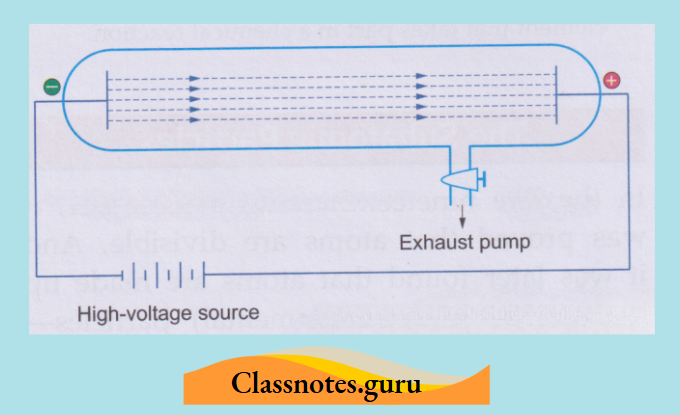
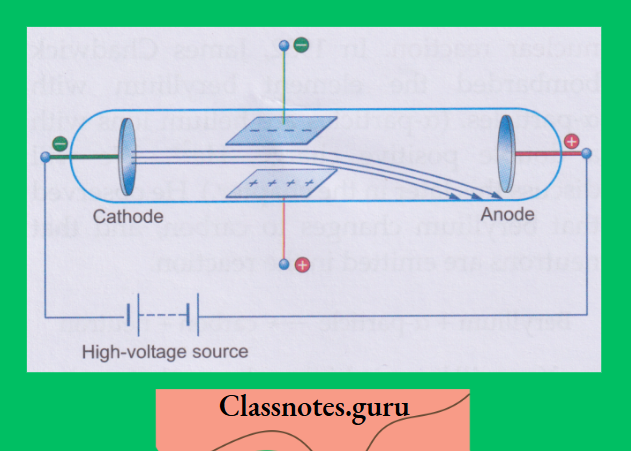
These conditions are achieved in what is called a discharge tube.
Cathode rays:
A discharge tube is a long glass tube at the two ends of which are sealed two metal plates.
Investigations have shown that some invisible rays, starting from the cathode, fall on the opposite wall of the tube, causing fluorescence. These rays were named cathode rays.
The characteristics of cathode rays:
Sir J J Thomson and others found that cathode rays have the following characteristics.
Atomic Structure NCERT Notes
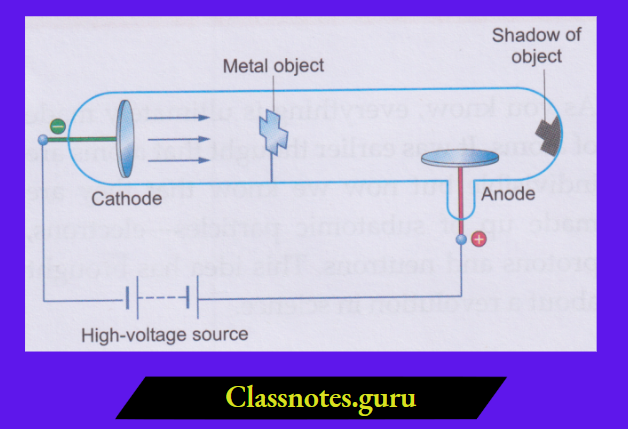
1. They originate at the cathode and travel in straight lines:
When an object is placed in the path of cathode rays, a shadow of the object falls on the wall opposite the cathode. A shadow can be formed only when the rays travel in straight lines.
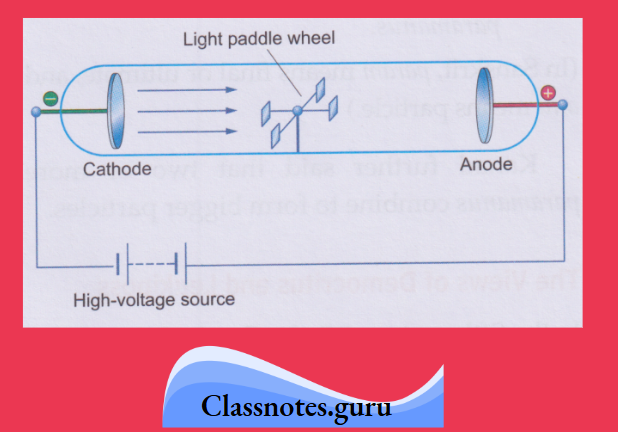
2. Cathode rays are a stream of particles:
A light paddle wheel, placed in the path of the cathode rays, rotates. This shows that some particles strike the plates of the wheel
3. The particles constituting cathode rays are negatively charged:
This is proved by the fact that the cathode rays bend towards the positive plate in an electric field.
4. The particles constituting cathode rays are O O all alike. They do not change with the gas, the electrodes and the kind of glass used for making the tube
Thus, Sir J J Thomson concluded that the particles constituting cathode rays are a universal constituent of all atoms. These particles were named electrons in 1897
Relative charge and mass:
The charge on an electron is taken as the unit of negative charge. So an electron is said to have a charge of -1 unit. The mass of an electron is about 1/1840th of a hydrogen atom, and so it is treated as negligible.
The Proton
An atom is electrically neutral. But the electrons present in it are negatively charged particles. Hence, the atom must also contain some positively charged particles so that the overall charge on it becomes zero. These particles should be found in the discharge tube itself, when cathode rays are formed.
NCERT Solutions for The Structure of the Atom
Anode rays:
Goldstein repeated the cathode-ray experiment using a perforated cathode.
It was found that these rays contained positively charged particles, and so J J Thomson called them positive rays
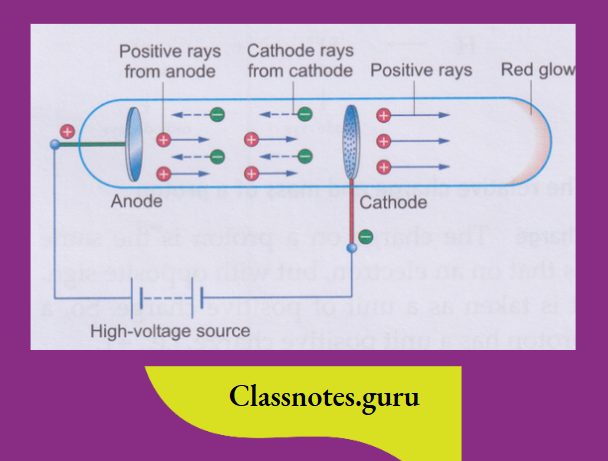
The characteristics of anode rays
The characteristics of anode rays were found by carrying out experiments similar to those with cathode rays.
The following features distinguish anode rays from cathode rays:
What constitutes anode rays?
The electrons constituting the cathode rays must come from the atoms of the gas inside the discharge tube. Electrons are negatively charged particles, so the atoms must be left with an equivalent amount of positive charge. It is these positively charged particles that constitute the anode rays.
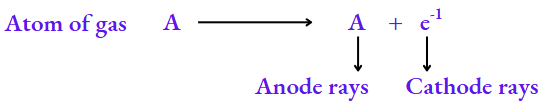
The Structure of the Atom Class 8 NCERT Notes
As an electron has a negligible mass, the particle A+ will have the same mass as A. So, the particles constituting anode rays will differ from gas to gas. When hydrogen, the lightest element, is taken in the discharge tube, the particles constituting the anode rays are called protons
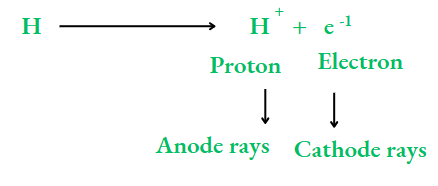
The relative charge and mass of a proton
Charge:
The charge on a proton is the same as that on an electron, but with opposite sign. It is taken as a unit of positive charge. So, a proton has a unit positive charge, i.e., +1. The mass of a proton is the same as that of a hydrogen atom, i.e., 1 u (u stands for unified mass, which is the unit of atomic mass). A proton is about 1840 times heavier than an electron
The Neutron
The Discovery of the Nucleus Till now, we have learnt of only one particle that can account for the mass of an atom —the proton.
This suggested that an atom must contain one more kind of particle, which should have
These particles were named neutrons as they should be electrically neutral. Experimentally, however, the neutron was observed much later in what is called a nuclear reaction. In 1932, James Chadwick bombarded the element beryllium with α-particles.
(α-particles are helium ions with a double positive charge, He2+. We will discuss this later in the chapter.) He observed that beryllium changes to carbon and that neutrons are emitted in the reaction.
Beryllium + α – particle → carbon + neutron
You will learn in higher classes that nuclear reactions are very different from chemical reactions. In a nuclear reaction, an atom can change altogether whereas in a chemical reaction, it will only rearrange itself but will not change.
Charge and mass of the subatomic particles:

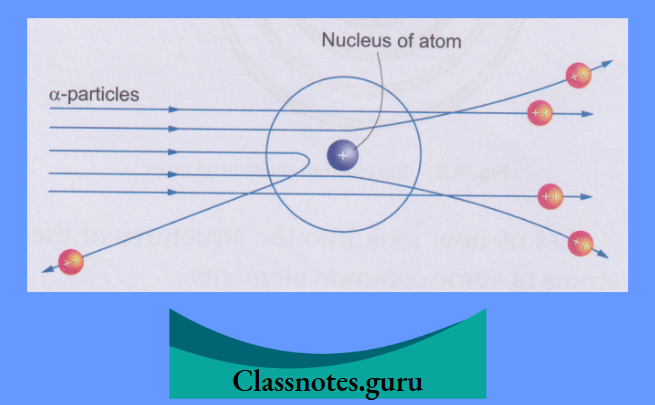
The concept of the nucleus was given by Ernest Rutherford in 1911. The idea was based on the results of his famous experiment, known as the α-particle scattering experiment.
Rutherford’s α-Particle Scattering Experiment
Rutherford bombarded a thin gold foil with α-particles. Alpha particles are emitted by radioactive substances like radium and polonium. (You will learn about radioactivity in higher classes.)
His observations and conclusions are described below:
The Atom and its Structure Class 8
Thus emerged the nuclear model of the atom from Rutherford’s α-particle scattering experiment
How Are the Subatomic Particles Placed in the Atom?
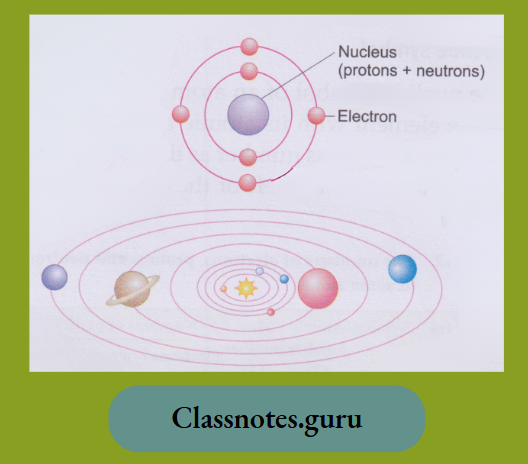
As we have just said, the mass of an atom is concentrated in its nucleus.
In 1913, Niels Bohr presented the atomic model. According to it, electrons revolve around the nucleus in their orbits, just as planets do in the solar system.
Atomic Number and Mass Number
To know the numbers of subatomic particles in an atom, one needs to know the atomic or proton number (Z) and the mass number (A) of the atom.
The number of electrons = Z,
The number of protons = Z, and
The number of neutrons
= mass number- number of protons
= mass number- atomic number
= A-Z
Atomic Models NCERT Notes
Nuclide symbol
The nuclide symbol of an atom is the symbol of the element with its atomic number as the subscript and mass number as the superscript, which are set to the left of the symbol of the element
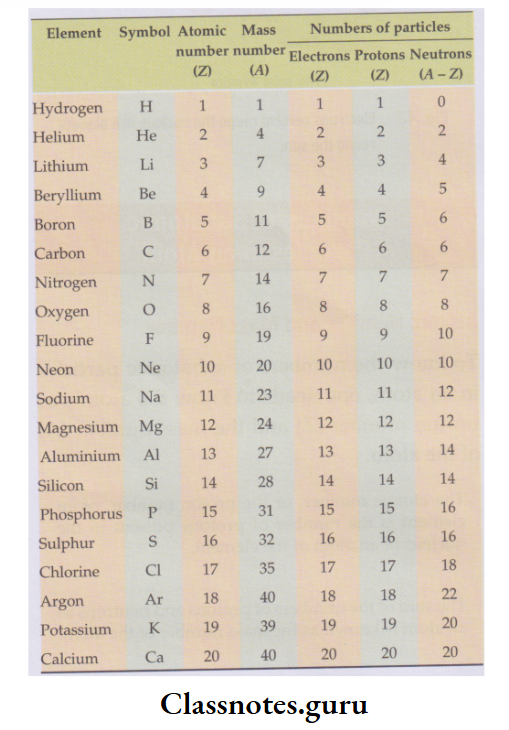
The numbers of electrons, protons and neutrons in some atoms:
The nuclide symbol is expressed as X.
For example:
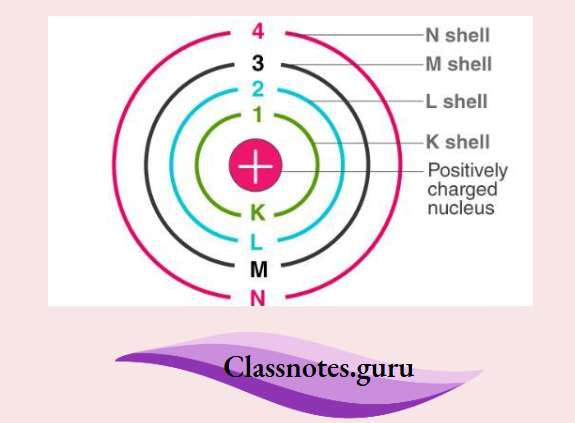
How Electrons Are Arranged
According to the Bohr model, the electrons revolve around the nucleus in shells, called K, L, M, N, … shells. They are named in this order, starting from the innermost shell. The first shell is called the K shell, the second is called the L shell, and so on. The numbers of electrons in these shells follow a set of rules. You will learn about the arrangement of electrons in detail in higher classes.
Let us now look into the structures of the atoms of some common elements.
Electron, Proton, Neutron Class 8 Chemistry
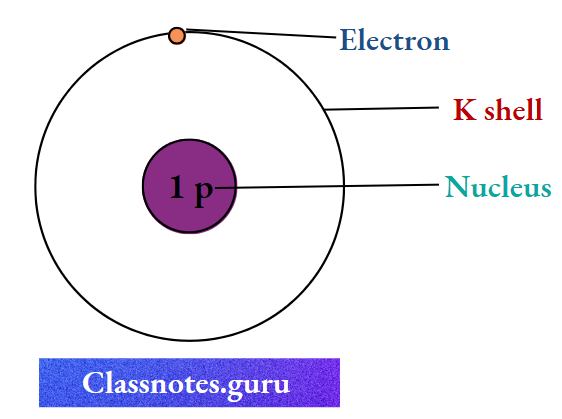
1. Hydrogen (1 H 1)
The number of electrons
Nucleus
The number of protons
= Z = 1
The number of neutrons = A- Z
= 1-1
= 0
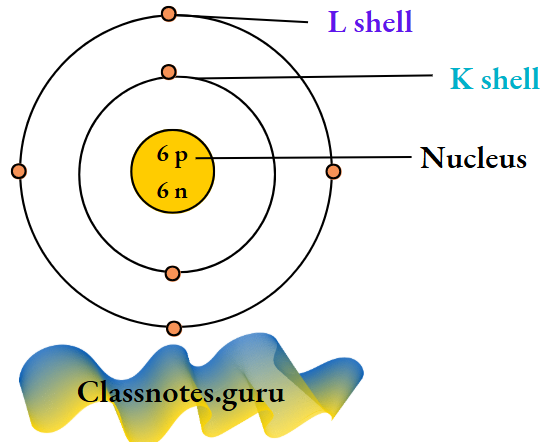
2. Carbon (12 C 6)
The number of electrons = Z = 6.
The number of protons = Z = 6.
The number of neutrons = A-Z
=12 – 6
= 6
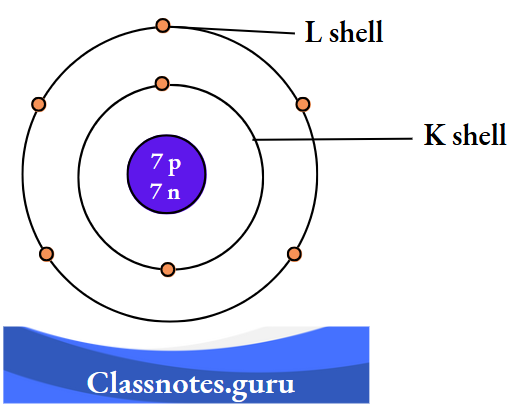
3. Nitrogen (12 N 7)
The number of electrons = Z = 7.
The number of protons = Z = 7.
The number of neutrons = A- Z
= 14-7
= 7.
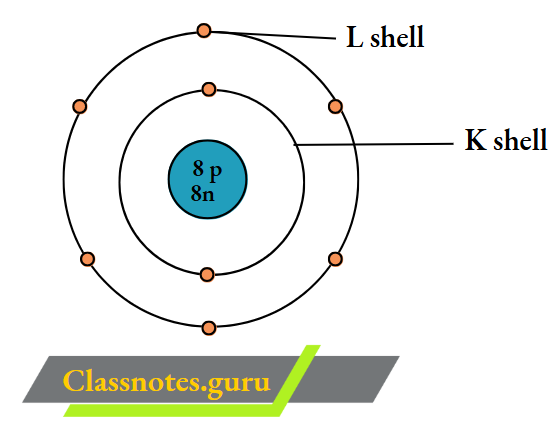
4. Oxygen (16 O 8)
The number of electrons = Z = 8.
The number of protons = Z = 8.
The number of neutrons = A- Z
= 16 – 8
= 8.
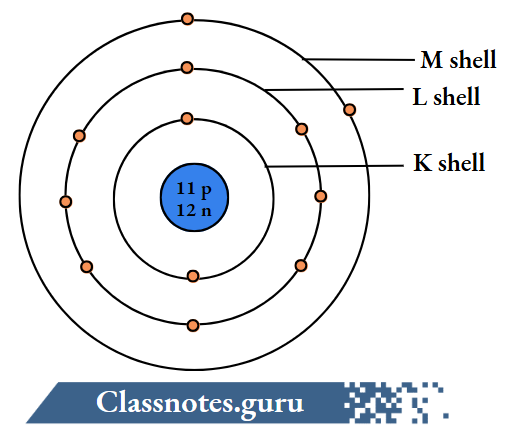
5. Sodium (23Na11)
The number of electrons = Z = 11.
The number of protons = Z = 11.
The number of neutrons = A-Z
= 23-11
= 12.
Atomic Number and Mass Number Class 8
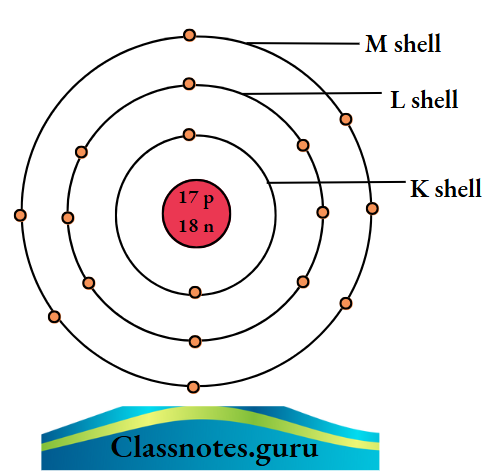
6. Chlorine (35Cl17)
The number of electrons = Z = 17.
The number of protons = Z = 17.
The number of neutrons = A- Z
= 35 – 17
= 18.
Ions
An atom is electrically neutral because the charge of the protons is balanced by that of the electrons. But what happens when an atom loses or gains an electron? If an atom loses an electron, the number of protons exceeds that of electrons. So the atom gets positively charged.
For example:
So, the atom becomes negatively charged.
NCERT Class 8 Atomic Structure Concepts
For example:
A chlorine atom contains 17 protons and 17 electrons. If it gains an electron, there are 18 electrons as against 17 protons.
As you know, substances are classified as elements, compounds and mixtures. This classification is of great interest to chemists as it helps describe the kind of matter anything contains
An element cannot be split into simpler substances by chemical means. For example, hydrogen, nitrogen, oxygen, carbon, sulphur, phosphorus, iron, copper, silver and gold are elements as they cannot be split into simpler substances by chemical means.
Read And Lean More Class 8 Chemistry
NCERT Solutions For Elements Compounds And Mixtures
So, an element is a pure substance.

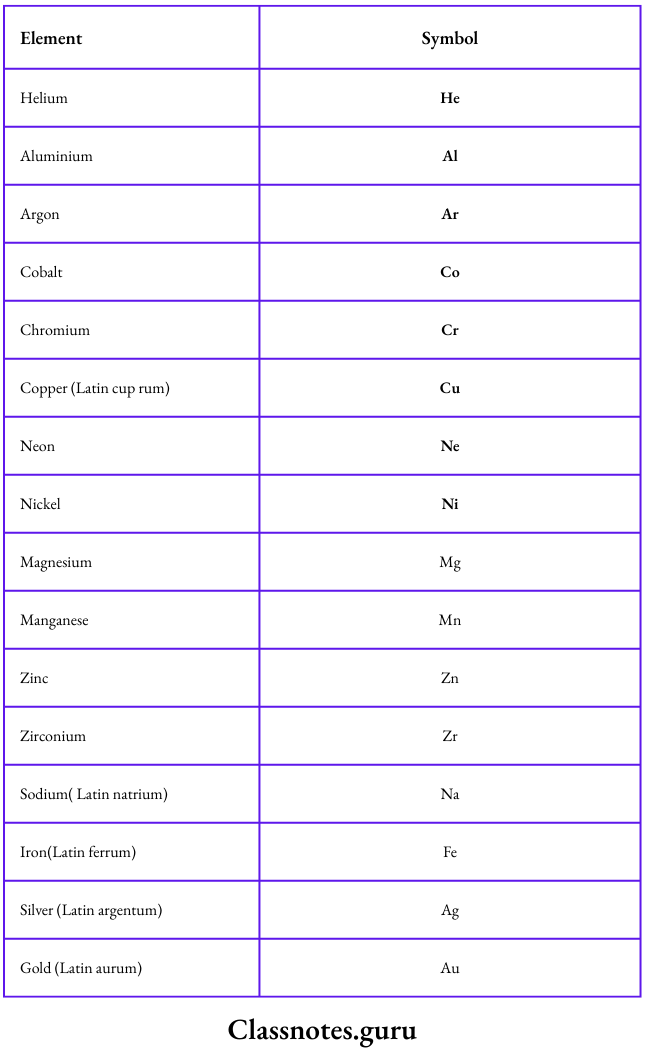
Symbols represent elements
Examples:
Elements And Compounds NCERT Notes
Some examples are given below:
For example, the compound:
Splitting of a Compound into Simpler Substances
As we have just said, a compound can be split into simpler substances by chemical means. Some examples are given below.
NCERT Class 8 Chemistry Chapter 3 Elements, Compounds, And Mixtures
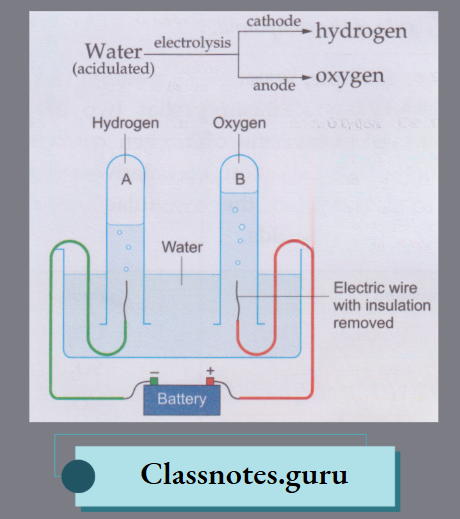
Electrolysis of Water:
Electrolysis is chemical decomposition brought about by passing an electric current through a liquid or a solution.
Thermal decomposition:
The splitting of a substance into simpler substances by the action of heat on it is called thermal decomposition.
For example:
Chalk, i.e., calcium carbonate (which contains calcium, carbon and oxygen), on being heated, gives calcium oxide (containing calcium and oxygen) and carbon dioxide (containing carbon and oxygen).
Both products are simpler than calcium carbonate, and so the latter is a compound.

There are other examples too:
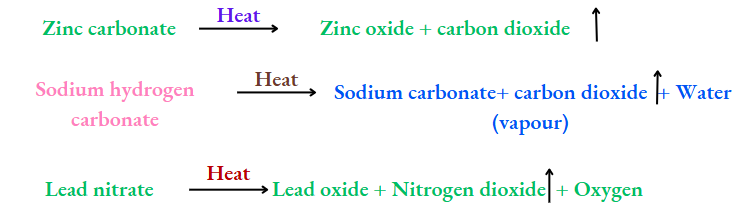
Compounds are Represented by Formulae
A compound is represented by a formula, which shows how many atoms of which element constitute a molecule of the compound.
Elements, Compounds And Mixtures NCERT Notes
For example:
Water is represented by the formula H2O, indicating that two atoms of hydrogen and one of oxygen constitute a molecule of it. You can derive similar information from the formulae of the following compounds.

Important Characteristics of a Compound
You should remember some important characteristics of a compound.
1. A compound can be split into its constituent elements only by chemical means and not by physical means like filtration, sublimation and distillation.
For example:
Water cannot be split into hydrogen and oxygen by any of the physical means mentioned above.
But it can be split by a chemical means like electrolysis. Hydrogen can also be obtained from water by the chemical action of an active metal like sodium on water.
⇒ \(\begin{aligned}\text { Sodium }+ \text { water } \rightarrow \text { sodium hydroxide + hydrogen} \uparrow\end{aligned}\)
Similarly, hydrogen can be obtained from an acid (like hydrochloric or sulphuric acid) by the action of a metal like magnesium, zinc or iron on the acid.
Magnesium + Hydrochloric acid( dilute) → MAgnesium chloride + Hydrogen ↑
Zinc + Sulphuric acid(dilute) → Zinc sulphate + Hydrogen ↑

2. The properties of a compound are entirely different from those of the constituent elements.
For example:
Hydrogen (a combustible gas) reacts with oxygen (a supporter of combustion) to form water (which is neither combustible nor a supporter of combustion).
You can also compare the properties of carbon dioxide with those of its constituent elements, carbon and oxygen.
3. Elements combine in a fixed proportion of atoms to form a compound.
For example:
2 atoms of hydrogen combine with 1 atom of oxygen to form
Atomic ratio of elements in some compounds:
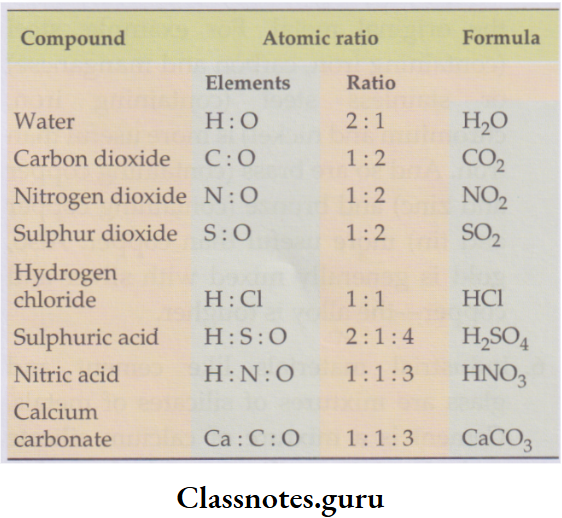
1 molecule of water. And the atomic ratio of hydrogen to oxygen in any sample of water will be 2:1.
4. A compound contains the constituent elements in a fixed proportion of mass.
For example:
By mass, water contains 1 part of hydrogen and 8 parts of oxygen.
You will learn in higher classes how to calculate the mass ratios of elements in a compound
Elements Compounds Mixtures Class 8
Elements and compounds are pure substances, but rarely are they found in the pure state in nature.
Some salts (chlorides, sulphates, hydrogencarbonates, etc.) of metals like magnesium and calcium are dissolved in it.

Similarly, sea water contains so many dissolved substances that it is unfit for industrial, agricultural or domestic purposes.
Examples of Mixtures
Most substances we come across in nature are mixtures. Fluman beings have also made many useful mixtures. Some examples are given below.
1. Air is the most commonly found mixture in nature. It contains nitrogen, oxygen, noble gases, carbon dioxide and moisture. We can separate the components by the physical means mentioned above.
2. Natural water is a mixture containing water, dissolved air and some salts which can be separated by physical means.
3. Foodstuffs
Example: Rice, wheat, pulses, vegetables, fruits, milk, butter, meat, and fish are all mixtures. They contain substances like starch, proteins, vitamins, salts, sugars and water in different proportions.
4. Medicines are mixtures containing some active ingredients in minute quantities (generally in milligrams) mixed with some other substances.
5. Alloys are very common examples of mixtures. It has been found that a metal, when alloyed with other metal(s) or nonmetal(s), is generally more useful than the original metal.
For example:
Steel (containing iron, carbon and manganese) or stainless steel (containing iron, chromium and nickel) is more useful than iron. And so are brass (containing copper and zinc) and bronze (containing copper and tin) more useful than copper. Also, gold is generally mixed with silver and copper—the alloy is tougher.
6. Industrial materials like cement and glass are mixtures of silicates of metals. Cement is a mixture of calcium silicate and aluminium silicate, and glass, that of sodium silicate, potassium silicate and lead silicate.
7. All minerals are mixtures
Characteristics of a Mixture
A mixture has the following characteristics.
1. The components of a mixture may be present in any proportion
For example:
Regardless of whether you dissolve one or two spoons of sugar in a glass of water, the resulting solution will be a sugar-water mixture.
2. The components of a mixture coexist without chemically reacting with one another.
Types Of Mixtures Class 8 Chemistry
For example:
3. The components of a mixture retain their properties. The constituent elements of a compound do not retain their properties, but the components of a mixture do.
For example:
Hydrogen and oxygen do not show their properties in water, but water and sugar do in a sugar solution. Isn’t a sugar solution as sweet as well as watery?
4. The components of a mixture can be separated by simple physical means. By simple physical means, we mean here methods like filtration, sublimation, distillation, etc. We will discuss these methods soon
Types of Mixtures
Mixtures are usually classified based on the state of their components.
One can see separately or distinguish between the different components of a heterogeneous mixture, but not ofa homogeneous mixture.

Thus, a fizzy drink that contains carbon dioxide, water and a sweetener in the same proportion throughout is a homogeneous mixture. But an oil-water mixture is a heterogeneous mixture.
Lists various kinds of mixtures, along with examples:
Types of mixtures with examples:

To obtain a pure substance, we often need to separate the components of a mixture.
It is only by doing so that we obtain
Principles of Separation
For separating the components of a mixture, we take advantage of the property of the components in which they differ the most. Such a distinguishing property can be the state, size, magnetic behaviour, solubility, melting point, boiling point or adsorbability.
Thus, it will be easy to separate
Difference Between Elements and Compounds
Methods of Separation
Scientists use varied methods of separation, from simple to sophisticated. But here we will discuss some simple ones only.
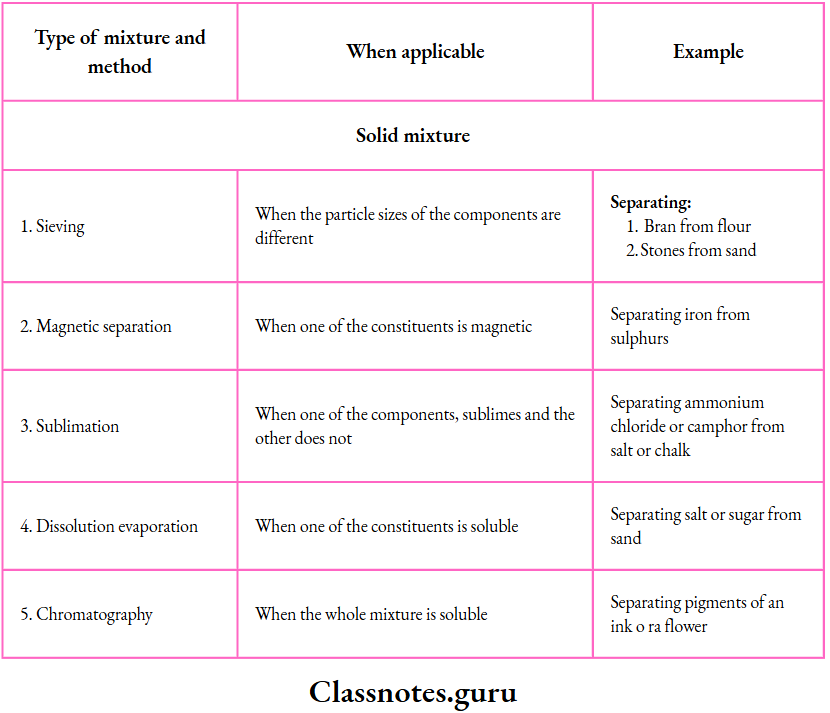
Separating the Components of Mixtures
1. Sieving:
This method is based on the difference in the particle size of the components.
2. Magnetic separation:
This method is used to separate a magnetic substance from a nonmagnetic substance.
The outer heap consists of the nonmagnetic component and the inner heap, of the magnetic component (as it is attracted by the pole).
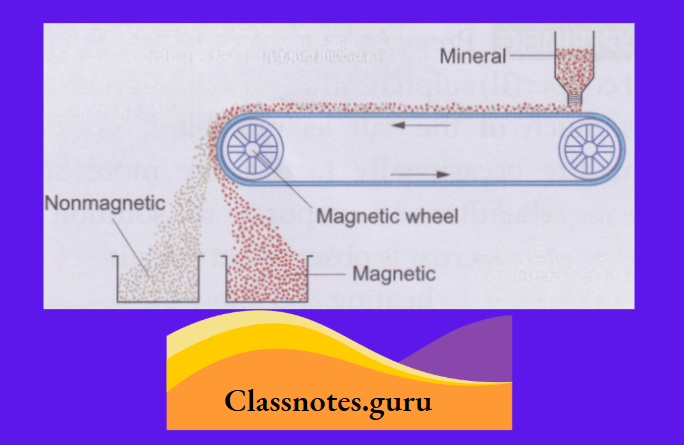
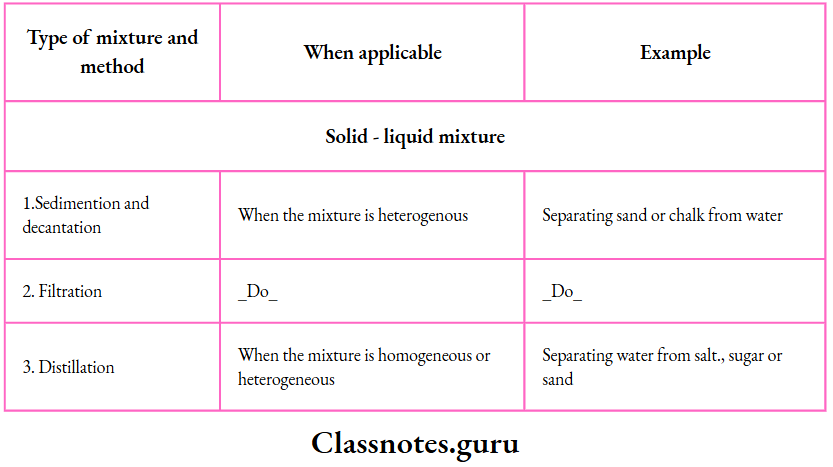
3. Sedimentation and decantation:
This method is useful for separating the components of a solid-liquid mixture in which the solid is heavier than the liquid.
Example:
Example: A sand-water mixture. If the mixture is allowed.


The process can be used to separate immiscible liquids from one another.
However, it is not suitable for the separation of the components of a solid-liquid mixture in which the solid is lighter than the liquid, as in a husk-water mixture
4. Filtration:
If you want to separate a liquid from an insoluble solid, filtration is a better method than decantation.
You have learnt how a filter paper cone is prepared and fitted to a funnel.
You can easily separate sand or chalk from water by filtration.

5. Dissolution followed by evaporation or crystallisation:
This method is useful for a solid mixture of which one component is soluble in a chosen solvent but the other is not.
Example: A mixture containing
Stir a salt-sand mixture in water and warm so that the salt dissolves. Filter the mixture. The salt passes into the filtrate, and the sand remains as residue.
Heat the mixture occasionally to dissolve more and more salt. Filter and evaporate the solution in a basin till a crust is observed at the surface of the liquid.
Mixtures And Their Types Class 8
Experiment:
Prepare a concentrated solution of copper (II) sulphate in a beaker by dissolving as much of the salt as possible.
Beautiful blue crystals of copper(II) sulphate pentahydrate will appear slowly and will grow in size with time.
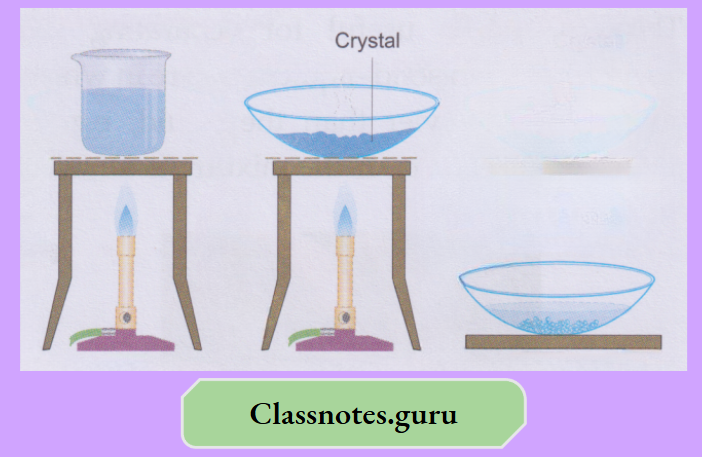
Similarly, sulphur can be separated from iron filings by stirring the mixture with carbon disulfide. The sulphur comes out with the solvent. Iron is filtered, and the filtrate gives sulphur on evaporation or crystallisation.
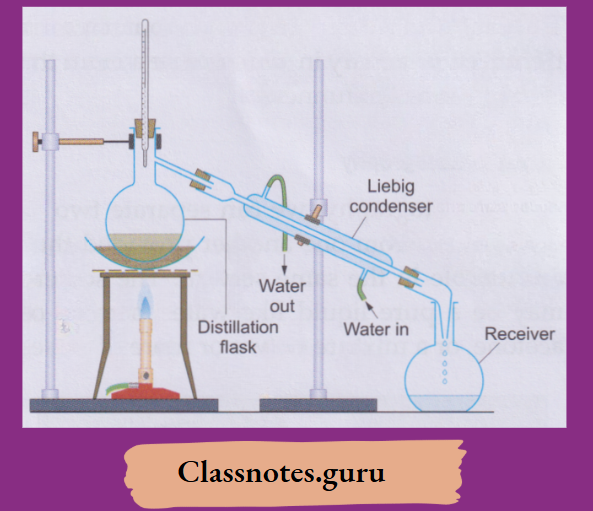
6. Distillation:
By distillation, we can separate a solid-liquid mixture—homogeneous
Example: A solution of salt in water) or heterogeneous
Example: A sandwater or a chalk-water mixture.
The apparatus is set up as shown. The mixture is boiled in a distillation flask.
The vapours coming out condense while passing through a Liebig condenser in which they are cooled by the water circulating in the outer jacket.
This is how distilled water is prepared in the laboratory
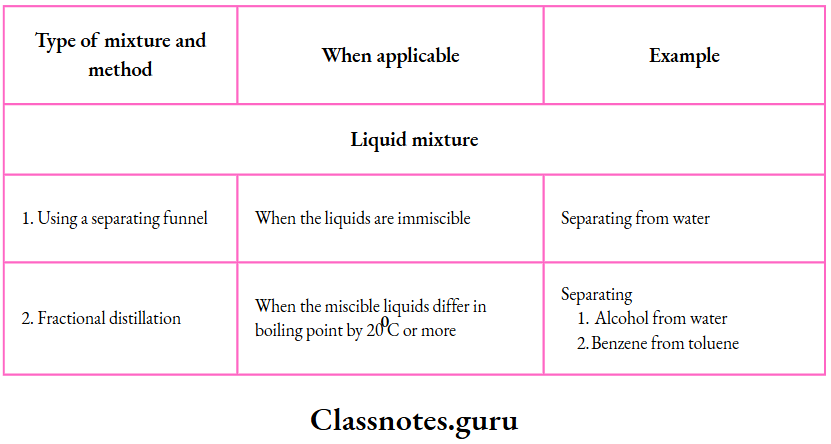
7. Fractional distillation:
By fractional distillation, we can separate liquids which differ in their boiling points by 20°C or more
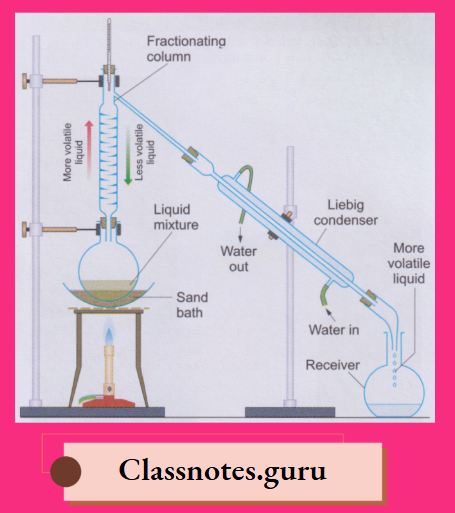
The liquid mixture is boiled in the distillation flask fitted with a fractionating column and a Liebig condenser
By this method, we can separate
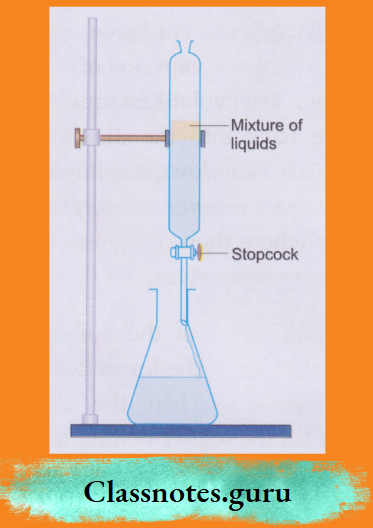
8. Using a separating funnel:
A separating funnel is used to separate two or more immiscible liquids.
9. Sublimation:
Using this method, we can separate a substance that sublimes
Example: Ammonium chloride, camphor or iodine)
From one that does not (Example: Salt, sand or chalk).
A funnel is inverted over the mixture placed in a china dish. A dry test tube is also inverted over the outlet of the funnel. The outlet of the funnel is loosely plugged with cotton. The mixture is heated.
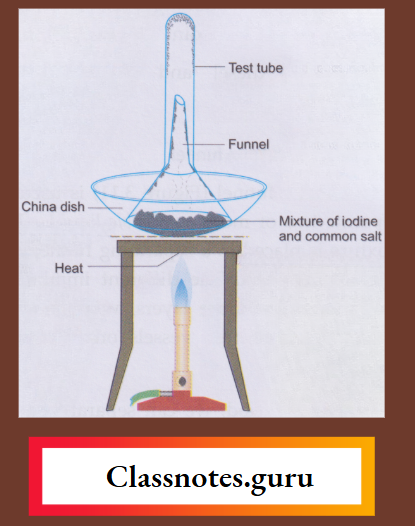
The sublimable component vapourises and the vapours solidify in the test tube and on the cooler part of the funnel.
10. Chromatography:
By chromatography, we can separate two or more solids from one another, provided they are soluble in the same solvent.
The solvent may be a pure liquid like water, alcohol or acetone, or a mixture of two or more of these.
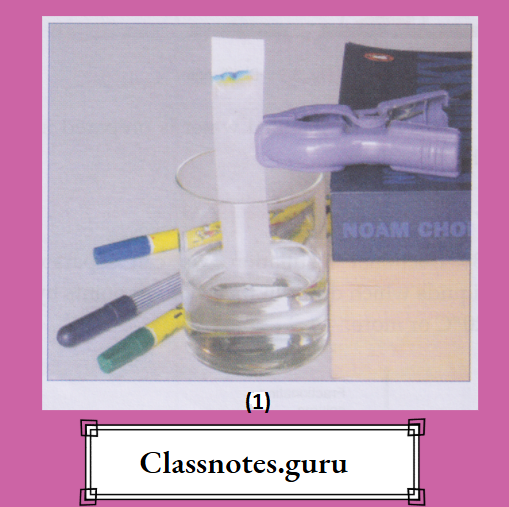

The method works on the principle of adsorption.
An example is a dye held on the surface of a fibre.
The substance that is adsorbed
(Example: A dye) is called the adsorbate, and the surface on which it is adsorbed
(Example: A fibre), The adsorbent.
In chromatography, we generally use cellulose, silica or alumina as an adsorbent.
This happens because the different dyes (pigments), i.e., the different colouring substances, are held (i.e., adsorbed) by the adsorbent with different forces—some by stronger and some by weaker forces.
The array of colours on a chromatographic paper is called a chromatogram.
In chromatography, the adsorbent part is called the stationary phase, and the things that move, i.e., the solvent and the solution, are collectively known as the mobile phase.
Separation Methods: A Summary
A summary of the methods ofseparationofthe components of mixtures is given in Table 3.3.
Methods of separating the components of different types of mixtures:
Separation of mixtures—a few examples
Through the following examples, you will learn how to choose a method for separating the components of a given mixture.
1. A sand-water mixture:
Sand can be separated from water by filtration or distillation. In distillation, the water distils out, leaving the sand as residue.
2. A salt solution:
By distillation, the water can be obtained as the distillate and the salt as the residue. (By evaporation to dryness, the salt can be obtained, but the water will be lost.)
3. A salt-sand mixture:
The salt can be dissolved in water, and the sand filtered out. The filtrate, on evaporation to dryness, yields the salt.
4. A sugar-chalk mixture:
Sugar is soluble in water, but chalk is not. So, the sugar can be dissolved in water, leaving the chalk behind. The mixture, on filtration, will give the chalk as the residue and the filtrate, on evaporation or crystallisation, will yield the sugar.
5. An iron filings-sawdust mixture:
As iron is magnetic and sawdust is not, magnetic separation will be a convenient method to separate them.
6. An iron filings—sulphur mixture:
Two methods can be used.
7. A carbon-sulphur mixture:
Knowing that sulphur is soluble in carbon disulfide but carbon is not, you can suggest the method.
8. A water-oil mixture.:
As water and oil are immiscible, they will form separate layers and can, therefore, be separated by using a separating funnel.
9. A benzene-toluene mixture:
As the difference in the boiling points of benzene (80°C) and toluene (110°C) is more than 20°C, the two miscible liquids can be conveniently separated by fractional distillation.
10. An ink mixture: By paper chromatography.
11. A salt-sand-sulphur mixture:
Among the three components, only sulphur is soluble in carbon disulphide and only salt in water, but sand in either of the two solvents.
12. A carbon-sulphur-nitre mixture (gunpowder):
Gunpowder is an explosive containing carbon, sulphur and potassium nitrate (nitre). Only sulphur is soluble in carbon disulphide, and only nitre in water.
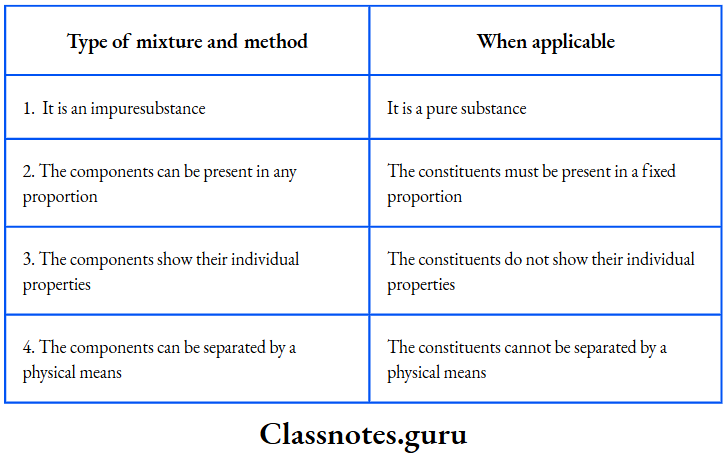
Difference between a Mixture and a Compound.
We can now conclude that a mixture differs from a compound, as shown in Table
How a mixture differs from a compound:
Changes occur every moment around us. They are an integral part of nature. In this chapter, we will see how changes are classified and study physical and chemical changes systematically.
Let us now discuss some ways in which changes are classified
Reversible and Irreversible Changes
A change is said to be reversible when the opposite change can be brought about by reversing the conditions.
Read And Lean More Class 8 Chemistry
Changes in state of matter are the most common examples of reversible change
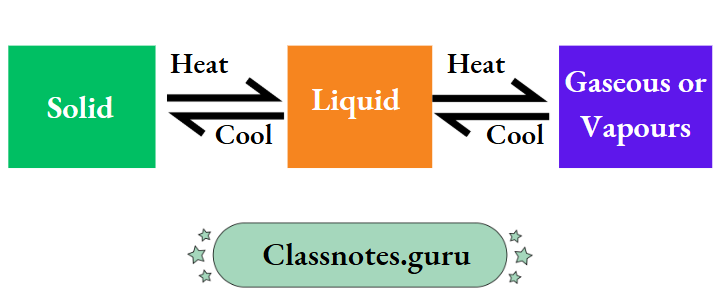
A solid melts on being heated and the melt solidifies (i.e., freezes) on being cooled. So, the melting of a solid and also the freezing of a liquid are reversible changes.
And so are the vaporisation of a liquid (on being heated) and the condensation of vapours (on being cooled). Also, the heating and cooling of the coil of an electric heater when the heater is switched on and off, respectively, are reversible changes
NCERT Solutions For Physical And Chemical Changes

A change is said to be irreversible when the opposite change cannot be brought about by reversing the conditions
For example:
Sugar, when heated, swells to form a brown substance which finally gets charred, i.e., forms a black mass. But the charred mass, on being cooled, does not give back sugar.
Similarly, milk can be changed into cottage cheese by boiling it with some lemon juice added to it. But cottage cheese, or paneer, cannot be changed into milk. Thus, the charring of sugar and the curdling of milk are irreversible changes. And so are the growth of plants and animals, digestion, photosynthesis, and the burning of a fuel
Activities:
1. With the help of an adult, place some sugar crystals in a china dish. Heat the solid slowly and observe the changes. It swells and becomes brown and finally black. The black mass is carbon.

2. Add a few drops of lemon juice to the milk and boil the mixture. In a short while, a thick white substance, called curd, is formed. This is called the curdling of milk. Curd is entirely different from milk. You will not be able to reverse this change.

Periodic and Nonperiodic Changes
Periodic changes are those which take place at fixed intervals of time.
NCERT Class 8 Chemistry Chapter 2 Physical And Chemical Changes
Desirable and Undesirable Changes
The changes that are useful to us are called desirable changes whereas those that are harmful are called undesirable changes.
Chemists are very interested in the classification of changes into physical and chemical changes. Therefore, we will study physical and chemical changes separately.
Physical Changes
A change in which no new substances are formed and which can be reversed by reversing the conditions is called a physical change. We will now discuss some examples of physical change.
Physical And Chemical Changes NCERT Notes
The glowing of a heater or a bulb:
An electric heater or a bulb glows when it is switched on.

Thermal expansion of a substance:
On being heated, a substance expands but on being cooled, it contracts. Also, no new substances are formed, and so the change is physical.
Changes in state of matter:
You have learnt that any change in the state of matter is reversible. And also that the kind of matter remains the same in any change in state of matter, be it solid-liquid or liquid-vapour (gas) interconversion.
For example:
The melting of ice is reversed by the freezing of water, and the vaporisation of water is reversed by the condensation of water vapour.
At the same time, the substances in the three states are the same, i.e., water (H2O). This is true for other substances too.
Example: Sulphur, sodium, iron, copper and zinc.
The kinetic theory of matter tells us that in the change
Physical vs Chemical Changes Class 8
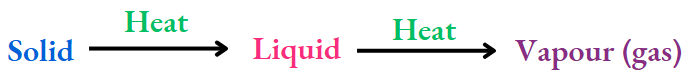
Only the kinetic energy (KE) of the molecules increases when heating. And in the opposite case,
The KE of the molecules decreases on cooling.

Sublimation:
This is also an example of state change. Insublimation, a solid like ammonium chloride, naphthalene, camphor or iodine directly forms vapours, without melting. And when cooled, the vapours are directly converted into the solid. Thus, the change is reversible. No new substances are formed either. So, sublimation is a physical change.
The dissolving of a substance in a liquid:
You know that a solid like sugar, salt, or glucose dissolves in water.
Chemical Changes
Types Of Changes In Matter Class 8
Burning:
When lighted, a combustible substance (Example: Hydrogen, coal, wood, paper, kerosene, diesel, petrol, LPG, CNG, spirit) burns in air.
This change is irreversible —on being cooled, carbon dioxide does not give back carbon and oxygen. Hence, the burning of coal is a chemical change.
Wood or paper, when burnt, combines with the oxygen of the air and forms new substances —carbon dioxide and water vapour.
The changes are irreversible, too. So, the burning of these fuels is a chemical change

Ethanol (i.e., ethyl alcohol, commonly called alcohol) is a compound containing carbon, hydrogen and oxygen. It is a liquid that catches fire when near a flame and burns to give carbon dioxide and water vapour. This is also a chemical change.
Physical And Chemical Changes Examples
Rusting
Iron rusts in moist air, forming a red-brown solid, called rust. The rust formed cannot be changed back to iron by reversing the condition, i.e., the change is irreversible. Thus, rusting is a chemical change.

The cooking of food
The taste of vegetables and pulses changes when they are cooked because new substances are formed in the process. Also, we cannot obtain green vegetables from a vegetable curry or grains from cooked pulses. So, the cooking of food is a chemical change.
The curdling of milk:
The charring of sugar:
Sugar belongs to a class of compounds called carbohydrates. A carbohydrate contains carbon and the elements of water. So, when sugar is heated, water gets loosened and vaporised, and the black residue of carbon is left behind. You have learnt that the change is also irreversible and is, therefore, a chemical change.
Digestion
Food, on being digested inside our body, is changed into several different things—some of which are taken up and some expelled by the body. The change cannot be reversed either. Thus, digestion is a chemical change.
Fermentation
Fermentation is a process employed for preparing alcohol from fruits containing sugar. In the presence of enzymes, a sugar solution changes into alcohol, liberating carbon dioxide.
Photosynthesis:
Green plants prepare their food (glucose) from carbon dioxide and water in the presence of chlorophyll in sunlight
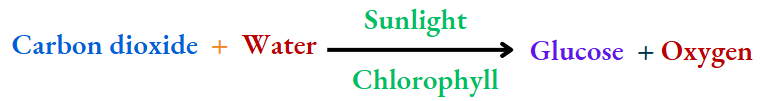
New substances (glucose and oxygen) are formed in the process. Also, the change is irreversible. So, photosynthesis is a chemical change.
Chemical Reactions NCERT Notes
Respiration:
In respiration, the glucose (a carbohydrate) formed by a living being combines with oxygen of the air, forming the new substances carbon dioxide and water. Energy is also released in the process, and the change is irreversible. So, respiration is a chemical change
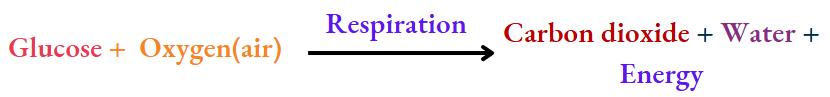
Conservation of mass in a chemical change
The mass of the individual substance(s) undergoing a chemical change is altered. But the total mass of the reactants is the same as that of the products.
For example:
When carbon is burnt in air, the mass of carbon is reduced, and finally, the carbon vanishes.
The mass of an iron nail increases when the nail rusts. But the mass of the original nail plus that of the oxygen and moisture taken up from the air is the same as that of the rusted nail.
Thus, a chemical change obeys the law of conservation of mass.
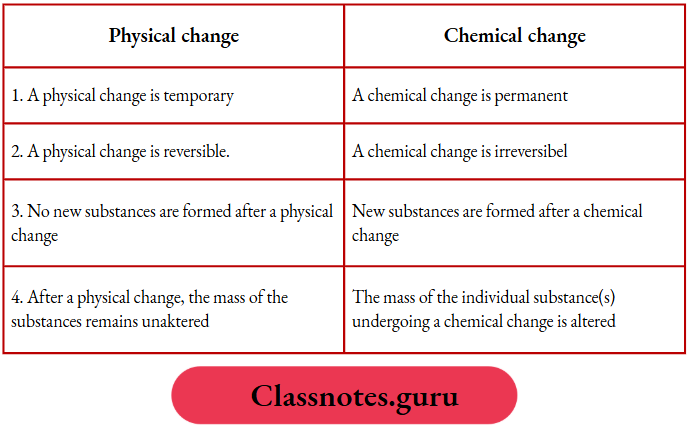
Comparison between physical and chemical changes:
Chemical Change NCERT Notes
Can Physical and Chemical Changes Occur Together?
Yes, they can. Let us examine the changes that occur when a candle burns.
1. The wax under the wick melts. The molten wax flows down and solidifies. These are changes in state and, therefore, physical changes

A part of the molten wax that vaporises burns to form carbon dioxide and water vapour. The burning of wax is a chemical change
Physical and Chemical Changes Involve Energy Change
There is a change in energy when a physical or chemical change occurs. Energy, usually in the form of heat, is either taken in (i.e., absorbed) or given out (i.e., evolved) by a substance undergoing a change
There are many changes which are neither exothermic nor endothermic. The formation of a heterogeneous mixture is a common example of this type of change. When sand is mixed with salt or water, no heat is given out or taken in.
Energy change in a physical change
State changes:
Activity:
Take a little water in a steel or glass bowl. Pour boiling water into a mug. Hold the bowl over the mug. Steam coming out of the mug will condense as it comes in contact with the bowl. After some time, you will find that the water in the bowl has become warmer than before.
On being boiled, water changes into water vapour. The water vapour condenses, transferring heat to the cold water in the bowl. So, the water in the bowl becomes warm.

Why an energy change in a change of state:
This happens because the kinetic energy possessed by the molecules of a substance is different in different states.
NCERT Class 8 Physical And Chemical Changes
Activity:
Hold a glass in your hand. Pour some water into the glass and stir it after adding a couple of spoons of glucose. The glass will become slightly colder. Thus, the dissolution of glucose in water is an endothermic change. You can get back the glucose by evaporating the water.
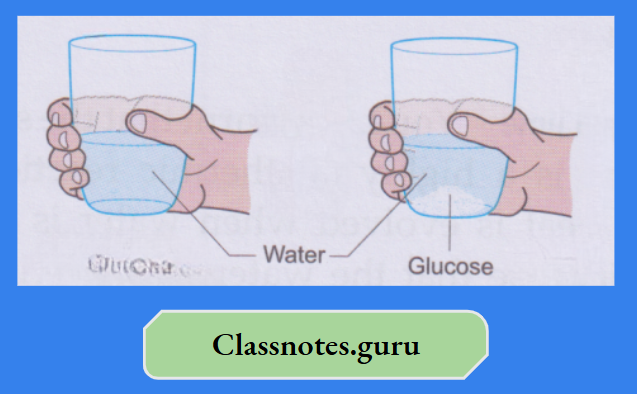
When you put some glucose on your tongue, your tongue feels cool. While dissolving in the moisture of the tongue, glucose absorbs heat from it. So, your tongue feels the cooling effect. On the other hand, if you add bathroom acid (concentrated hydrochloric acid) to water, heat is evolved.
Why an energy change in dissolution:
During dissolution, the solute particles break up from the main bulk and hide themselves in the intermolecular space of the solvent.
Energy change in a chemical change
All chemical changes involve a change in energy.
Burning:
You know that both heat and light are emitted when anything burns

Photosynthesis:
In photosynthesis, chlorophyll —the green pigment of leaves—absorbs sunlight (energy), and helps the plant produce glucose and oxygen from water and carbon dioxide.
Slaking of lime. You have learned that the slaking of lime is a highly exothermic reaction. So much heat is evolved when water is added to quicklime that the water boils during the reaction.
Quicklime + water→slaked lime + heat
Why an energy change in a chemical change:
A chemical change involves a rearrangement of atoms. The reactant molecules break up, and the product molecules are formed.
Energy Requirement for the Completion of a Change
The energy requirements are different for the completion of the two kinds of change: endothermic and exothermic
Endothermic change:
You know that energy is absorbed in an endothermic change. In other words, a substance essentially requires energy from outside to undergo an endothermic change. Hence, an endothermic change continues only till energy is pumped in.
For example:
A solid melts or a liquid boils only when it is heated. And the reaction between nitrogen and oxygen forming nitric oxide (NO)—a highly endothermic reaction—takes place only in the presence of an electric spark. So, the reaction takes place in the sky only when there is lightning
Exothermic change:
Heat is evolved in an exothermic change. So, at first instance, it appears that no energy should be required from outside by the substance undergoing such a change. And actually, there are many exothermic changes—especially the exothermic physical changes—which do not need energy from outside at any stage of the change.
A common example is the condensation of vapour or the freezing of a liquid. Similarly, when dilute hydrochloric acid is added to a solution of sodium hydroxide, an instant reaction takes place (forming sodium chloride and water), liberating heat.
However, many exothermic reactions do not take place without being initiated by heating, igniting, illumination and sparking. But once initiated, exothermic reactions go to completion on their own.
NCERT Class 8 Physical And Chemical Changes
Some examples are given below:
Thus, we can conclude that an exothermic reaction, once initiated, continues. The heat evolved is more than enough to sustain it.
You know that matter is made up of tiny particles called molecules. Molecules are held together by a force of attraction called an intermolecular force. There is also a space between the particles called the intermolecular space.
Matter And Its Properties
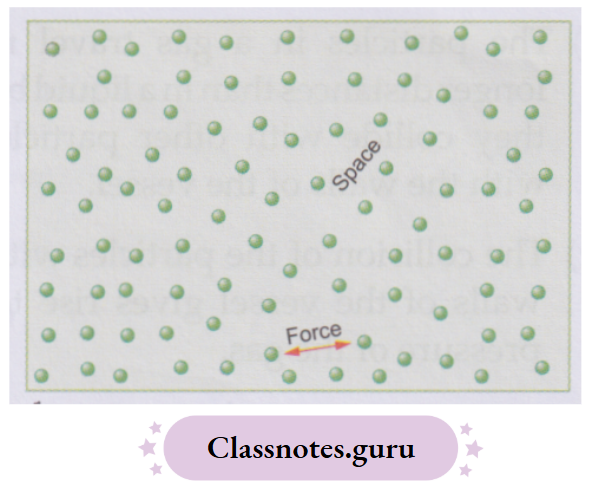
The force of attraction and the space between the particles (i.e., molecules) differ from substance to substance. But for any substance,
Read And Lean More Class 8 Chemistry
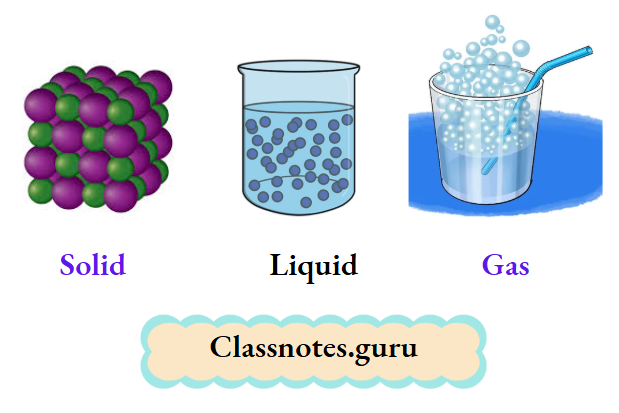
You have learnt earlier that matter exists in three states
For example:
NCERT Solutions For Matter Class 8
Water exists in three states— ice (solid), water (liquid), and water vapour (gaseous). Here we must understand that the kind of matter, i.e., the molecules constituting ice, water, or water vapour, is the same, i.e., water (H2O).
What Causes the Difference in States
For any substance, the molecules making up the substance do not change in the three states.
And so, the intermolecular space is the smallest in the solid state, larger in the liquid state, and the largest in the gaseous state
Thus, the molecules are most tightly held in solids, loosely held in liquids, and almost independent of each other in gases. This explains the general behaviour of solids, liquids, and gases.
Interconversion of States of Matter
As you know,
NCERT Class 8 Chemistry Chapter 1 Matter
Such a change in state may be brought about in other substances too, without a change in the composition
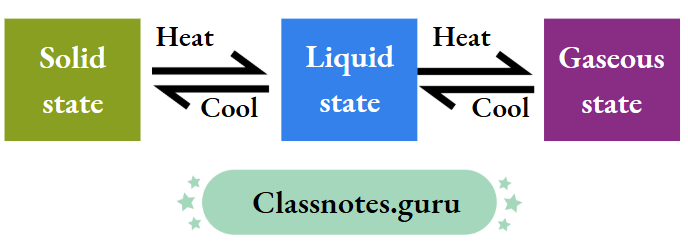
This is called the interconversion of states of matter.
For example:
For example:
States Of Matter NCERT Notes
The kinetic theory of matter explains the behaviour of the three states of matter.
It is summarised below.
How Kinetic Theory Explains the Interconversion of States of Matter
1. Solid-liquid interconversion
Solid to liquid:
Liquid to solid:
Types Of Matter Class 8
2. Liquid-gas (vapour) interconversion
Liquid to gas:
The particles in a liquid are in continuous motion, during which they collide among themselves.
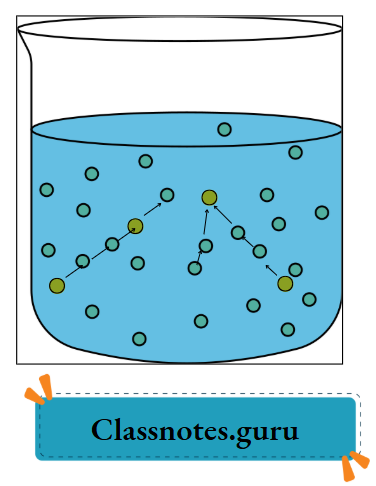
Gas (vapour) to liquid:
In the gaseous state, the particles move very fast, independently of each other. As the temperature is lowered, the KE of the particles is also lowered. When low-energy gaseous particles collide with each other, they may form bigger lumps or clusters, and the gas may condense into a liquid.
Solid Liquid Gas NCERT Notes
The Conditions of Change of State Differ from Substance to Substance
A liquid freezing at 0°C and boiling at 100 °C is water.
You know that only a rearrangement of atoms or molecules takes place when a change occurs. And as the atoms or molecules of a substance have fixed masses, the total mass of the substances involved in a change should remain the same.
Law of Conservation of Mass
Lavoisier, in the late eighteenth century, gave a law, called the law of conservation of mass, which can be stated as follows.
Conservation of Mass Physical changes:
It is easily understood that there is no change in the mass of a substance when it undergoes a physical change.
Properties Of Matter Class 8
For example:
The mass of an electric bulb does not change after it remains lit for some time.
Conservation of Mass Chemical changes:
According to the law of conservation of mass, in a chemical change, the sum of the masses of the reactants is the same as that of the products.
We must look at the total mass of the substances before and after the reaction.
Mass of the reactants = Mass of the products
Matter Science Notes
Let us consider the burning of a piece of paper. During the process, it appears that there is a loss of mass.
Mass of paper + oxygen = Mass of ash + carbon dioxide + water vapour
You can verify the law with the help of a simpler experiment, as described below.
Experiment:
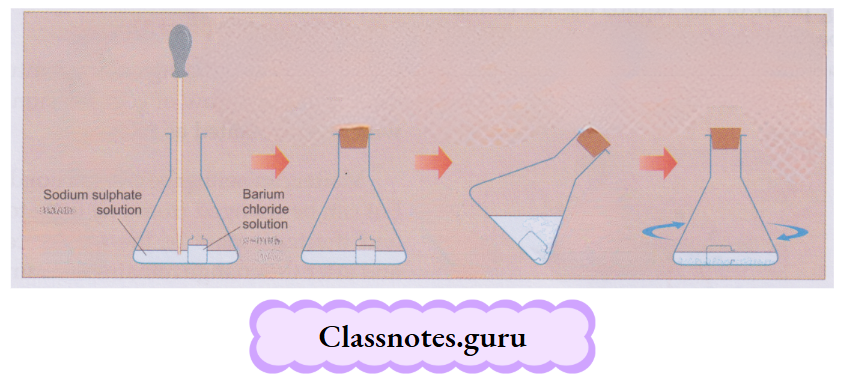
Put a small tube or bottle containing a solution of barium chloride into a conical flask.
NCERT Chemistry Matter Chapter
Hydrogen is the lightest element known. It was first prepared by Robert Boyle by diluting sulphuric acid on iron nails. Cavendish studied the gas and called it inflammable air, as the gas burns when kindled

The occurrence of Hydrogen Stars (including our sun) is mainly composed of hydrogen. Hydrogen is the most abundant element in the universe. On the earth, however, hydrogen occurs in very small amounts in the free state —in the air, in volcanic gases and the earth’s crust.
Read And Lean More Class 8 Chemistry

But there is plenty of hydrogen on Earth in combination with other elements, i.e., as part of compounds. Water is an important source of hydrogen. Every nine parts by mass of water contains one part of hydrogen. In combination with carbon, hydrogen is present in natural gas and petroleum as well as in all living things. Acids and alkalis also contain hydrogen.
Preparation of Hydrogen
Hydrogen can be obtained by the following methods.
1. By displacement reactions:
Since hydrogen is available in the combined state, it can be obtained by displacement reactions. You have learnt earlier that
Thus, metals above hydrogenin the activity series can displace hydrogen from water and acids. However, remember that tin and lead do not undergo this reaction with water and nor does lead with acids.
NCERT Solutions for Hydrogen Class 8
From water:
Highly active metals like sodium and calcium vigorously react with water, displacing hydrogen from it even in cold conditions.
2 Na + 2H2O → 2NaOH (sodium hydroxide) + H2↑
Ca + 2H2O → Ca2 (OH)(Calcium hydroxide) + H2↑
A less active metal, like magnesium, does so when steam is passed over it.
⇒ \(\mathrm{Mg}+\underset{\text { (steam) }}{\mathrm{H}_2 \mathrm{O}} \quad \rightarrow \underset{\text { magnesium oxide }}{\mathrm{MgO}}+\mathrm{H}_2 \uparrow\)
A still less active metal like iron, when steam is passed over the red-hot metal.
3 Fe + 4H2O(steam) ⇌ Fe3O4 (ferrosoferric oxide) +4H2 ↑
From an acid:
All metals above hydrogen (except lead) in the activity series displace hydrogen from acid, like dilute hydrochloric or sulphuric acid. (We do not consider nitric acid for this reaction because it gives the oxides of nitrogen.)
⇒ \(\left.\begin{array}{c}
2 \mathrm{Na}+2 \mathrm{HCl} \longrightarrow 2 \mathrm{NaCl}+\mathrm{H}_2 \dagger \\
\mathrm{Mg}+2 \mathrm{HCl} \longrightarrow \mathrm{MgCl}_2+\mathrm{H}_2 \uparrow
\end{array}\right] \text { Vigorous }\)
⇒ \(\left.\begin{array}{l}
\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \underset{\text { zinc sulphate }}{\mathrm{ZnSO}_4}+\mathrm{H}_2 \uparrow \\
\mathrm{Fe}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \underset{\text { iron(II) sulphate }}{\mathrm{FeSO}_4}+\mathrm{H}_2 \uparrow
\end{array}\right] \text { Moderate }\)
2. By the electrolysis of water:
You have learnt earlier that acidulated water decomposes into hydrogen and oxygen when an electric current is passed through it
Example:
NaCl, which is made of Na+ and Cl– ions) or form ions in solution. Pure water does not allow an electric current to pass through it. But water acidulated with (a very small amount of) dilute hydrochloric or sulphuric acid does because it dissociates.
NCERT Class 8 Chemistry Chapter 7 Hydrogen
⇒ \(\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}\)
During electrolysis, the H+ ions move towards the negative electrode, i.e., the cathode, and get discharged (lose their charge) there by the negative charge of the electrode. And the OH” ions move to the positive electrode, i.e., the anode, and get discharged to give oxygen

You will learn in higher classes that, instead of the H+ ion, it is the H3O+ ion (i.e., H++ H2O), called the hydronium ion, that exists in an aqueous solution
The laboratory method
Principle:
Hydrogen is prepared in the laboratory by the action of dilute hydrochloric or sulphuric acid on granulated zinc. We do not use nitric acid, as the oxides of nitrogen are formed
⇒ \(\mathrm{Zn}+2 \mathrm{HCl} \longrightarrow \mathrm{ZnCl}_2+\mathrm{H}_2 \uparrow\)
⇒ \(\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{ZnSO}_4+\mathrm{H}_2 \uparrow\)
As the gas is almost insoluble in water, it is collected by the displacement of water.
Procedure:
A conical flask is fitted with a thistle funnel and a delivery tube. The other end of the delivery tube passes through a beehive shelf placed in a water trough. A gas jar full of water is inverted over the beehive shelf. Some granulated zinc is placed in the conical flask.
Dilute hydrochloric or sulphuric acid is added through the thistle funnel till the lower end of the funnel dips in the liquid. Hydrogen then begins to evolve.
The gas is collected by the downward displacement of water. It is not collected by the downward displacement of air since a mixture of hydrogen and air is explosive.
Initially, the air inside the flask and the delivery tube is driven out. So, whatever is collected in the first one or two jars is air, and is rejected. The gas collected afterwards is hydrogen.

Why we prefer zinc to other metals
In the above method, we could have used any metal more active than hydrogen (except lead). We do not use highly active metals like sodium, calcium and magnesium because the reactions of these metals with an acid are too vigorous to control and these metals are expensive too.
The moderately active metals—zinc and iron, which are also cheaper—should be more suitable. We do not use iron either, because it forms some foul-smelling poisonous gases of silicon, sulphur and phosphorus present in it as impurities. So, we prefer zinc to other metals.
Hydrogen Class 8 NCERT Notes
Large-scale preparation
Hydrogen is prepared on a large scale, i.e., in the industry, by the following methods.
Let us discuss the principles of the Bosch method.
The Bosch method:
When steam is passed over red-hot coke, what is known as water gas is formed. Water gas is a mixture of mainly carbon monoxide (CO) and hydrogen (H2), containing some carbon dioxide (CO2).
⇒ \(\underset{\text { (steam) }}{\mathrm{H}_2 \mathrm{O}}+\underset{\text { (red hot) }}{\mathrm{C}} \longrightarrow \underbrace{\mathrm{CO}+\mathrm{H}_2}_{\text {water gas }}\)
The water gas is mixed with excess steam and heated to 450 °C. The mixture is passed over a catalyst consisting of iron(III) oxide (Fe2 O3) mixed with some chromium(III) oxide (Cr2O3). The CO abstracts oxygen from Thistle funnel H2O to form CO2 and releases more H2
⇒ \(\underbrace{\mathrm{CO}+\mathrm{H}_2}_{\text {water gas }}+\underset{\text { (steam) }}{\mathrm{H}_2 \mathrm{O}} \frac{450^{\circ} \mathrm{C}}{\mathrm{Fe}_2 \mathrm{O}_3 \text { catalyst }} \mathrm{CO}_2+2 \mathrm{H}_2\)
The CO2 is removed from the mixture by dissolving it in water under pressure. (Remember that ordinarily, CO2 is only slightly soluble in water but highly soluble under pressure.)
Physical Properties
Chemical Properties
1. Reaction with oxygen (or air):
When kindled, hydrogen bums in air or oxygen to form water
⇒ \(2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}\)
A large amount of heat is produced in this reaction. So, a mixture of hydrogen and oxygen may explode.
2. Reaction with chlorine:
When kindled, hydrogen burns in chlorine to form hydrogen chloride gas. Also, a mixture of hydrogen and chlorine, when placed in sunlight, explodes to form the same product
⇒ \(\mathrm{H}_2+\mathrm{Cl}_2 \longrightarrow \underset{\text { hydrogen chloride gas }}{2 \mathrm{HCl} \uparrow}\)
Hydrogen chloride gas can be dissolved in water to obtain hydrochloric acid.
Properties of Hydrogen Class 8
3. Reaction with sulphur:
Hydrogen reacts with molten sulphur to give hydrogen sulphide gas, which smells like rotten eggs.
⇒ \(\mathrm{H}_2+\underset{\text { (molten) }}{\mathrm{S}} \longrightarrow \underset{\text { hydrogen sulphide }}{\mathrm{H}_2 \mathrm{~S} \uparrow}\)
4. Reaction with nitrogen:
Hydrogen reacts with nitrogen only under special conditions to form an appreciable amount of ammonia.

The ammonia gas formed, in turn, decomposes back to hydrogen and nitrogen. So, this reaction occurs both ways. Such reactions are called reversible reactions.
5. Reactions with some metal oxides:
When passed over some hot metal oxides like zinc oxide, iron oxide and copper oxide, hydrogen gas converts them into the corresponding metals.

The oxides of metals like potassium, calcium, sodium and magnesium are not converted into the metals.
Hydrogen is a reducing agent. The addition of hydrogen to or the removal of oxygen from a substance is called reduction.
On the other hand, the addition of oxygen to or the removal of hydrogen from a substance is called’ oxidation. Also, a substance causing reduction is known as a reducing agent, and one causing oxidation is called an oxidising agent.
You have just seen that hydrogen
Thus, hydrogen is a reducing agent. It reduces
By adding H:
By removing O:
Hydrogen as a Fuel Class 8
Hydrogen burns with a characteristic sound, or ‘pop’. This property is used as a test for hydrogen
1. For manufacturing ammonia:
Hydrogen is used in large quantities to manufacture ammonia.
2. For the hydrogenation of oils:
Vegetable oils react with hydrogen in the presence of a catalyst (like nickel) to form solid fats. Such addition of hydrogen is called hydrogenation.
3. As a fuel:
On the combustion of hydrogen, i.e., when hydrogen is burnt, heat is produced along with water

So, hydrogen can be used as a fuel. The product of the reaction is water, which does not pollute the environment. Hence, hydrogen is a clean fuel.
Hydrogen produces the maximum heat among all the known fuels. Liquid hydrogen is used as rocket fuel. However, it is difficult to handle and store.
4. The oxyhydrogen flame:
Hydrogen is used to produce an oxyhydrogen flame, which is employed for welding and cutting metals. The two gases—oxygen and hydrogen—passing through different pipes, mix at a point where the mixture is kindled. A high-temperature (2800 °C) flame, called an oxyhydrogen flame, is produced. The metal melts at this temperature, enabling it to be cut or welded.
5. As a reducing agent:
Hydrogen is used as a reducing agent in the burning laboratory and industry
6. For filling balloons:
As hydrogen is lighter than air, a balloon filled with hydrogen rises in the air and drifts in the wind. If a weather instrument is placed in it, the balloon can be used for studying weather conditions.
There was a time when such weather balloons were much in use. But the practice was stopped as such weather balloons often caught fire due to the inflammability of hydrogen. As helium, the next lightest gas, is available in plenty, it is preferred to hydrogen. Helium does not catch fire.
Hydrogen and its Compounds Class 8
Weather balloons:

Fill balloons:

In the 1930s, airships using hydrogen to float were used for transport. But they were discontinued after the airship Hindenburg caught fire in 1937, killing 36 people.
We have discussed a large number of chemical reactions. Some are accompanied by a change in colour, some by the evolution of a gas, some by the formation of a precipitate, and so on. At the molecular level, different types of changes occur. In some reactions, smaller parts add up to form bigger entities, whereas in some others, bigger entities break into smaller ones.

Again, in some reactions, one element displaces another from a compound whereas in some others, compounds exchange radicals among themselves. For a systematic study, therefore, it is essential that we classify the reactions into different types.
NCERT Solutions for Chemical Reactions Class 8
Read And Lean More Class 8 Chemistry
However, we will discuss first the change in energy in chemical reactions, which is common to all types
All reactions are accompanied by a change in energy. Energy (in the form of heat or light) is either given out or taken in during a chemical reaction.
As we know, any two atoms in a molecule are held together by a force of attraction called a chemical bond. And also that a chemical bond is much stronger than an intermolecular force. We also know that it is the atoms that take part in a chemical reaction.
Thus, we can consider a chemical reaction to be a result of the following phenomena.
The energy required for the breaking of the bonds of the reactant molecules is not the same as that given out in the formation of the bonds between the new partners.
However, when the energy required for the breaking of the bonds is greater than that given out in the formation of the bonds, the difference in energy has to be compensated for from outside.
In other words, energy will be absorbed from the surroundings for the reaction to happen, and the process will be endothermic.

NCERT Class 8 Chemistry Chapter 6 Chemical Reactions
Examples of Exothermic Reactions
We will now discuss some common exothermic reactions
1. Burning:
The burning of a substance is exothermic. It is the exothermicity of burning that makes a combustible substance a fuel. For example, hydrogen, carbon (coal), CNG(CH4), and LPG(C4H10) are fuels.

2. How some active metals react with water:
The reaction of a highly active metal like K, Na or Ca is vigorous and exothermic. Hydrogen is liberated, and the metal hydroxide is formed in the reaction.
2K(s) (potassium) + 2H2O(l) → 2 KOH(aq)(Potassium hydroxide) + H2(g)
2Na(s) + 2H2O(l) → 2NaOH(aq)(sodiumhydroxide) + H2(g)
Ca(s) + 2H2O(l) → Ca (OH)2(calcium hydroxide)(aq) + H2(g)
3. The dissolution of metal oxides (bases) in water:
Bases like sodium oxide and calcium oxide vigorously react with water to form their hydroxides, releasing a large amount of heat.
The hydroxides dissolve in an excess of water. The reaction of calcium oxide (CaO, commercially known as quicklime) with water, giving calcium hydroxide [Ca(OH)2, commercially known as slaked lime] is called the slaking of lime.

4. The dissolution of nonmetal oxides (acidic) in water:
The oxides of nonmetals are acidic and many of them dissolve in water to form acids exothermically.
⇒ \(\begin{aligned}
&\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \mathrm{H}_2 \mathrm{CO}_3(\mathrm{aq}){ carbonic acid }\\\end{aligned}\)
⇒ \(\begin{aligned}
&\mathrm{SO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \mathrm{H}_2 \mathrm{SO}_3(\mathrm{aq})sulphurous acid \\\end{aligned}\)
⇒ \(\underset{\text { sulphur trioxide }}{\mathrm{SO}_3(\mathrm{l})}+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \underset{\text { sulphuric acid }}{\mathrm{H}_2 \mathrm{SO}_4(\mathrm{aq})}\)
5. The reaction between a basic and an acidic oxide:
A basic oxide reacts with an acidic oxide, forming a salt and evolving heat
⇒ \(\begin{aligned}
&\mathrm{Na}_2 \mathrm{O}(\mathrm{~s})+\mathrm{CO}_2(\mathrm{~g}) \longrightarrow \mathrm{Na}_2 \mathrm{CO}_3(\mathrm{~s})(sodium carbonate)\\
&\text { }
\end{aligned}\)
⇒ \(\mathrm{CaO}(\mathrm{~s})+\mathrm{CO}_2(\mathrm{~g}) \longrightarrow \underset{\substack{\text { calcium carbonate } \\ \text { (limestone) }}}{\mathrm{CaCO}_3(\mathrm{~s})}\)
6. Neutralisation reactions:
In a neutralisation reaction, an acid reacts with a base, forming a salt and water. All such reactions are exothermic
⇒ \(\text { Base }+ \text { acid } \longrightarrow \text { salt + water }\)
⇒ \(\mathrm{NaOH}(\mathrm{aq})+\mathrm{HCl}(\mathrm{aq}) \longrightarrow \mathrm{NaCl}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
⇒ \(\mathrm{KOH}(\mathrm{aq})+\mathrm{H}_2 \mathrm{SO}_4(\mathrm{aq}) \longrightarrow \mathrm{K}_2 \mathrm{SO}_4(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
Chemical Reactions Class 8 NCERT Notes
7. Reaction of an acid with a metal carbonate:
By performing a similar activity as above, you can conclude that heat is evolved when an acid acts on a carbonate or a hydrogencarbonate, producing CO2
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq})\) → \(2 \mathrm{NaCl}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{CO}_2(\mathrm{~g})\)
⇒ \(\mathrm{CaCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \longrightarrow\)\(\mathrm{CaCl}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{CO}_2(\mathrm{~g})\)
8. Respiration:
You know that, in respiration, the glucose formed in plants and animals combines with the oxygen of the air to give C02 and H20. The reaction, being exothermic, provides living beings with the energy to sustain their life processes.
⇒ \(\begin{aligned}
&\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})+6 \mathrm{O}_2(\mathrm{~g}) \longrightarrow 6 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l})\\
&\text { glucose }
\end{aligned}\)
9. Rusting
In rusting, iron slowly combines with oxygen (air) in the presence of moisture to form the brownish red hydrated iron(III) oxide, called rust
⇒ \(4 \mathrm{Fe}(\mathrm{~s})+3 \mathrm{O}_2(\mathrm{~g})+2 x \mathrm{H}_2 \mathrm{O}(\mathrm{~g}) \longrightarrow\) \(\underset{\text { rust }}{2\left[\mathrm{Fe}_2 \mathrm{O}_3 \cdot x \mathrm{H}_2 \mathrm{O}\right](\mathrm{s})}\)
The reaction is exothermic, but too slow to let you feel its heat at any point in time.
Examples of Endothermic Reactions
Endothermic reactions are much fewer in number than exothermic ones. Some examples are given below
1. Combination of nitrogen with oxygen:
Nitrogen combines with oxygenforming nitric oxide (NO) when an electric spark is passed through a mixture of the two gases (as during lightning). The reaction is endothermic.
⇒ \(\mathrm{N}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow[\text { spark }]{\text { electric }} 2 \mathrm{NO}(\mathrm{~g})\)
2. Thermal decomposition of metal carbonates:
The breaking down of a substance into simpler substances on being heated is called thermal decomposition. The carbonates of many metals decompose on being heated to give the metal oxides and carbon dioxide. These are endothermic reactions. Remember that, among the common carbonates, sodium carbonate (Na2C03) and potassium carbonate (K2C03) do not undergo such a reaction
⇒ \(\mathrm{CaCO}_3(\mathrm{~s}) \xrightarrow{\text { heat }} \mathrm{CaO}(\mathrm{~s})+\mathrm{CO}_2(\mathrm{~g})\)
⇒ \(\mathrm{MgCO}_3(\mathrm{~s}) \xrightarrow{\text { heat }} \mathrm{MgO}(\mathrm{~s})+\mathrm{CO}_2(\mathrm{~g})\)
3. Photosynthesis:
We know that a plant prepares its food, glucose, from atmospheric C02 and soilmoisture in the presence of chlorophyll by absorbing sunlight.
The reaction is endothermic
⇒ \(6 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \xrightarrow[\text { chlorophyll }]{\text { sunlight }}\) \(\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})+6 \mathrm{O}_2(\mathrm{~g})\)
Endothermic physical processes:
Many physical processes are endothermic. We have learnt that the melting of ice requires heat from outside and so does the boiling of water.
Some common observations:
Chemical reactions have the following general characteristics.
1. Exothermic reactions are more common than endothermic reactions:
This is because exothermic reactions do not need energy from the surroundings.
Types of Chemical Reactions Class 8
2. Once they begin, exothermic reactions continue on their own but endothermic reactions do not:
This is because some heat is required to keep a reaction going. And that heat is generated on its own during an exothermic reaction.
For example:
When you light a combustible substance, it burns on its own.
For example:
Nitrogen combines with oxygen to form nitric oxide (NO) only as long as an electric spark is passed through a mixture of nitrogen and oxygen. The reaction stops in the absence of a spark
⇒ \(\mathrm{N}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow[\text { spark }]{\text { electric }} 2 \mathrm{NO}(\mathrm{~g})\)
Also, photosynthesis takes place in the presence of sunlight. Does it ever happen in the dark?
3. Most exothermic reactions too have to be initiated:
For example:
A substance burns when ignited, hydrogen reacts with oxygen when ignited, and so on. You will learn the reason for this in higher classes.
Among the different types of chemical reactions, combination, decomposition, displacement, double displacement and neutralization reactions are the ones we commonly come across. We will discuss them here.
Combination Reactions
In a combination reaction (synthesis), two or more reactants combine to form a product. Some examples are given below. The burning of some elements Elements like H, C, S, P, Ca and Mg, on being burnt in air, form their oxides
By direct combination:
1. \(2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { burn }} 2 \mathrm{H}_2 \mathrm{O}(\mathrm{~g})\)
2.\(\mathrm{C}(\mathrm{~s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { burn }} \mathrm{CO}_2(\mathrm{~g}) \\\)
3. \(\mathrm{S}(\mathrm{~s}) +\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { burn }} \mathrm{SO}_2(\mathrm{~g}) \\\)
4. \(2 \mathrm{Ca}(\mathrm{~s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { burn }} 2 \mathrm{CaO}(\mathrm{~s})\\\)
5. \(2 \mathrm{Mg}(\mathrm{~s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { burn }} 2 \mathrm{MgO}(\mathrm{~s})\)
The burning of carbon:
Burn some charcoal (carbon) in a deflagrating spoon. Introduce the spoon into an open gas jar near the mouth of the jar so that the burning charcoal gets a clear supply of air.

The burning of magnesium:
When ignited in air, say in an open gas jar, it burns with a dazzling white flame. The magnesium oxide formed deposits as a white, smoky solid on the walls of the jar

Chemical Reactions and Equations NCERT Notes
Rusting
A piece of iron, when left in moist air, slowly gets a crust of a brown-red solid called rust. Rust —a hydrated iron(III) oxide product of the direct combination of iron with the oxygen and moisture of the air.
4Fe(s) + 3O2(g) + Moisture → 2[Fe2O3. xH2O](s)(rust brown- red)
(x can vary).
The combination of elements with chlorine
Chlorine is a very active nonmetal—a greenish-yellow gas. Most metals and some nonmetals combine with chlorine to form their chlorides
⇒\(2 \mathrm{Na}(\mathrm{~s})+\mathrm{Cl}_2(\mathrm{~g}) \longrightarrow \underset{\text { sodium chlorid }}{2 \mathrm{NaCl}(\mathrm{~s})}\)
⇒ \(2 \mathrm{Fe}(\mathrm{~s})+3 \mathrm{Cl}_2(\mathrm{~g}) \xrightarrow{\text { heat }} \underset{\text { iron }(\text { III }) \text { chloride }}{2 \mathrm{FeCl}_3(\mathrm{~s})}\)
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \xrightarrow{\text { ignite }} \underset{\text { hydrogen chloride }}{2 \mathrm{HCl}(\mathrm{~g})}\)
⇒ \(\underset{\text { phosphorus }}{\mathrm{P}_4(\mathrm{~s})}+10 \mathrm{Cl}_2(\mathrm{~g}) \xrightarrow{\text { burn }} \underset{\begin{array}{c}
\text { phosphorus } \\
\text { pentachloride }
\end{array}}{4 \mathrm{PCl}_5(\mathrm{~s})}\)
The combination of iron with sulphur
You have already learnt that iron combines with sulphur, when heated, to form iron(II) sulphide.
⇒ \(\underset{\substack{\text { iron } \\ \text { (grey) }}}{\mathrm{Fe}}+\underset{\substack{\text { sulphur } \\ \text { (yellow) }}}{\mathrm{S}} \xrightarrow{\text { heat }} \underset{\substack{\text { iron(II) sulphide } \\ \text { (greyish black) }}}{\mathrm{FeS}}\)
The combination of oxides with water
The oxide of a nonmetal generally dissolves in water to form an acid.
⇒ \(\begin{aligned}
&\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{H}_2 \mathrm{CO}_3 (carbonic acid )\\
&\text { }
\end{aligned}\)
⇒ \(\begin{aligned}
&\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_3( sulphurous acid)\\
&\text { }
\end{aligned}\)
Balancing Chemical Reactions Class 8

And the oxides of many metals combine with water to form the basic hydroxides.
⇒ \(\underset{\text { sodium oxide }}{\mathrm{Na}_2 \mathrm{O}}+\mathrm{H}_2 \mathrm{O} \longrightarrow \underset{\text { sodium hydroxide }}{2 \mathrm{NaOH}}\)
⇒ \(\underset{\text { calcium oxide }}{\mathrm{CaO}}+\mathrm{H}_2 \mathrm{O} \longrightarrow \underset{\text { calcium hydroxide }}{\mathrm{Ca}(\mathrm{OH})_2}\)
When carbon dioxide is passed through water for some time, the water turns blue litmus wine red, showing that it contains an acid.
Decomposition Reactions
In a decomposition reaction, one substance breaks down into two or more simpler substances.
Some examples are given below:
Decomposition of water on electrolysis Electrolysis is a process in which a substance is decomposed, or broken down into simpler substances, by passing an electric current through it
Water mixed with a very small amount of an acid breaks down into hydrogen and oxygen on electrolysis
⇒ \(\underset{\text { water }}{\mathrm{H}_2 \mathrm{O}} \xrightarrow{\text { electrolysis }} \underset{\substack{\text { hydrogen } \\
\text { (negative } \\
\text { electrode) }}}{2 \mathrm{H}_2}+\underset{\begin{array}{c}
\text { oxygen } \\
\text { (positive } \\
\text { electrode) }
\end{array}}{\mathrm{O}_2}\)
Take some water, mixed with a few drops of dilute sulphuric acid, in a beaker. Invert two test tubes full of water into it. Remove the insulation from the ends of two thick wires. Introduce them into the test tubes as shown in. Connect the wires to a battery and pass current for some time. Gases start collecting in the test tubes. You will observe that the volume of the gas collected over the negative electrode is twice that of the gas collected over the positive electrode.

Stop passing current when there is enough gas in the test tubes. Cork the test tubes inside the water and take them out. Perform the following experiments.
The gas collected at the negative electrode:
Bring a lighted match near the mouth of the tube and open its mouth. The gas burns with a ‘pop’, and so it is hydrogen.
The gas collected at the positive electrode:
Bring a glowing matchstick near the mouth of the test tube and remove the cork. The matchstick gets lighted. So, the gas is oxygen
The experiment also shows that the volume ratio of hydrogen and oxygen in water is 2:1
Chemical Reactions and Their Types Class 8
The decomposition of baking soda
Sodium hydrogencarbonate (NaHC03) is called baking soda. On strong heating, baking soda decomposes into sodium carbonate, water (vapour) and carbon dioxide.
⇒ \(\underset{\begin{array}{c}
\text { sodium } \\
\text { hydrogencarbonate }
\end{array}}{2 \mathrm{NaHCO}_3(\mathrm{~s})} \xrightarrow{\text { heat }} \underset{\begin{array}{c}
\text { sodium } \\
\text { carbonate }
\end{array}}{\mathrm{Na}_2 \mathrm{CO}_3(\mathrm{~s})}+\underset{\begin{array}{c}
\text { water } \\
\text { vapour }
\end{array}}{\mathrm{H}_2 \mathrm{O}(\mathrm{~g})}\)
The decomposition of potassium chlorate
When strongly heated, potassium chlorate gives potassium chloride and oxygen.
⇒ \(\underset{\begin{array}{c}
\text { potassium } \\
\text { chlorate }
\end{array}}{2 \mathrm{KClO}_3(\mathrm{~s})} \xrightarrow{\text { heat }} \underset{\begin{array}{c}
\text { potassium } \\
\text { chloride }
\end{array}}{2 \mathrm{KCl}(\mathrm{~s})}+\underset{\text { oxygen }}{3 \mathrm{O}_2(\mathrm{~g})}\)
Displacement Reactions
In a displacement reaction, one element displaces another from its compound and takes its place therein.
Displacement reactions are best studied using the activity series. In the activity series, metals, along with hydrogen, are arranged in order of their activity. The higher an element is placed in the series, the more active it is. Thus, you can easily find out the relative activity of the metals along with hydrogen by consulting the series. For example, Na is more active than M,g and Mg is more active than Fe and so they fall in the following order of activity:
Na > Mg > Fe.
Could you say in what order will the activity of copper, silver, magnesium, iron and aluminium decrease? It has been observed that a more active metal displaces a less active one from its compounds.
Some common examples are given below.
The displacement of hydrogen from water by a metal:
An active metal like potassium (K), sodium (Na) or calcium (Ca) reacts with water, displacing hydrogen even in cold conditions.
2K(s) + 2H2O(1) — 2KOH(aq) + H2(g)
2Na(s) + 2H2O(1) — 2NaOH(aq) + H2(g)
Ca(s) + 2H2O(1) — Ca(OH)2(aq) + H2 (g)
Magnesium, which is less active than the above-mentioned metals, reacts only at high temperatures.
When placed in steam, a burning magnesium ribbon continues to bum, forming magnesium oxide and hydrogen.
⇒ \(\underset{\text { magnesium }}{\mathrm{Mg}(\mathrm{~s})}+\underset{\text { water }(\text { steam })}{\mathrm{H}_2 \mathrm{O}(\mathrm{~g})} \longrightarrow \underset{\text { magnesium oxide }}{\mathrm{MgO}(\mathrm{~s})}+\underset{\text { hydrogen }}{\mathrm{H}_2(\mathrm{~g})}\)
In this reaction, a magnesium atom displaces two hydrogen atoms and takes their place

A less active metal like magnesium, zinc or iron easily displaces hydrogen from dilute hydrochloric or sulphuric acid.
⇒ \(\underset{\text { zinc }}{\mathrm{Zn}(\mathrm{~s})}+2 \mathrm{HCl}(\mathrm{aq}) \longrightarrow \underset{\text { zinc chloride }}{\mathrm{ZnCl}}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})\)
⇒ \(\underset{\substack{\text { iron }}}{\mathrm{Fe}(\mathrm{~s})}+\mathrm{H}_2 \mathrm{SO}_4(\mathrm{aq}) \longrightarrow \underset{\text { iron(II) sulphate }}{\mathrm{FeSO}_4(\mathrm{aq})}+\mathrm{H}_2(\mathrm{~g})\)
The displacement of copper from copper sulphate by iron or zinc:
When an iron knife or nail is placed in a copper sulphate solution, there is a brown-red deposit of copper over the iron object

After some time, the blue colour of the solution changes to green, owing to the formation of iron(II) sulphate
⇒ \(\underset{\substack{\text { iron } \\ \text { igrey) }}}{\mathrm{Fe}(\mathrm{~s})}+\underset{\substack{\text { copper sulphate } \\ \text { (blue) }}}{\mathrm{CuSO}_4(\mathrm{aq})} \longrightarrow \underset{\text { copper }}{\text { (brown-red) }} \underset{\text { cuper }}{\mathrm{Cu}(\mathrm{~s})}+\underset{\text { (iron(I) sulphate }}{\mathrm{FeSO}_4}(\mathrm{aq})\)
Reactants and Products Class 8 Chemistry
Also, zinc displaces copper from copper sulphate, but the resultant solution is colourless. You can use a piece of granulated zinc in place of iron and it will be coated by copper
⇒ \(\underset{\substack{\text { zinc } \\ \text { (white) }}}{\mathrm{Zn}(\mathrm{~s})}+\underset{\substack{\text { copper sulphate } \\ \text { (blue) }}}{\mathrm{CuSO}_4(\mathrm{aq})} \rightarrow \underset{\substack{\text { copper } \\ \text { (brown-red) }}}{\mathrm{Cu}(\mathrm{~s})}+\underset{\substack{\text { zinc sulphate } \\ \text { (colourless) }}}{\mathrm{ZnSO}_4(\mathrm{aq})}\)
In a double displacement reaction, the positive and negative radicals of two reactants are exchanged, leading to the precipitation of a product.
AgNO3 (aq) silver nitrate (colourless) + NaCl(aq) sodium chloride (colourless) → AgCl(s) + NaNO3 (aq)

The reaction between barium chloride and sodium sulphate:
When a solution of barium chloride is mixed with that of sodium sulphate, a white precipitate of barium sulphate is formed along with a solution of sodium chloride.
The solution initially appears white but gradually becomes colourless as the precipitate settles down
\(\underset{\substack{\text { barium chloride } \\ \text { solution } \\ \text { (colourless) }}}{\mathrm{BaCl}_2(\mathrm{aq})}+\underset{\substack{\text { sodium sulphate } \\ \text { (colution } \\ \text { (colourless) }}}{\mathrm{Na}_2 \mathrm{SO}_4(\mathrm{aq})}\) → \(\underset{\substack{\text { barium sulphate } \\ \text { precipitate } \\ \text { (white) }}}{\mathrm{BaSO}_4(\mathrm{~s})}+\underset{\substack{\text { sodium chloride } \\ \text { solution } \\ \text { (colourless) }}}{2 \mathrm{NaCl}(\mathrm{aq})}\)
Neutralisation Reactions
In a neutralisation reaction, an acid reacts with a base, forming a salt and water. The acid as well as the base lose their properties to form a salt and water, which are neither acidic nor basic, i.e., they are neutral. So, the reaction is called a neutralisation reaction.
Some examples are given below:
Acid + Base → Salt+ Water
HCl(aq) + NaOH(aq)- NaCl(aq) + H2O(I)
H2SO4(aq) + 2KOH(aq)→ K2SO4(aq) + 2H2O(I)
H2SO4(aq) + 2NaOH(aq)→ Na2SO4(aq) + 2H2O(I)
2HCl(aq) + Ca(OH)2(aq)→CaCl2(aq) + 2H2O(I)
3HCl(aq) + Al(OH)3(aq) → AlCl3(aq) + 3H2O(I)
Activity:
Take about 5 ml of dilute sodium hydroxide in a conical flask and dilute it with a test tube of water. Swirl the flask to ensure thorough mixing. Add a drop of phenolphthalein solution to it.
The contents of the flask turn pink. (Phenolphthalein is an indicator which turns red in a basic solution and colourless in a neutral or acidic solution.)
Add dilute hydrochloric acid dropwise with the help of a long dropper and swirl the contents after each addition. Add the acid till the pink colour just vanishes. The solution in the flask is neutral —neither acidic nor basic —and the reaction is neutralisation.’
Chemical Reactions in Everyday Life Class 8

Oxides are an important class of compounds. We know that the two simple oxides, water (H2O) and carbon dioxide (CO2), play important roles in our life processes, agriculture and industry. Let us discuss the preparation and the acid-base nature of oxides in general.
Preparation
They can be prepared by the following processes.
By direct combination:
An element generally forms its oxide when heated or burned in air or oxygen. The reaction is exothermic and hence continues on its own when initiated.
Nonmetals:
⇒ \(2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { ignite }} 2 \mathrm{H}_2 \mathrm{O}(\mathrm{~g})\)
⇒ \(\mathrm{C}(\mathrm{~s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { burn }} \mathrm{CO}_2(\mathrm{~g}) \\\)
⇒ \(\mathrm{S}(\mathrm{~s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { burn }} \mathrm{SO}_2(\mathrm{~g})\)
Metals:
⇒ \(2 \mathrm{Mg}(\mathrm{~s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { ignite }} 2 \mathrm{MgO}(\mathrm{~s}) \\\)
⇒ \(2 \mathrm{Ca}(\mathrm{~s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { ignite }} 2 \mathrm{CaO}(\mathrm{~s})\)
By the thermal decomposition of some compounds
The oxides of both metals and nonmetals are formed when the hydroxides, carbonates, sulphates and nitrates of metals are strongly heated
1. Hydroxides:
1. \(\mathrm{Zn}(\mathrm{OH})_2(\mathrm{~s}) \xrightarrow{\text { heat }} \mathrm{ZnO}(\mathrm{~s})+\mathrm{H}_2 \mathrm{O}(\mathrm{~g}) \\\)
2. \({\text {(bluish white) }}{\mathrm{Cu}(\mathrm{OH})_2(\mathrm{~s}) \xrightarrow{\text { heat }} \underset{\text { (black) }}{\mathrm{CuO}(\mathrm{~s})}+\mathrm{H}_2 \mathrm{O}(\mathrm{~g})}\)
2. Carbonates:
1.\(\underset{\text { (white) }}{\mathrm{CaCO}_3(\mathrm{~s})} \xrightarrow{\text { heat }} \underset{\text { (white) }}{\mathrm{CaO}(\mathrm{~s})}+\mathrm{CO}_2(\mathrm{~g}) \\\)
2.\(\underset{\text { (white) }}{\mathrm{ZnCO}_3(\mathrm{~s})} \xrightarrow{\text { heat }} \underset{\text { (white) }}{\mathrm{ZnO}(\mathrm{~s})}+\mathrm{CO}_2(\mathrm{~g}) \\\)
3.\(\underset{\text { (white) }}{\mathrm{PbCO}_3(\mathrm{~s})} \xrightarrow{\text { heat }} \underset{\text { (yellow) }}{\mathrm{PbO}(\mathrm{~s})}+\mathrm{CO}_2(\mathrm{~g}) \\\)
4.\( \mathrm{CuCO}_3(\mathrm{~s}) \xrightarrow[\text { (green) }]{\text { heat }} \underset{\text { (black) }}{\mathrm{CuO}(\mathrm{~s})}+\mathrm{CO}_2 \text { (s) }\)
Exceptions: Na2CO3 and K2CO3
3. Sulphates:
1.\(\mathrm{Fe}_2\left(\mathrm{SO}_4\right)_3(\mathrm{~s}) \xrightarrow{\text{heat}}\mathrm{Fe}_2\mathrm{O}_3(\mathrm{~s})+3 \mathrm{SO}_3(\mathrm{~g}) \\\)
2. \(\underset{\text { (white) }}{\mathrm{CuSO}_4(\mathrm{~s})} \xrightarrow{\text { heat }} \underset{\text { (black) }}{\mathrm{CuO}(\mathrm{~s})}+\mathrm{SO}_3(\mathrm{~g})\)
Exceptions: Na2SO4, K2SO4, CaSO4 and many others
4. Nitrates:
1. \(\begin{aligned}\mathrm{Ca}\left(\mathrm{NO}_3\right)_2(\mathrm{~s}) \xrightarrow{\text { heat }} 2 \mathrm{CaO}(\mathrm{~s})+4 \mathrm{NO}_2(\mathrm{~g})&\text {(brown) }+\mathrm{O}_2(\mathrm{~g})\\\end{aligned}\)
2.\(\underset{\text { (colourless) }}{2 \mathrm{~Pb}\left(\mathrm{NO}_3\right)_2(\mathrm{~s})} \xrightarrow[\text {}]{\text { (heat) }} \underset{\text { (yellow) }}{2 \mathrm{PbO}(\mathrm{~s})}+\underset{(brown)}{4 \mathrm{NO}_2(\mathrm{~g})}+\mathrm{O}_2(\mathrm{~g})\)
Exceptions:
NaNO3 and KNO3, on being heated, decompose to form sodium nitrite (NaNO2) and potassium nitrite (KNO2), respectively, plus oxygen.
It is left to you to write the chemical equations for the reactions.

Types of Reactions: Combination, Decomposition, and Displacement
Classification
Oxides have been classified in many ways. Here we will take up a classification based on their acid-base character

Neutral oxides:
Neutral oxides, which are only four in number, are neither acidic nor basic. They are:
They do not change the character of an acid or a base.
Acidic oxides:
The oxides of nonmetals, in general, are acidic. Reaction with water They dissolve in water to form acids, which neutralise bases to form salts and water.
⇒ \(\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \underset{\text { Carbonic acid }}{\mathrm{H}_2 \mathrm{CO}_3(\mathrm{aq})}\)
⇒ \(\mathrm{SO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \underset{\text { Sulphurous acid }}{\mathrm{H}_2 \mathrm{SO}_3(\mathrm{aq})}\)
⇒ \(\underset{\substack{\text { sulphur } \\ \text { trioxide }}}{\mathrm{SO}_3(\mathrm{l})}+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \underset{\text { sulphuric acid }}{\mathrm{H}_2 \mathrm{SO}_4(\mathrm{aq})}\)
⇒ \(\underset{\text { dinitric pentoxide }}{\mathrm{N}_2 \mathrm{O}_5(\mathrm{~s})}+\underset{\text { }}{\text { }} \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \underset{\text { }}{2 \mathrm{HNO}_3(\mathrm{aq})}\)
A compound that is obtained by removing the elements of water from an acid is called the anhydride of the acid. In other words, the anhydride of an acid is the compound which adds water to form the acid. Thus, these oxides are acid anhydrides

You know that an acid turns blue litmus red. Acidic oxides turn moistened blue litmus paper red—they first add up water (moisture of the litmus paper) to form the acids. Else you can use a solution of blue litmus.
Reaction with bases: They directly react with bases to form salts.
⇒ \(\mathrm{CO}_2(\mathrm{~g})+\mathrm{Na}_2 \mathrm{O}(\mathrm{~s}) \longrightarrow \underset{\text { Sodium carbonate}}{\mathrm{Na}_2 \mathrm{CO}_3(\mathrm{~s})}\)
⇒ \(\mathrm{SO}_2(\mathrm{~g})+2 \mathrm{KOH}(\mathrm{~s}) \longrightarrow \underset{\substack{\text { potassium } \\ \text { sulphite }}}{\mathrm{K}_2 \mathrm{SO}_3(\mathrm{~s})}+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
Basic oxides
The oxides of metals are generally basic and react with acids to form salts and water. They also react with acidic oxides to form salts.
⇒ \(\mathrm{Na}_2 \mathrm{O}(\mathrm{~s})+\mathrm{H}_2 \mathrm{SO}_4(\mathrm{aq}) \longrightarrow \mathrm{Na}_2 \mathrm{SO}_4(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\\)
⇒ \(\mathrm{MgO}(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \longrightarrow \mathrm{MgCl}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
⇒ \(\mathrm{CaO}(\mathrm{~s})+\mathrm{CO}_2(\mathrm{~g}) \longrightarrow \mathrm{CaCO}_3(\mathrm{~s})\)
Basic oxides which are soluble at least to some extent turn moistened red litmus paper blue. Thus, a freely soluble metal oxide like Na2O, a less soluble oxide like CaO and an only slightly soluble oxide like MgO turn moistened red litmus paper blue, but the insoluble CuO does not. All these are bases
Amphoteric oxides
An amphoteric oxide is one which behaves like a base in the presence of an acid and like an acid in the presence of a base. These are metal oxides like Al2O3, ZnO and PbO. (Remember that the corresponding hydroxides are also amphoteric.)
An amphoteric oxide reacts with an acid as well as with a base, forming a salt and water.
⇒ \(\text { Base }+ \text { acid } \rightarrow \text { salt }+ \text { water }\)
⇒ \(\mathrm{ZnO}(\mathrm{~s})+\mathrm{H}_2 \mathrm{SO}_4(\mathrm{aq}) \rightarrow \underset{\text { zinc sulphate }}{\mathrm{ZnSO}_4(\mathrm{aq})}+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
⇒ \(\mathrm{PbO}(\mathrm{~s})+2 \mathrm{HNO}_3(\mathrm{l}) \rightarrow \underset{\text { lead nitrate }}{\mathrm{Pb}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})}+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
⇒ \(\text { Base }+ \text { acid } \rightarrow \text { salt }+ \text { water }\)
⇒ \(2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{ZnO}(\mathrm{~s}) \rightarrow \underset{\text { sodium zincate }}{\mathrm{Na}_2 \mathrm{ZnO}_2(\mathrm{aq})}+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
⇒ \(2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{PbO}(\mathrm{~s}) \rightarrow \underset{\text { sodium plumbite }}{\mathrm{Na}_2 \mathrm{PbO}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})}\)
Activities:
1 . Take about 3-5 g of zinc carbonate (ZnCO3) in a dry test tube. Heat it strongly on a flame till the white solid turns yellow. Cool the solid and it will turn white again. This colour change can be observed again and again on heating and cooling. This is the special property of zinc oxide (ZnO). On being strongly heated, ZnCO3 decomposes into Zno (and CO2).
Now, divide the residue of ZnO into two parts and treat them as follows.
2. Add some dilute sulphuric acid (or dilute hydrochloric acid) to one part. The solid quickly dissolves (to form the soluble salt ZnSO4)
Add some dilute sodium hydroxide solution to the other part and shake well. The solid will dissolve (forming the soluble salt Na2ZnO2).

2. Heat, as above, about 3-5 g of PbCO3. This time, the residue is the yellow PbO yellow in hot as well as cold conditions. Divide the residue into two parts and treat them as follows
Add dilute nitric acid to one part. The yellow solid dissolves to form a colourless solution of Pb(NO3)2. Do not use dilute HCI or H2SO4, because the salts PbCI2 or PbSO4 would be formed, which, being insoluble, would form a crust over the solid PbO, preventing it from reacting
Add some dilute NaOH solution to the other part and shake well. The solid will dissolve to form a colourless solution of sodium plumbite, Na2PbO2.
The language of chemistry is different from 3. One or two letters of the Latin name of an element in ordinary language. Instead of using words and sentences, we use symbols, formulae (formulas) and chemical equations to represent substances and the changes they undergo.

Read And Lean More Class 8 Chemistry
Berzelius laid the foundation of this language in the early nineteenth century. And this language gradually developed into its present form.
Symbols of different elements have been derived in three ways.
NCERT Solutions for The Language of Chemistry
1. The first letter (in capital) of the English name of an element:

2. The first letter, along with one more letter of the English name of an element:

3. One or two letters of the Latin name of an element:

What Does a Symbol Represent?
The symbol of an element represents the following.
1. An element in particular:
As you know, the symbol of sodium is Na, and that of chlorine is Cl. Instead of saying that the compound common salt is made up of the elements sodium and chlorine, you can say that it is made up of Na and Cl. You can also say that Cu is red-brown, whereas Au is yellow, and that Ca is a metal, whereas Cl is a nonmetal
2. An atom of an element:
In formulae and equations, a symbol represents one atom of an element. A numerical subscript shows more than one atom in a molecule. This is explained in the next section
The formula of a molecule gives the number(s) of atoms of the same or different elements present in the molecule. In other words, it tells us how many atoms of which elements have combined to form the molecule.
NCERT Class 8 Chemistry Chapter 5 Language of Chemistry
Formulae of Elements
When an atom of an element combines with another atom(s) of the same element, a molecule of the element is formed. The number of atoms contained in a molecule is called the atomicity of the molecule
Molecules of nitrogen, oxygen, fluorine and chlorine contain two atoms of the element, so they are represented as N2, O2, F2 and Cl2, respectively and are said to be diatomic. A common example of a triatomic gas is ozone(O3).
An atom of a noble-gas element,
Example:
Helium (He), neon (Ne), argon (Ar), etc., are highly inactive and do not combine with other atoms.
Hence, a molecule of a noble gas contains only one atom of the element. In other words, noble gases are monoatomic. The atomicity of phosphorus is 4(P4) and that of sulphur is 8(S8).
The Valency of an Element
The combining capacity of an element with other elements is known as its valency.
Valencies of the first twenty elements:

When elements are arranged in increasing order of atomic number, we find that their valencies are also arranged in order,
The Valency of an Element below:
You will later learn that the above kind of trend in a property is known as the periodic nature or the periodicity of the property. The term periodic means appearing at certain intervals. Don’t you find that valency has a periodic nature?
Language of Chemistry NCERT Notes

Formulae of Compounds
You have learnt earlier that the formula of a binary compound (i.e., a compound formed by only two elements) is obtained by transposing the valencies. Thus, the formula of the compound formed by the elements A (valency y) and B (valency x) is AxBy

The numeral subscripts are divided by a common factor, if any.
For example:
1. \(\stackrel{4}{\mathrm{C}} \stackrel{2}{\mathrm{O}} \Rightarrow \mathrm{C}_2 \mathrm{O}_4 \Rightarrow \mathrm{CO}_2\)
2. \(\stackrel{2}{\mathrm{Ca}}{\stackrel{2}{\mathrm{O}} \Rightarrow \mathrm{Ca}_2 \mathrm{O}_2 \Rightarrow \mathrm{CaO}}\)
3. \(\stackrel{3}{\mathrm{Al}} \stackrel{3}{\mathrm{~N}} \Rightarrow \mathrm{Al}_3 \mathrm{~N}_3 \Rightarrow \mathrm{AlN}\)
There are some exceptions
Example:
H2O (hydrogen peroxide), C2H2 (acetylene) and C4H10 (butane).
In which the numeral subscripts are not divided by a common factor. You will learn the reason in higher classes.
Symbols and Formulae in Chemistry Class 8
Variable valency
Some elements show variable valency,
Example:
Cu (1, 2), iron (2, 3), phosphorus (3, 5) and sulphur (2, 4, 6).
The valency of such an element in a compound is often indicated in Roman numerals in the name of the compound, as shown below
1. \(\stackrel{1}{\mathrm{Cu}}{\stackrel{2}{\mathrm{O}} \Rightarrow \mathrm{Cu}_2 \mathrm{O} \text { copper(I) oxide }}\)
2. \(\stackrel{2}{\mathrm{Cu}} \stackrel{2}{\mathrm{O}} \Rightarrow \mathrm{CuO} \text { copper(II) oxide }\)
3. \(\stackrel{2}{\mathrm{Fe}} \stackrel{2}{\mathrm{O}} \Rightarrow \mathrm{FeO} \text { iron(II) oxide }\)
4. \(\stackrel{3}{\mathrm{Fe}} \stackrel{2}{\mathrm{O}} \Rightarrow \mathrm{Fe}_2 \mathrm{O}_3 \text { iron(III) oxide }\)
As an exercise, you can guess the valencies of P in PCl3 and PCl5, and those of S in H2S, SO3 and SO3
Compounds containing radicals
You remember that a radical is a kind of entity that can be an atom with a charge on it or a group of atoms behaving as a single atom with a charge on the group.
It has a valency which is the same as its charge (without sign).
Positive radicals Examples:
Na+, K+, NH+4, Mg2+, Ca2+, Cu2+, Fe2+, Fe3+and Al3+)
Combine with Negative radicals. Examples:
Cl–, OH– , NO–3 , HCO–3 , CO2-3, SO2-4 , O2- , S2- and PO3-4) to form compounds.
Chemical Symbols and Formulae Class 8
The formula of such a compound can again be obtained by transposing the valencies (i.e., charges without sign) of the radicals and dividing the numbers of radicals by a common factor, if any.
Some examples are given below:

Radicals carry a charge over them, but the compounds they form do not. The compounds are electrically neutral. Hence, the positive and negative radicals must be present in a compound in such numbers that the opposite charges cancel each other.
For example:
In Al2 (SO4)3, the total positive charge is 2 × 3 = 6 for two Al3+ ions, and the total negative charge is 3 × 2 = 6 for three SO2-4 ions. You can understand this by using valency cards, also given in below.

A chemical change, i.e., a chemical reaction, is represented by a chemical equation. You know that in a chemical reaction, the substances we start with are called reactants, and those we end up with are called products. In an equation, we mention the reactants on the left-hand side and the products on the right-hand side, with an arrow in between.
Reactants → Products
In the previous class, you have learnt about the word equations in which we mention the reactants and products by name. Here, we will learn to write equations using symbols and formulae instead of words
Equations Using Symbols and Formulae
Such equations are quantitative and much more informative than word equations. They are written in the following three steps.
1. Writing the skeleton:
The skeleton of an equation is first written by noting the symbols and formulae of the reactants on the left side and those of the products on the right side, with an arrow in between.
For example:
Carbon, when burnt in a sufficient supply of air, forms carbon dioxide. The skeleton of the equation is written as follows.
C + O2 → CO2
But carbon, when burnt in an insufficient supply of air, forms carbon monoxide. And the skeleton for the equation is
2C + O2 → 2CO
Some more examples are given below:

2. Balancing a chemical equation:
According to the law of conservation of matter, matter can neither be created nor destroyed.
Understanding Chemical Language Class 8
After writing the skeleton, you should proceed as follows:
Step 1:
Count the atoms of each element on both sides of the arrow. If they tally, the equation is balanced. If not, proceed to step 2.
Step 2:
Make the number of atoms of each element equal on the both sides by using proper coefficients. (If O or N appears in the equation, balancing it first makes the task easier.)
From the examples discussed below, it will be clear that some of the skeletons written above are already balanced chemical equations and some others are not.
Example 1: Is thefollowing equation balanced? C + O2 → CO2
Solution:
Yes, because the number of atoms of C and O is equal on both sides of it.

Example 2: Is thefollowing equation balanced? CO2+H2O →H2CO3
Solution:
Yes, because the atom counts of C, H and O are the same on both sides of the equation

Example 3: Balance the equation C + O2 → CO
Solution:
Follow the following steps.
Step 1:
The atom count shows that the equation is not balanced.

Step 2:
To balance O, place the coefficient 2 before the product
C + O2 → 2CO
Step 3:
Now, the atom count is as follows.

Chemical Formulas NCERT Class 8
Step 4:
Balance C by placing the coefficient 2 before C on the LHS.
2C + O2 → 2CO
Step 5:
The atom count is now as follows

As the atom count of the elements on both sides is the same, the balanced equation is
2C + O2 → 2CO
Example 4: Is the following equation balanced? If it is not, balance it.
H2 + Cl2 → 2HCl
Solution:
Follow the following steps.
Step 1:
The atom count shows that the equation is not balanced.

Step 2:
Place the coefficient 2 before the product
H2 + Cl2 → 2HCl
Step 3:
The number of atoms on the two sides is now as follows.

The numbers tally.
Therefore, the balanced chemical equation for the given reaction is
H2 + Cl2 → 2HCl
Example 5: Balance the following equation. H2 +O2 → H2O
Solution:
Follow the following steps
Step 1:
Count the number of atoms of each element on both sides.

The atom count shows that the equation is not balanced.
Step 2:
To balance the number of oxygen atoms on both sides, place the coefficient 2 before H2O.
H2 +O2 → 2H2O
Step 3:
Again, count the atoms ofeach element on both sides.

The atom count shows that the equation is not balanced.
Step 4:
To balance the number of oxygen atoms on both sides, place the coefficient 2 before H2O.
2H2 +O2 → 2H2O
Step 5:
Tally the number of atoms of each element on both sides.

The numbers tally. Therefore, the balanced chemical equation for the reaction is
2H2 +O2 → 2H2O
Example 6: The numbers tally. Therefore, the balanced chemical equation for the reaction is.
Solution:
Let us now see if we can balance a chemical equation without writing the steps so elaborately. The reactants and the product may be written as follows.
Mg +O2 → MgO
Balance O: Mg + O2 → 2MgO
Balance Mg: 2Mg + O2 → 2MgO
Therefore, the balanced chemical equation for the reaction is
2Mg + O2 → 2MgO
Molecules and Their Representations Class 8
Example 7: On being strongly heated, potassium chlorate (KClO3 ) gives potassium chloride and oxygen. Write a balanced chemical equation for the reaction
Solution:
The reactant and the products can be written as follows.
KCIO3→ KCI+O2
Balance O:
2KCIO3→ KCI + 3O2
Balance K and Cl:
2KClO3 → 2KCl + 3O2
Hence, the balanced chemical equation is
2KClO3 → 2KCl + 3O2
Example: 8 Balance the equation
N2 +H2 → NH3
Solution:
Balance N: N2 + H2 → 2 NH3
Balance H: N2 + 3H2→2NH3
Thus, the balanced equation is
N2 + 3H2 → 2NH3
Atoms and Molecules in NCERT Class 8
Example 9: Is the following equation balanced? If not, balance it
Na2CO3 + HCl → NaCl + H2O + CO2
Solution:
The equation is not balanced as the atom counts of Na, H and Cl on the two sides do not tally.
Balance Na: Na2CO3 + HCl→ 2NaCl + H2O + C2O
Balance H and Cl: Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Hence, the balanced chemical equation is
Na2CO3+ 2HCl → 2NaCl + H2O+ CO2
Making a Chemical Equation More Informative
A balanced chemical equation tells us how many atoms and molecules of which reactants give how many atoms of which products. Had you known the masses of the atoms of different elements, you could have calculated the quantities of these substances. Keeping such calculations aside for higher classes, let us learn here how to make a chemical equation more informative.
Mentioning the conditions and catalysts:
The conditions under which a reaction takes place and the catalysts needed, if any, are mentioned at the arrow —generally, the conditions above and the catalyst below the arrow.
Mentioning the states of the reactants and products:
The state of each reactant and product is mentioned along with it, using the following symbols:
(s) for the solid state
(l) for the liquid state
(g) for the gaseous state, and
(aq) for an aqueous solution
When these symbols are used, a downward arrow (↓) for a precipitate and an upward arrow ( ↑ ) for a gas on the product side are not used. Instead, we use (s) for (↓) and (g) for ( ↑ ).
Mentioning the name and colour of a substance, if needed:
The name and/or colour of a substance is mentioned, if needed, below the symbol or formula of the substance in the equation—the name outside and the colour within brackets
Examples
The following examples will show how informative a chemical equation becomes when we include the points mentioned above.
1. Hydrogen reacts with chlorine in the presence of light to form hydrogen chloride gas.

2. When ignited, a mixture of hydrogen and oxygen (in the volume ratio 2:1) explodes to form water vapour

3. Solid potassium chlorate, when heated at 200-300 °C in the presence of manganese dioxide as a catalyst, gives oxygen, leaving behind a residue of potassium chloride.

4. Carbon dioxide turns limewater milky.
(Lime water) Ca(OH)2(aq) + CO2(g) → CaCO3 (s) (Milky)+ H2O (I)
5. A burning piece of magnesium continues to burn in a jar of carbon dioxide, forming white, smoky magnesium oxide with some black carbon particles.
![]()
Present in every living being, carbon is a very important element. It has many uses. The food we eat has compounds of carbon. Coal and hydrocarbons are widely used as fuels. Carbon compounds are also used in medicines. The clothes we wear have compounds of carbon. In the form of a diamond, carbon is used as a gemstone.
In the form of graphite, carbon finds a wide application in the industry. And you will learn in higher classes that in the form of carbon nanotubes, it plays a great role in nanotechnology.
Read And Lean More Class 8 Chemistry
In one form or another, carbon forms a significant part of the mineral world.

In the free or combined state, carbon is widely distributed on earth.
In the Free State
Carbon exists in the free state in coal, diamond and graphite.
1. Coal:
Coal is a decomposition product of plants buried millions of years ago due to some natural phenomena. Plants contain carbon compounds, slowly converted their buried remains into carbon. The conversion of a carbon compound into carbon is called carbonisation.
2. Diamond and graphite:
Diamond and graphite are the crystalline forms of carbon found in nature. Graphite is more abundant than diamond
In the Combined State
Carbon is widely distributed in the combined state.
1. Carbon dioxide:
Air contains about 0.03% carbon dioxide (CO2).
2. Living organisms:
All living organisms—plants and animals— have carbon compounds. Hence, everything we eat, which is derived from plants and animals, contains carbon compounds. The essential ingredients of food—carbohydrates, proteins, fats and vitamins—are compounds of carbon.
3. Minerals:
All carbonate minerals contain carbon. For example, limestone, calcite and marble are calcium carbonate (CaCO3), and dolomite is a mixed carbonate of magnesium and calcium (MgCO3CaCO3).
4. Natural gas and petroleum
Natural gas and petroleum contain mainly hydrocarbons, i.e., compounds which have only carbon and hydrogen. Natural gas is mostly methane (CH4), and petroleum is a mixture of various hydrocarbons containing a large number of carbon atoms.
Some common compounds which have carbon:

Common Compounds Which Have Carbon
Compounds containing carbon are of two types
All carbon-containing compounds, except carbon monoxide, carbon dioxide, carbonates and hydrogencarbonates, are called organic compounds.
And all noncarbon compounds, along with carbon monoxide, carbon dioxide, carbonates and hydrogencarbonates, are called inorganic compounds
Some examples of organic and inorganic compounds are given in Table
Before we take up allotropy, let us learn about crystalline and amorphous solids.
Crystalline and Amorphous Solids
Solids are divided into two classes:

1. Characteristics of crystalline solids:
1. The particles constituting a crystalline solid are arranged in an ordered manner in three dimensions.

2. When crystalline solids are broken or cut with a sharp knife, we get pieces with sharp edges and plane faces.

2. Characteristics of amorphous solids:
1. The particles constituting an amorphous solid are not arranged in an ordered manner.

2. When amorphous solids are broken or cut with a sharp knife, we get pieces with curved faces.

What is Allotropy?
The phenomenon of some elements existing in different forms is called allotropy
The term allotropy has been derived from the Greek words alios (meaning ‘other’) and tropos (meaning ‘form’).
The different forms of an element are known as allotropes or allotropic modifications. The allotropes of an element are chemically the same but different from each other in structure, atomicity or both.

Carbon, oxygen, phosphorus and sulphur are some common elements that show allotropy. Diamond, graphite, the fullerenes etc., are allotropes of carbon. Dioxygen (O2) and ozone (O3) are allotropes of oxygen. You will learn about the allotropy of other elements in higher classes.
The allotropes of an element differ in physical properties. And though they are chemically the same, they differ in some chemical properties too.
For example:
Graphite is a good conductor of heat and electricity, whereas diamond is not. And, though both of them are chemically carbon, graphite burns in air to give carbon dioxide at 700°C, whereas diamond does so at 900 °C. Again, ozone (O3) absorbs UV rays, whereas dioxygen (02) does not. And though both of them are oxygen, ozone is a much stronger oxidising agent than dioxygen.
Allotropy of Carbon
Carbon exists in crystalline as well as amorphous forms.
The crystalline forms of carbon:
In its crystalline forms, a carbon atom is bonded to three or four other carbon atoms. These atoms, in turn, are bonded to other carbon atoms. In the different crystalline forms of carbon such as diamond and graphite, the atoms are arranged in a different manner.
1. Diamond:
Diamond is the costliest gemstone and the hardest natural substance known. It is so hard that it can only be cut by another diamond.
The colour arises due to metallic impurities. The less costly varieties—grey and black —have no use as gemstones, and are used for cutting glass and drilling rocks

Diamond Properties:
1. Diamond is the hardest solid known.
2. It has a density of 3.51 g/cm3.
3. A properly cut diamond bends back a great percentage of the light falling on it. That is why it sparkles.The ability of a substance to bend light depends upon a property called refractive index. Diamond has a very high refractive index.

4. It has a very high melting point—3930 °C.
5. It is a bad conductor of electricity, i.e., it does not allow electric current to pass through it.
6. When ignited, it burns in air at 900 °C and in oxygen at 700 °C to give carbon dioxide

Diamond Structure:
In diamond, each carbon atom is bonded to four other carbon atoms. As shown in, the central carbon atom is bonded to four carbon atoms placed at the vertices of a tetrahedron. The other carbon atoms, in turn, are also tetrahedrally bonded to four carbon atoms each.

This kind of bonding results in the formation of a giant molecule, in which the carbon atoms are packed closely. The fact that the carbon atoms are so closely packed in diamond accounts for its high density and hardness. The strong chemical bonding between the atoms gives diamond its high melting point.
Diamond:

2. Graphite
Graphite is a black, opaque solid, found in large deposits in many countries like China, South Korea and India It is artificially prepared by strongly heating coke with silica in an electric furnace.

Graphite Properties:
Graphite Structure:
Graphite contains layers of hexagonal rings of carbon atoms, joined together.
You will learn more about this in higher classes.

Graphite Uses:
Fullerenes and carbon nanotubes
The amorphous forms of carbon:
Unlike the crystalline form, the amorphous form of a substance contains loosely held particles.

The charcoals:
Charcoals are prepared by a process known as destructive distillation or pyrolysis. In destructive distillation, a substance is heated in the absence of air with a view to breaking larger molecules into smaller ones. During the process, certain substances distil out, and may be collected.
Wood charcoal:
Wood charcoal is prepared by the destructive distillation of wood. A mixture of gases and vapours evolves, and charcoal is left as a residue.
The mixture of gases (CO2, CO, CH4 and H2) is combustible, and is known as wood gas. On being condensed, the vapours separate into a tar and a liquid. The liquid, called pyroligneous acid, contains some organic compounds.
The destructive distillation of wood can be carried out on a small scale in the laboratory.

Activity:
You can make wood charcoal at home, too. Put some wood shavings in a can, and cover it with a lid.

Being a form of carbon, wood charcoal shows the general properties of carbon, which we will study soon.
Activated charcoal:
Activated charcoal is prepared by heating wood charcoal at 900°C in a limited supply of air or steam.
Bone, or animal, charcoal:
Animal bones are first boiled with water to remove fatty substances and then subjected to destructive distillation in a retort.
The solid product in the retort is washed thoroughly with hydrochloric acid. The residual substance is bone charcoal.
Sugar charcoal:
Sugar charcoal is the purest form of carbon. It is prepared by the destructive distillation of cane sugar or by the dehydration of sugar with concentrated sulphuric acid
⇒ \(\begin{aligned}
&\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11} \rightarrow 12 \mathrm{C}+11 \mathrm{H}_2 \mathrm{O}\\
&\text { cane sugar }
\end{aligned}\)
Lampblack (soot):
When substances like oil and wax burn, soot is given out.
Activity:
You can prepare lampblack at home by burning mustard oil with the help of a wick and collecting the soot on a dish. The soot is used for preparing kajal and printer’s ink.

Coke:
Coke is obtained when coal is heated strongly in the absence of air. Coke is more porous and, therefore, more active than coal.
Gas carbon:
Gas Carbon deposits on the walls of a retort when a hydrocarbon is heated in it in the absence of air. Carbon is a good conductor of electricity and is, therefore, used as an electrode.
We have already discussed some properties and uses of the different forms of carbon. Let us talk about a few more of its properties and uses.
Adsorption—A Physical Property
You have learnt that in adsorption, a thin layer of a substance is formed on the surface of another substance.
Adsorption by charcoals
As charcoals have a large surface area in powder form, they are good adsorbents.
The following uses of charcoals are based on this property:
The reaction between chlorine and hydrogen in the dark is otherwise extremely slow
Chemical Properties and Their Uses
1. Reaction with oxygen or air:
On being lit, carbon burns in an excess of oxygen or air to form carbon dioxide
C + O2 → CO2+ heat
In an insufficient supply of air, carbon monoxide is formed.
2C + O2 → 2CO + heat
Carbon monoxide also burns in air to form carbon dioxide
2CO + O2 → 2CO2 + heat
The heat produced in these reactions makes carbon a good fuel.
2. Reducing properties:
Carbon has a great affinity for oxygen. So, it combines with the oxygen present in many compounds, and thus acts as a reducing agent.
Reduction of metal oxides. When heated with coke or charcoal, the oxides of metals below aluminium in the activity series (e.g., zinc, iron, tin, lead and copper) are reduced to the corresponding metals
1. \(\underset{\text { zinc(II) oxide }}{\mathrm{ZnO}}+\mathrm{C} \xrightarrow{\text { heat }} \underset{\text { zinc }}{\mathrm{Zn}}+\mathrm{CO} \uparrow\)
2. \(\underset{\text { iron(III) oxide }}{\mathrm{Fe}_2 \mathrm{O}_3}+3 \mathrm{C} \xrightarrow{\text { heat }} \underset{\text { iron }}{2 \mathrm{Fe}}+3 \mathrm{CO} \uparrow\)
3. \(\underset{\text { tinc(I) oxxide }}{\mathrm{SnO}_2}+2 \mathrm{C} \xrightarrow[\text { heat }]{\mathrm{Sn}}+2 \mathrm{CO} \uparrow\)
4. \(\underset{\text { copper(II) oxide }}{\mathrm{CuO}}+\mathrm{C} \xrightarrow{\text { heat }} \underset{\text { copper }}{\mathrm{Cu}}+\mathrm{CO} \uparrow\)
This kind of reduction, called carbon reduction, is of great importance in metallurgy, i.e., the science of extracting metals from their ores and modifying them for use.
Reduction of water:
When steam is passed over red-hot coke, a mixture of carbon monoxide and hydrogen is formed. This mixture is called water gas
⇒ \(\underset{\text { lead(II) oxide }}{\mathrm{PbO}}+\mathrm{C} \xrightarrow{\text { heat }} \underset{\text { lead }}{\mathrm{Pb}}+\mathrm{CO} \uparrow\)
⇒ \(\underset{\text { copper(II) oxide }}{\mathrm{CuO}}+\mathrm{C} \xrightarrow{\text { heat }} \underset{\text { copper }}{\mathrm{Cu}}+\mathrm{CO} \uparrow\)
Water gas is of great industrial importance
Carbon dioxide is present in the air (-0.03%), and its amount is greater in cities than in the countryside.
Carbon dioxide is used by plants for photosynthesis and given out by plants and animals during respiration. It is present in the form of carbonates in minerals like limestone or marble (CaCO3), dolomite (MgCO3.CaCO3), calamine (ZnCO3), etc.
Obtaining CO2
Carbon dioxide is formed in the following reactions
1. Burning of C and C-based fuels:
CO2 is formed when C is burnt in an excess of air or oxygen
⇒ \(\mathrm{C}+\underset{\text { (excess) }}{\mathrm{O}_2} \xrightarrow{\text { burn }} \mathrm{CO}_2\)
In an insufficient supply of air, carbon monoxide is formed, which is a poisonous gas
⇒ \(2 \mathrm{C}+\mathrm{O}_2 \xrightarrow{\text { burn }} \mathrm{CO}\)
Carbon-based fuels like CNG (compressed natural gas, which is mainly methane) and LPG (liquefied petroleum gas, which is mainly butane) burn smoothly to form CO2 and H2O (vapour).
⇒ \(\underset{\substack{\text { methane } \\(\mathrm{CNG})}}{\mathrm{CH}_4}+2 \mathrm{O}_2 \xrightarrow{\text { burn }} \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\underset{\substack{\text { butane } \\ \text { (LPG) }}}{2 \mathrm{C}_4 \mathrm{H}_{10}}+13 \mathrm{O}_2 \xrightarrow{\text { burn }} 8 \mathrm{CO}_2+10 \mathrm{H}_2 \mathrm{O}\)
Fuels like petrol, kerosene and diesel are mixtures of hydrocarbons containing larger numbers of C atoms. They also burn to give CO2 and H2O
2. Thermal decomposition of carbonates:
The thermal decomposition of metal carbonates (except a few like Na2CO3 and K2CO3) gives CO2
1. \(\underset{\begin{array}{c}
\text { magnesium } \\
\text { carbonate }
\end{array}}{\mathrm{MgCO}_3} \xrightarrow{\text { heat }} \underset{\substack{\text { magnenium } \\
\text { oxide }}}{\mathrm{MgO}}+\mathrm{CO}_2\)
2. \(\underset{\text { limestone }}{\mathrm{CaCCO}_3} \xrightarrow{\text { heat }} \underset{\text { quicklime }}{\mathrm{CaO}}+\mathrm{CO}_2\)
3. Action of an acid on a carbonate or a hydrogencarbonate:
When a carbonate or a hydrogencarbonate is treated with an acid, CO2 is evolved with effervescence
1. \(\underset{\substack{\text { sodium } \\ \text { carbonate }}}{\mathrm{Na}_2 \mathrm{CO}_3}+\underset{\substack{\text { sulphuric } \\ \text { acid }}}{\mathrm{H}_2 \mathrm{SO}_4} \rightarrow \underset{\substack{\text { sodium } \\ \text { sulphate }}}{\mathrm{Na}_2 \mathrm{SO}_4}+\mathrm{CO}_2 \uparrow+\mathrm{H}_2 \mathrm{O}\)
2. \(\underset{\substack{\text { sodium } \\ \text { ndrogencarbonate }}}{\mathrm{NaHCO}_3}+\underset{\substack{\text { hydrochloric } \\ \text { acid }}}{\mathrm{HCl}} \underset{\mathrm{NaCl}}{ }+\mathrm{CO}_2 \uparrow\)
4. Fermentation of a sugar:
CO2 is evolved during the fermentation of a sugar (in the presence of yeast), a process in which alcohol is formed. (This is how ethanol —an alcohol—is manufactured from the molasses obtained from the sugar industry.)
⇒ \(\underset{\text { (from the molasses) }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6} \xrightarrow{\text { yeast }} \underset{\text { ethanol }}{2 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}+2 \mathrm{CO}_2\)
Laboratory preparation
Principle CO2 is prepared in the laboratory by the action of dilute hydrochloric acid on marble (CaCO3) chips.
⇒ \(\underset{\substack{\text { calcium carbonate marble } \\ \text { } \\ \text { }}}{\mathrm{CaCO}_3}+\underset{\substack{\text { hydrochloric acid (dilute) } \\ \text { } \\ \text { }}}{2 \mathrm{HCl}} \rightarrow \underset{\substack{\text { calcium chloride} \\ \text { }}}{\mathrm{CaCl}_2}+\mathrm{CO}_2 \mathrm{}+\mathrm{H}_2 \mathrm{O}\)
As the gas is heavier than air (~1.6 times), it is collected by the upward displacement of air.
Procedure:
Some marble chips are placed in a conical flask and covered by water. The flask is fitted with a thistle funnel and a delivery tube bent at 90° .
Dilute HCl is poured through the thistle funnel into the flask. A brisk reaction takes place. The gas evolved displaces the air inside the flask and the delivery tube first, and then collects in the gas jar by displacing the air upwards.
To test whether the jar is filled with CO2, a lighted matchstick is brought near its mouth from time to time. When the flame gets extinguished, we infer that the jar is filled with CO2. The delivery tube is taken out and the jar is closed by putting the lid in place.


(You could use sodium carbonate in place of marble chips and then dilute H2SO4 in place of dilute HCI. The reaction with marble is, however, smoother.)
Could we use H2SO4 instead of HCI with marble?
No, because calcium sulphate (CaSO4) would be formed then. And the salt, being insoluble, would deposit on the chips , preventing them from coming in contact with the acid.
⇒ \(\mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \underset{\text { (insoluble) }}{\mathrm{CaSO}_4}+\mathrm{CO}_2 \uparrow+\mathrm{H}_2 \mathrm{O}\)
How about using H2SO4 with Na2CO3?
This is all right because the salt Na2SO4 formed is soluble.
Does the action of dilute HCI on marble chips give pure CO2 ?
No, the gas is contaminated with HCI as the latter gets volatilised by the evolving CO2. The gas can be freed from HCI vapours by passing it through a small amount of water before collection. Water dissolves its own volume of CO2, but large volumes of HCl.

Properties of CO2
Na2CO3 CO2 has the following properties
Physical properties:
Chemical properties:
1. Combustion CO2 is neither combustible nor a supporter of combustion. So, it is used in extinguishing a fire. Being heavier than air, the gas displaces air from the vicinity, and the fire gets extinguished. You can easily understand this by doing the following activity.
Activity:
Take a spoonful of baking powder in glass A and add some vinegarto it.
This shows that CO2 is heavier than air. And so it can be ‘poured’ from one glass to another and then over the flame, which gets extinguished.

2. Reaction with active metals:
An active metal like Na, K or Mg abstracts oxygen from CO2 (setting C free) when burnt in the gas.
Experiment:
In the reaction, some black carbon particles are also formed, which can be seen more clearly if the smoky scales of MgO are dissolved in dilute HCl.

3. Action on litmus:
The aqueous solution of the gas is weakly acidic and turns blue litmus wine-red. The acidic nature of the gas is generally tested by placing a moist blue litmus paper in the gas. An aqueous solution of C02 contains carbonic acid
⇒ \(\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \underset{\text { carbonic acid }}{\mathrm{H}_2 \mathrm{CO}_3}\)
4. Reaction with metal oxides:
Metal oxides are generally basic in nature, and CO2 is acidic. So, CO2 slowly reacts with metal oxides to form the metal carbonates, which are salts.
1. \(\underset{\substack{\text { sodium } \\ \text { oxide }}}{\mathrm{Na}_2 \mathrm{O}}+\mathrm{CO}_2 \rightarrow \underset{\substack{\text { sodium } \\ \text { carbonate }}}{\mathrm{Na}_2 \mathrm{CO}_3}\)
2. \(\underset{\substack{\text { magnesium } \\ \text { oxide }}}{\mathrm{MgO}}+\mathrm{CO}_2 \longrightarrow \underset{\substack{\text { magnesium } \\ \text { carbonate }}}{\mathrm{MgCO}_3}\)
3. \(\underset{\substack{\text { calcium } \\ \text { carbonate }}}{\mathrm{CaOClcium}}+\mathrm{CO}_2 \rightarrow \underset{\text { oxide }}{\mathrm{CaCO}_3}\)
5. Reaction with limewater:
Limewater is a dilute solution of calcium hydroxide. The reaction of CO2 with limewater may be studied in three parts.
1. The clear limewater turns milky when CO2 is passed through it. This is because the insoluble, white substance calcium carbonate is formed.
⇒ \(\underset{\text { limewater }}{\mathrm{Ca}(\mathrm{OH})_2}+\mathrm{CO}_2 \longrightarrow \underset{\substack{\text { calcium } \\ \text { carbonate } \\ \text { (causing milkiness) }}}{\mathrm{CaCO}_3 \downarrow}+\mathrm{H}_2 \mathrm{O}\)
2. The milkiness disappears when an excess of CO2 is passed through the liquid. This is because the soluble, colourless compound calcium hydrogencarbonate is formed.
⇒ \(\mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 \rightarrow \underset{\substack{\text { calcium hydrogencarbonate } \\ \text { (soluble) }}}{\mathrm{Ca}\left(\mathrm{HCO}_3\right)_2}\)
3. The milkiness reappears when the above solution is boiled. This is because the hydrogencarbonate, on being heated, decomposes back to give the carbonate, causing the milkiness.
⇒ \(\mathrm{Ca}\left(\mathrm{HCO}_3\right)_2 \xrightarrow{\text { heat }} \mathrm{CaCO}_3 \downarrow+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 \dagger\)
Carbon Dioxide and the Environment
In low concentrations in the air, CO2 is essential for life but in high concentrations, it is a cause of great concern. Let us see how.
1. Photosynthesis:
We know that green plants use C02 for photosynthesis and manufacture their food glucose in the process

Thus, life seems to have originated on this planet from C02 and moisture.
2. The greenhouse effect:
A greenhouse is a glasshouse inside which we can grow plants. During the day, the rays enter the glasshouse and keep the plants warm. But the glass traps a part of the heat of the rays and keeps on radiating it back, even during the night, keeping the plants warm

The carbon dioxide present in the Earth’s atmosphere plays the same role as the glass in a greenhouse. The CO2 traps the heat of the rays and keeps radiating it back so that the warmth of the Earth is retained. The rays heat the Earth directly, but a large portion of the rays are reflected back. So, without the warmth provided by the C02 of the atmosphere, the Earth would have been much colder during the nights.
A gas that traps heat in the environment and keeps the surroundings warm is known as a greenhouse gas and the whole effect as the greenhouse effect. Thus, CO2 is a greenhouse gas and other such gases in our environment are mainly methane (emitted by cattle dung) and flurocarbons (used as coolants in fridges and air conditioners).
Global warming
What will happen if the concentration of CO2 increases in our environment?
All this will happen due to a rise in Earth’s average temperature, i.e., due to global warming.
Afforestation can bring down the dangers of global warming by using up the increased CO2 in photosynthesis. But the opposite activity, i.e., deforestation, is on the increase due to increased population.
With increasing deforestation as well as industrialisation, the proportion of CO2 in our environment has increased a lot. And we are already facing the dangers of global warming. The world community is making all-out efforts to bring the proportion of CO2 down to permissible levels.
3. Action on natural water
Natural water is slightly acidic to slightly alkaline (ph 6.5-8.5). pH is a measure of the acidic or alkaline nature of a dilute solution. It is measured with the help of a pH-meter or a pH paper.
Pure water is neutral, i.e., neither acidic nor alkaline and has pH 7.0. Acidic solutions have pH < 7, and alkaline solutions, pH > 7. You know that, on dissolving in water, CO2 forms the weak acid H2CO3.
So, the gas tends to bring down the pH of water that is open to the atmosphere. For drinking purposes, slightly acidic water is better than alkaline water. Acidic water (pH 6.5) is soft and palatable but alkaline water (pH 8) is hard and unpalatable. Also, the pH inside our digestive system is acidic.
On the other hand, marine organisms thrive better in alkaline water than in acidic water. And it has been found that the increasing proportion of dissolved CO2 is lowering the pH of seawater. This is posing a threat to marine life
Uses of CO2
1. CO2 is used in the industry for the manufacture of
2. It is used in fire extinguishers.
3. It is extensively used for making soda water and fizzy drinks
4. In welding, CO2 is used to prevent the oxidation of the metal by air.
5. In the form of dry ice, CO2 is used as a coolant and for depicting smoke in plays, movies, and so on.
How a fire extinguisher works

Common fire extinguishers produce carbon dioxide by the action of sulphuric acid on a solution of sodium carbonate or sodium hydrogencarbonate.
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
⇒ \(2 \mathrm{NaHCO}_3+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+\underbrace{2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{CO}_2}_{\text {gushes out }}\)
A solution of sodium carbonate or sodium hydrogencarbonate is placed in a fire extinguisher. A sealed bottle containing sulphuric acid is also placed there. In case of fire, the acid bottle is broken by striking the plunger against a hard surface.
The acid reacts with the sodium carbonate or sodium hydrogencarbonate, and carbon dioxide is formed. A mixture of water and carbon dioxide gushes out, which is directed towards the burning object. Carbon dioxide, being heavier than air, surrounds the burning object and cuts off the supply of air. As a result, the fire is put out
Carbon monoxide (CO) is a common product of the combustion of carbon-based fuels. Being a poisonous gas, it pollutes air. But, at the same time, the gas is used for many industrial purposes too.
So, we will look into both these aspects of the gas here
Poisonous Nature of CO:
Let us see how the gas affects us and how we can get rid of its effects.
Concentration of CO:
The concentration of a substance, expected to be present in very small quantities in a mixture, is often expressed in parts per million (ppm). This tells us the number of parts of a substance present in 1 million (1,000,000) parts of the mixture.
The concentration of CO in air is also expressed in ppm.
For example:
How does CO affect us?
As you know, blood transports oxygen in our body. When we inhale air, the oxygen of the air combines with haemoglobin of the blood to form oxyhaemoglobin.
Effects of CO poisoning:

Curing CO poisoning
Carboxyhaemoglobin slowly loses CO on being exposed to an excess of oxygen, and oxyhaemoglobin is again formed. So, a person suffering from CO poisoning should be kept on oxygen till he or she recovers
Industrial Uses of CO
Carbon monoxide is an industrial gas. Some important uses of this gas are given below.
1. Itis usedasa reducingagentin metallurgy. It can abstract the oxygen from many metal oxides and set the metal free.
⇒ \(\underset{\text { metal oxide }}{\mathrm{MO}}+\mathrm{CO} \xrightarrow{\text { heat }} \underset{\text { metal }}{\mathrm{M}}+\mathrm{CO}_2\)
For example:
The mineral bauxite (which contains Fe203) is reduced by CO in a blast furnace to give iron.
⇒ \(\underset{\text { iron(III) oxide }}{\mathrm{Fe}_2 \mathrm{O}_3}+\mathrm{CO} \xrightarrow[\text {}]{\text { heat }} \underset{\text {iron oxide }}{2 \mathrm{FeO}}+\mathrm{CO}_2\)
⇒ \(\mathrm{FeO}+\mathrm{CO} \xrightarrow{\text { heat }} \underset{\text { iron }}{\mathrm{Fe}}+\mathrm{CO}_2\)
2. Carbon monoxide is also used in metallurgy for the refining of nickel.
3. It is used on a large scale in the industrial preparation of several organic compounds such as
Phosgene (COCl2, used in the synthesis of some polymers)
We know that heat (usually accompanied by light) is produced when a substance burns. A substance that is burnt to obtain heat or light from it is called a fuel. Thus, wood, cow-dung cakes, coal, petrol, diesel, kerosene, CNG and LPG are fuels.
Hydrogen is also a good fuel, but it is difficult to handle due to its explosive nature. These fuels (except hydrogen) contain carbon.
Calorific Value of a Fuel:
The amount of heat given out by 1 g of a fuel in air or oxygen is known as the calorific value of the fuel. It is expressed in kj/g.
The calorific value of hydrogen is the highest, and that of common fuels, much lower
The calorific values of some fuels:

That the calorific value of LPG is much higher than that of the traditional fuels can be realised by doing the following activity with the help of an adult.
Activity:
Take the same volume of water in two similar vessels. Heat one of them on an LPG stove and the other on burning cow-dung cakes. You will find that the water boils on the LPG stove much sooner than that on the burning cow-dung cakes.
(You could use thermometers to observe the rate at which the temperature of water increases in the two vessels).
Thus, attaining the cooking temperature on an LPG stove will be faster than on a conventional fire as that of cow-dung cakes

Of the C-based fuels, CNG is mainly methane (CH4 ) and LPG, butane (C4 H10 ). Methane and butane contain only hydrogen and carbon, so they belong to a class of compounds known as hydrocarbons. Hydrocarbons burn to give CO2 and H2O.
Promoting Cleaner Fuels
The use of fuels like wood, cow-dung cake and coal for cooking and of petrol and diesel in motor vehicles is accompanied by the emission of pollutants like
CNG and LPG are considered to be much cleaner fuels than those mentioned above because, on complete burning, they form C02 and H20 only.