NCERT Solutions For Class 11 Chemistry Chapter 8 Redox Reactions Long Questions And Answers
Question 1. In the following redox reactions, identify the oxidation half-reactions and reduction half-reactions along with the oxidants and reductants
1. \(\mathrm{Cl}_2(g)+2 \mathrm{I}^{-}(a q) \rightarrow 2 \mathrm{Cl}^{-}(a q)+\mathrm{I}_2(g) \)
2. \( \mathrm{Sn}^{2+}(a q)+2 \mathrm{Fe}^{3+}(a q) \rightarrow \mathrm{Sn}^{4+}(a q)+2 \mathrm{Fe}^{2+}(a q)\)
3. \( 2 \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3(a q)+\mathrm{I}_2(s) \rightarrow \mathrm{Na}_2 \mathrm{~S}_4 \mathrm{O}_6(a q)+2 \mathrm{NaI}(a q)\)
Read and learn More NCERT Class 11 Chemistry
4. \(\mathrm{Fe}(s)+2 \mathrm{H}^{+}(a q) \rightarrow \mathrm{Fe}^{2+}(a q)+\mathrm{H}_2(g)\)
5. \( \mathrm{H}_2 \mathrm{~S}(a q)+\mathrm{Cl}_2(g) \rightarrow \mathrm{S}(s)+2 \mathrm{HCl}(a q)\)
6. \(2 \mathrm{FeCl}_2(a q)+\mathrm{Cl}_2(g) \rightarrow 2 \mathrm{FeCl}_3(a q)\)
7. \(2 \mathrm{Hg}^{2+}(a q)+\mathrm{Sn}^{2+}(a q) \rightarrow \mathrm{Hg}_2^{2+}(a q)+\mathrm{Sn}^{4+}(a q)\)
Answer:
1.
Oxidation half-reaction: 2l-(aq)→I2(s) + 2e
Reduction half-reaction: Cl2(g) + 2e→2Cl-(aq)
Oxidant: Cl2(g); Reductant: l-(aq)
2.
Oxidation half-reaction: Sn2+(aq)→Sn4+(aq) + 2e
Reduction half-reaction: Fe3+(aq) + e→Fe2+(aq)
Oxidant: Fe3+(aq); Reductant: Sn2+(aq)
3.
Oxidation half-reaction:\(\mathrm{S}_2 \mathrm{O}_3^{2-}(a q) \rightarrow \mathrm{S}_4 \mathrm{O}_6^{2-}(a q)+2 e\)
Reduction half-reaction: I2(s) + 2e→2l-(aq)
Oxidant: I2(s); Reductant: Na2S2O3(aq)
4.
Oxidation half-reaction: Fe(s)→Fe2+(aq) + 2e
Reduction half-reaction: 2H+(aq) + 2e→H2(g)
Oxidant: H+(aq); Reductant: Fe(s)
Redox Reactions Class 11 Long Questions and Answers
5.
Oxidation half-reaction: S2-(aq)→S(s) + 2e
Reduction half-reaction: Cl2(g) + 2e→CI-(aq)
Oxidant: Cl2(g); Reductant: H2S(aq)
6.
Oxidation half-reaction: Fe2+(aq)→Fe3+(aq)
Reduction half-reaction: Cl2(g) + 2e→2Cl-(aq)
Oxidant: Cl2; Reductant: FeCl2
7.
Oxidation half-reaction: Sn2+(aq)→Sn4+(aq) + 2e
Reduction half-reaction: \(2 \mathrm{Hg}^{2+}(a q)+2 e \rightarrow \mathrm{Hg}_2^{2+}(a q)\)
Oxidant: Hg2+(aq); Reductant: Sn2+(aq)
Question 2. A compound is composed of three elements A, B, and C. The oxidation numbers of A, B, and C in the compound are +1, +5, and -2, respectively. Which one of the following formulas represents the molecular formula of the compound?
- A2BC4
- A2(BC3)2.
Answer:
The algebraic sum of the oxidation numbers of all atoms present in a molecule is equal to zero.
In an A2BC4 molecule, the algebraic sum ofthe oxidation numbers of all the constituent atoms
= 2 × (+1) +1 × (+5) + 4 × (-2) = -1
In A2(BC3)2 molecule, the algebraic sum of the oxidation numbers of all the atoms
= 2 × (+ 1) + 2[5 + 3 × (-2)] = 0
Therefore, A2(BC3)2 represents the molecular formula ofthe compound.
Question 3. Among the reactions given below, identify the redox reactions and also mention the oxidant and the reductant in each case
1. \(\mathrm{Fe}_2 \mathrm{O}_3(\mathrm{~s})+3 \mathrm{CO}(\mathrm{g}) \rightarrow 2 \mathrm{Fe}(\mathrm{s})+3 \mathrm{CO}_2(\mathrm{~g})\)
2. \(2 \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3(a q)+\mathrm{I}_2(s) \rightarrow \mathrm{Na}_2 \mathrm{~S}_4 \mathrm{O}_6(a q)+2 \mathrm{NaI}(a q)\) →\(\)
3. \(\left(\mathrm{NH}_4\right)_2 \mathrm{~S}(a q)+\mathrm{Cu}\left(\mathrm{NO}_3\right)_2(a q)\) →\(\mathrm{CuS}(s)+2 \mathrm{NH}_4 \mathrm{NO}_3(a q)\)
4. \(\mathrm{Cu}_2 \mathrm{~S}(s)+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{Cu}(s)+\mathrm{SO}_2(g)\)
5. \(\mathrm{Ca}(\mathrm{OH})_2(a q)+\mathrm{H}_2 \mathrm{SO}_4(a q) \rightarrow \mathrm{CaSO}_4(s)+2 \mathrm{H}_2 \mathrm{O}(l)\)
6. \(\mathrm{H}_2 \mathrm{~S}(g)+\mathrm{HNO}_3(a q)\) →\(\mathrm{H}_2 \mathrm{SO}_4(a q)+\mathrm{NO}_2(g)+\mathrm{H}_2 \mathrm{O}(l)\)
Answer:
1.

The Oxdination number of Fe decreases from +3 to 0 while that of C increases from +2 to +4. so in this reaction, Fe2O3 undergoes reduction and Co undergoes Oxdination. Hence, it’s a redox reaction in which Fe2O3 acts as an Oxdiant and Co as Reductant.
2.

The oxidation number of S increases from +2 to +2.5 while that of decreases from 0 to -l. So, in this reaction, Na2S2O3 undergoes oxidation and I2 undergoes reduction. Hence, it is a redox reaction in which Na2S2O3 acts as a reductant and I2 as an oxidant.
3. In this reaction, there occurs no change in oxidation number for any element. So, it is not a redox reaction.
4.

The oxidation number of Cu decreases from +1 to 0 while that of S increases from -2 to +4. Also, the oxidation number of 0 decreases from O to -2. So, in this reaction, Cu2S undergoes oxidation as well as reduction and O2 undergoes reduction. Hence, it is a redox reaction in which Cu2S serves as both oxidant and reductant and O2 acts as an oxidant.
5. In this reaction, no change in oxidation number for any element takes place. So, it is not a redox reaction.
6.

The oxidation number of S increases from -2 to +6 while that of N decreases from +5 to +4. So, in this reaction, H2S undergoes oxidation and HNO3 undergoes reduction. Hence, it is a redox reaction in which H2S acts as a reductant and HNO3 acts as an oxidant
Hence, it is a redox reaction in which H2S acts as a reductant and HNO3 acts as an oxidant.
Question 4. Identify the following half-reactions as oxidation half¬ reactions and reduction half-reactions:
1. \(\mathrm{Cr}_2 \mathrm{O}_7^{2-} \rightarrow 2 \mathrm{Cr}^{3+}\)
2. \(\mathrm{Cr}(\mathrm{OH})_4^{-}(a q) \rightarrow \mathrm{CrO}_4^{2-}(a q)\)
3. \(\mathrm{IO}_3^{-}(a q) \rightarrow \mathrm{IO}_4^{-}(a q)\)
4. \(\mathrm{ClO}^{-}(a q) \rightarrow \mathrm{Cl}^{-}(a q)\)
5. \(\mathrm{MnO}_4^{-}(a q) \rightarrow \mathrm{MnO}_2(s)\)
Answer:
1.

The oxidation number of Cr changes from +6 to +3. This indicates that the reaction involves the reduction of Cr2O7-2 Hence, it is a reduction half-reaction.
2.

The reaction involves the oxidation of Cr(OH)4 because the oxidation number of Cr increases from +3 to +6. So, this reaction represents an oxidation half-reaction.
3.

This reaction represents an oxidation half-reaction since the oxidation number of I increases from +5 to +7 in the reaction.
4.

This reaction represents a reduction half-reaction because the oxidation number of Cl decreases from +1 to -1 in the reaction.
5.

This is a reduction half¬ reaction because the oxidation number of Mn decreases from +7 to +4.
Question 5. Give an example of a disproportionation reaction. Calculate the volume of /0.225(M) KMnO4 solution that can completely react with 45mL of a 0.125(M) FeSO4 solution in an acid medium.
Answer:
⇒ \(\left[\mathrm{Fe}^{2+}(a q) \rightarrow \mathrm{Fe}^{3+}(a q)+e\right] \times 5\)
Reduction reaction:
⇒ \(\mathrm{MnO}_4^{-}(a q)+8 \mathrm{H}^{+}(a q)+5 e\) → \(\mathrm{Mn}^{2+}(a q)+4 \mathrm{H}_2 \mathrm{O}(l) \times 1\)
Net reaction:
⇒ \(5 \mathrm{Fe}^{2+}(a q)+\mathrm{MnO}_4^{-}(a q)+8 \mathrm{H}^{+}(a q)\) → \(5 \mathrm{Fe}^{3+}(a q)+\mathrm{Mn}^{2+}(a q)+4 \mathrm{H}_2 \mathrm{O}(l)\)
5 mol of FeSO4 = 1 mol of MnO4
or, 1000mL of 5 M FeSO4 = 1000 ml limit of 1M KMnO4
or, 1mL of 5M FeSO4 s lmL of lM KMnO4
or, 1mL of 1M FeSO4 \(\equiv \frac{1}{5} \mathrm{~mL}\) of 1M KMno4
or, 45mL of 0.125M FeSO4 = 45 × 0.125 ,\(\times \frac{1}{5} \mathrm{~mL}\) of 1M KMnO4 \(\equiv \frac{9 \times 0.125}{0.225} \mathrm{~mL}\) = 5mL of0.225M KMnO4
= The volume of KMnO4 required = 5mL
NCERT Class 11 Chemistry Chapter 8 Redox Reactions Long Questions & Answers
Question 6. Identify the following reactions as disproportionation and comproportionation reactions
1. \(\mathrm{Ag}^{2+}(a q)+\mathrm{Ag}(s) \rightarrow 2 \mathrm{Ag}^{+}(a q)\)
2. \(2 \mathrm{H}_2 \mathrm{O}_2(l) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(l)+\mathrm{O}_2(g)\)
3. \({O}(\mathrm{l})+\mathrm{O}_2(\mathrm{~g})\)
4. \(4 \mathrm{KClO}_3(s) \rightarrow \mathrm{KCl}(s)+3 \mathrm{KClO}_4(s)\) → \(2 \mathrm{MnO}_4^{-}(a q)+\mathrm{MnO}_2(s)+4 \mathrm{OH}^{-}(a q)\)
5. \(2 \mathrm{NH}_4 \mathrm{NO}_3(s) \rightarrow \mathrm{N}_2(g)+4 \mathrm{H}_2 \mathrm{O}(g)+\mathrm{O}_2(g)\)
6. \(\mathrm{IO}_3^{-}(a q)+5 \mathrm{I}^{-}(a q)+6 \mathrm{H}^{+}(a q) \rightarrow 3 \mathrm{I}_2(s)+3 \mathrm{H}_2 \mathrm{O}(l)\)
Answer:
1.

In this reaction, the resulting species, Ag+, exists in an oxidation state (+1) that lies between the oxidation states of Ag2+(+2) and Ag(0). Hence, it is a comproportionation reaction.
2.

In this reaction, H2O2 (oxidation number of 0 is -1 ) decomposes to form H2O (oxidation number of O is -2) and O2 (oxidation number of O is zero).
The oxidation state -1 lies between 0 and -2. So, this reaction represents a disproportionation reaction.
3.

In this reaction, the oxidation number of Cl decreases (+5 to-1 ) as well as increases (+5 to +7). This means KClO3 undergoes both oxidation and reduction in the reaction. Hence, this reaction is a disproportionation reaction.
4.

In this reaction, the oxidation number of Mn increases (+6 to +7) and decreases (+6 to +4) as well. ‘Hus’ means MnO– undergoes both oxidation and reduction in the reaction. Therefore, this reaction is a disproportionation reaction.
5.
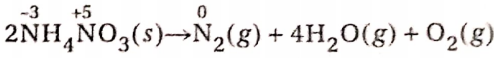
NH4NO3 molecule has two N-atoms, out of which one has an oxidation number of -3 and the other has an oxidation number of +5—the decomposition of NH4NO3 results in N2(g).
So, in this reaction, the oxidation number of one N-atom increases from -3 to 0 while the oxidation number of other N-atom decreases from +5 to 0. Since the oxidation number 0 lies between the oxidation numbers -3 and +5, the reaction represents a comproportionation reaction.
6.

In this reaction, 10J (oxidation number of I = +5) reacts with I- (oxidation number of = -1 ) to form I2 (oxidation number of = 0 ). Since the oxidation number 0 lies between -1 and +5, the reaction represents a comproportionation reaction.
Question 7. Determine the equivalent masses of the following underlined compounds by both oxidation number and electronic methods
1. SO2 + 2H2O→H2SO4
2. HNO3→NO2 + H2O
3. HNO3 + 3H+→ NO + 2H2O
4. MnO2 + 4H+→ Mn2+→ + 2H2O
5. \(\mathrm{KMnO}_4+\mathrm{FeSO}_4+\mathrm{H}_2 \mathrm{SO}_4\) → \(\mathrm{K}_2 \mathrm{SO}_4+\mathrm{MnSO}_4+\mathrm{Fe}_2\left(\mathrm{SO}_4\right)_3+\mathrm{H}_2 \mathrm{O}\)
Answer:
1. Oxidation number method: \(\stackrel{+4}{\mathrm{SO}_2}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4\)
In this reaction, the oxidation number of S increases from +4 to +6. The change in oxidation number per molecule of SO2 = 6 – 4
= 2 units.
∴ Equivalent mass of SO2
⇒ \(\frac{\text { Molecular mass of } \mathrm{SO}_2}{2}=\frac{64}{2}=32\)
Electronic method: SO, + 2H2O→4H++ SO4 2-+ 2e
Number of electrons lost by a molecule of SO2 for its oxidation = 2.
∴ Equivalent mass of SO2 \(=\frac{64}{2}=32\)
Class 11 Chemistry Chapter 8 Redox Reactions NCERT Long Questions
2. Oxidation number method: \(\stackrel{+5}{\mathrm{HN}} \mathrm{O}_3 \rightarrow \stackrel{+4}{\mathrm{~N}} \mathrm{O}_2+\mathrm{H}_2 \mathrm{O}\)
The change in oxidation number per molecule of HNO3 = 5-4 = unit:
∴ Equivalent mass of HNO3
⇒ \(\frac{\text { Molecular mass of } \mathrm{HNO}_3}{1}=\frac{63}{1}=63\)
Electronic method: NO–3 + 2H+ + e→ NO2+ H2O.
The number of electrons involved in the die reduction of NO–3 is 1.
∴ Equivalent mass of HNO3
⇒ c\(\frac{\text { Molecular mass of } \mathrm{HNO}_3}{1}=\frac{63}{1}=63\)
3. Oxidation number method: \(\mathrm{H}^{+5} \mathrm{~S}_3+3 \mathrm{H}^{+} \rightarrow \stackrel{+2}{\mathrm{~N} O}+2 \mathrm{H}_2 \mathrm{O}\)
In this reaction, the oxidation number of N decreases from +5 to +2. So, the change in oxidation number per molecule of HNO3 = 5-2
= 3 units.
∴ Equivalent mass of HNO3
⇒ \(=\frac{\text { Molecular mass of } \mathrm{HNO}_3}{1}=\frac{63}{3}=21\)
Electronic method: \(\mathrm{NO}_3^{-}+4 \mathrm{H}^{+}+3 e \rightarrow \mathrm{NO}+2 \mathrm{H}_2 \mathrm{O}\)
The number of electrons involved in the reduction of 1 molecule of HNOg = 3
∴ Equivalent mass of HNO3
⇒ \(\frac{\text { Molecular mass of } \mathrm{HNO}_3}{1}=\frac{63}{3}=21\)
4. Oxidation number method: \(\stackrel{+4}{\mathrm{MnO}_2}+4 \mathrm{H}^{+} \rightarrow \stackrel{+2}{\mathrm{M}}{ }^{2+}+2 \mathrm{H}_2 \mathrm{O}\)
In this reaction, the oxidation number decreases from +4 to +2. So, the change in oxidation number per molecule of MnO2 = 4-2
= 2 units.
∴ Equivalent mass of MnO2
⇒ \(=\frac{\text { Molecular mass of } \mathrm{MNO}_2}{1}=\frac{87}{2}=43.5\)
Electronic method: \(\mathrm{MnO}_2+4 \mathrm{H}^{+}+2 e \rightarrow \mathrm{Mn}^{2+}+2 \mathrm{H}_2 \mathrm{O}\)
The number of electrons involved in the reduction of a molecule of MnO2 =-2
⇒ \(=\frac{\text { Molecular mass of } \mathrm{MnO}_2}{1}=\frac{87}{2}=43.5\)
5. Oxidation number method:
⇒ \(\stackrel{+7}{\mathrm{~K}} \mathrm{nO}_4+\stackrel{+2}{\mathrm{~F}} \mathrm{eSO}_4+\mathrm{H}_2 \mathrm{SO}_4\)
⇒ \(\mathrm{~K}_2 \mathrm{SO}_4+\stackrel{+2}{\mathrm{MnSO}_4+\stackrel{+3}{\mathrm{Fe}} \mathrm{e}_2}\left(\mathrm{SO}_4\right)_3+\mathrm{H}_2 \mathrm{O}\)
In this reaction, KMnO4 undergoes reduction because the oxidation number of Mn decreases from +7 to +2. So, the change in oxidation number of Mn= 7-2
=5 units.
∴ Equivalent mass of KMnO4
⇒ \(\frac{\text { Molecular mass of } \mathrm{KMnO}_4}{\begin{array}{c}
\text { Change in oxidation number per molecule } \\
\text { of } \mathrm{KMnO}_4 \text { because of its reduction }
\end{array}}\)
⇒ \(\frac{158}{5}\)
= 31.6
In the reaction, FeSO4 undergoes oxidation because the oxidation number of Fe increases from +2 to +3. So, the change in oxidation number of Fe = 3-2
= 1 unit.
∴ Equivalent mass of FeSO4
⇒ \(\frac{\text { Molecular mass of } \mathrm{FeSO}_4}{\begin{array}{c}
\text { Change in oxidation number per molecule } \\
\text { of } \mathrm{FeSO}_4 \text { because of its oxidation }
\end{array}}\)
⇒ \(\frac{151. 85}{1}\)
= 151.85
∴ Electronic method:
MnO4 + 8H++ 5e→ Mn2+ + 4H2O
Equivalent mass of KMnO4
⇒ \(=\frac{\text { Molecular mass of } \mathrm{KMnO}_4}{\begin{array}{c}
\text { Number of electrons gained in reduction of } \\
\text { one molecule of } \mathrm{KMnO}_4
\end{array}}\)
= \(\frac{158}{5}\)
31.6
Fe2+ →Fe3+ + e
∴ Equivalent mass of FeSO4
⇒ \(=\frac{\text { Molecular mass of } \mathrm{FeSO}_4}{\begin{array}{c}
\text { Number of electrons lost in oxidation of } \\
\text { one molecule of } \mathrm{FeSO}_4
\end{array}}\)
=\(\frac{151.85}{1}\)
Question 8. Determine the equivalent mass of Br2(l) [Molecular mass =159.82 in the given reaction:
⇒ \(2 \mathrm{MnO}_4^{-}(a q)+8 \mathrm{H}^{+}(a q)+\mathrm{Br}_2(l)\)→ \(2 \mathrm{Mn}^{2+}(a q)+2 \mathrm{BrO}_3^{-}(a q)+2 \mathrm{H}_2 \mathrm{O}(l)\)
In the given reaction, the oxidation half-reaction is —
⇒ \(\mathrm{Br}_2(l)+6 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 2 \mathrm{BrO}_3^{-}(a q)+12 \mathrm{H}^{+}(a q)+10 e\)
The number of electrons involved in the oxidation of one molecule of Br2 = 10 Equivalent mass of Br2.
⇒ \(=\frac{\text { Molecular mass of } \mathrm{Br}_2}{\begin{array}{c}
\text { Number of electrons involved per } \\
\text { molecule of } \mathrm{Br}_2 \text { in its oxidation }
\end{array}}\)
=\(\frac{159.82}{10}\)
= 15.982
Question 9. \(\mathrm{MnO}_4^{2-}\) undergoes a disproportionation reaction in an acidic medium but MnO4 docs do not. Give reason.
Answer:
The oxidation number of Mn in MnO4 is +7, which is the highest oxidation number that Mn can possess. So, it does not undergo the disproportionation reaction.
Again, in the case of \(\mathrm{MnO}_4^{2-}\) the oxidation number of Mn is +6. Therefore, Mn in \(\mathrm{MnO}_4^{2-}\) can increase its oxidation number to +7 or decrease it to some lower value. So, \(\mathrm{MnO}_4^{2-}\) undergoes a disproportionation reaction as given below—

In the above reaction, the oxidation number of Mn increases from +6 in \(\mathrm{MnO}_4^{2-}\) to +7 in MnO4 and decreases to +4 in MnO4.
Question 10. What amount of K2Cr2O7? (in mmol) is required to oxidize 24 mL 0.5 M Mohr’s salt?
Answer:
The number of mmol of Mohr’s salt in 24 mL 0.5MMohr’s salt solution =24 x 0.5 = 12.
So, the balanced redox reaction is
⇒ \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+6\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \cdot \mathrm{FeSO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O}+7 \mathrm{H}_2 \mathrm{SO}_4\)
⇒ \(\mathrm{~K}_2 \mathrm{SO}_4+6\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4+3 \mathrm{Fe}_2\left(\mathrm{SO}_4\right)_3+\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3+43 \mathrm{H}_2 \mathrm{O}\)
So, from the balanced equation, we see that 6mmol Mohr’s salt gets oxidized by 1mmol K2Cr2O7.
12 mmol Mohr’s salt gets oxidized by \(\frac{1}{6} \times 12\) = 2 mmol K2Cr2O7.
Question 11. Explain with reaction mechanism why the reaction between 03 and H2O2 is written as—
⇒ \(\mathrm{O}_3(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}_2(l) \rightarrow \mathrm{H}_2 \mathrm{O}(l)+\mathrm{O}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})\)
Answer:
The reaction between O3 and H2O2 is as follows
First step: O3(g)→O2(g) + O(g)’
Second step: H2O2(g) + O(g)→H2O(g) +O2(g)
In the first step, ozone produces nascent oxygen which is H2O2 in the second step. So, the overall reaction is as follows H2O2 + O3→H2O + O2 + O2
So, in the overall reaction, O2 Is written twice because a total of two molecules of O2 are produced during the reaction.
Question 12. 12.53 cm3 0.051M SeO2 reacts completely with 25.5 cm3 0.1 M CrSO4 to produce Cr2(SO4)3. What is the change in the oxidation number of Se in this redox reaction?
Answer:
Let the oxidation number of Se in the newly produced compound be x.
The redox reaction is as follows:
⇒ \({\left[\mathrm{Se}^{4+}+x e \longrightarrow \mathrm{Se}^{4-x}\right] \times 1}\)
⇒\({\left[\mathrm{Cr}^{2+} \longrightarrow \mathrm{Cr}^{3+}+e\right] \times x}\)
⇒ \(\mathrm{Se}^{4+}+x \mathrm{Cr}^{2+} \longrightarrow \mathrm{Se}^{4-x}+x \mathrm{Cr}^{3+}\)
Now, 12.53 cm3 0.051M SeO2 = 12.53 x 0.051 = 0.64 mmol SeO2
25.5 cm3 0.1 M CrSO4= 25.5 × 0.1 = 2.55 mmol CrSO4 However according to the balanced equation, 1 mol SeO2 gets reduced by x mol CrSO4.
2.55 mmol CrSO4 is reduced by \(\frac{2.55}{x}\) mmol SeO2
But 0.64 mmol SeO2 gets reduced
⇒ \(\text { So, } \frac{2.55}{x}=0.64 \quad \text { or, } x=4\)
The change in oxidation number of Se -atom = 4- (4- x) = x = 4.
Question 13. 30 ml 0.05 M KMnb4 is required for the complete oxidation of 0.5 g oxalate in an acidic medium. Calculate ) tl,e percent amount of oxalate in that salt sample:
Answer:
⇒ \(2 \mathrm{MnO}_4^{-}+5 \mathrm{C}_2 \mathrm{O}_4^{2-}+16 \mathrm{H}^{+} \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}\)
According to the equation, 2 mol MnO-4 = 5 mol C2O4
⇒ \(1 \mathrm{~mol} \mathrm{MnO}_4^{-} \equiv \frac{5}{2} \mathrm{~mol} \mathrm{C}_2 \mathrm{O}_4^{2 \mathrm{ris}}\)
Again, 1000 mL 0.05(M)KMnO4 =→ 0.05 mol KMnO4
30 mL 0.05(M)MnO4 \(\Rightarrow \frac{0.05 \times 30}{1000}\)
= 1.5×10-3 mol KMnO4
Now, 1 mol \(\mathrm{MnO}_4=\frac{5}{2} \mathrm{~mol} \mathrm{C}_2 \mathrm{O}_4\)
⇒ \(1.5 \times 10^{-3} \mathrm{~mol} \mathrm{MnO}_4=\frac{5}{2} \times 1.5 \times 10^{-3} \mathrm{~mol} \mathrm{C}_2 \mathrm{O}_4^2\)
⇒ \(=\frac{5}{2} \times 1.5 \times 10^{-3} \times 38 \mathrm{~g} \mathrm{C}_2 \mathrm{O}_4^{2-}=0.33 \mathrm{~g} \mathrm{C}_2 \mathrm{O}_4^{2-}\)
⇒ \(\text { Percentage of } \mathrm{C}_2 \mathrm{O}_4^{2-} \text { in the sample }=\frac{0.33 \times 100}{0.5}=66 \%\)
Long Answer Questions for Redox Reactions Class 11 Chemistry
Question 14. What will be the nature of the suit formed when 2 mol Nil Is added to the pigeon’s solution of mol pyrophosphoric? Clive equation?

Answer:
From the structure of pyrophosphoric acid, it Is clear that it contains four replaceable hydrogen atoms.
So, the reaction between and 2 mol NaOH will be as follows:
⇒ \(-\mathrm{H}_4 \mathrm{P}_2 \mathrm{O}_7+2 \mathrm{NaOH} \rightarrow \mathrm{Na}_2 \mathrm{H}_2 \mathrm{P}_2 \mathrm{O}_7+2 \mathrm{H}_2 \mathrm{O}\)
There are two replaceable 11-atoms in Na2H2P2O7 fonned due to the above reaction. So, it is an acidic salt.
Question 15. Oxidation million of the elements A, It mid-C are 12,1 to mid -2 respectively. Which one will lie the formula of the compound containing these three elements?
⇒ \(\mathrm{A}_2\left(\mathrm{BC}_2\right)_2, \mathrm{~A}_3\left(\mathrm{~B}_2 \mathrm{C}\right)_2, \mathrm{~A}_3\left(\mathrm{BC}_4\right)_2\)
Answer:
The total oxidation number of all the elements in a compound should be zero (0).
In A2(BC2) molecule, the sum of oxidation numbers of all the atoms
=2 ×(+ 2) + 2 × (+ 5) + 4 × (-2) = + 6
In the A2(B2C)2 molecule, the sum of oxidation numbers of all the atoms
= 3 × (+ 2) + 4 × (+ 5) + 2 × (-2) = + 22
In A3(BC4)2 molecule, the sum of oxidation numbers of all the atoms
= 3 × (+ 2) + 2 × (+ 5) + 8 ×(-2) = 0.
∴ The correct formula of the compound will be A3(BC4)2
Question 16. In an acidic medium, for the reduction of each NO3 ion in the given reaction, how many electrons will be required? NO3 NH2OH
Answer:
NO3— NH2OH; For equalizing the number of O -atoms on both sides, two H2O molecules are added to the right side (having a lesser number of O -atoms) and two H+ ions are added to the left side for each molecule of H2O added.
⇒ \(\mathrm{NO}_3^{-}+4 \mathrm{H}^{+} \longrightarrow \mathrm{NH}_2 \mathrm{OH}+2 \mathrm{H}_2 \mathrm{O}\)
For equalizing the number of H -atoms on both sides, three additional H+ ions are required on the left side
so we get, \(\mathrm{NO}_3^{-}+7 \mathrm{H}^{+} \longrightarrow \mathrm{NH}_2 \mathrm{OH}+2 \mathrm{H}_2 \mathrm{O}\)
To balance the charge on both sides, 6 electrons are added to the left side ofthe equation.
⇒ \(\mathrm{NO}_3^{-}+7 \mathrm{H}^{+}+6 e \longrightarrow \mathrm{NH}_2 \mathrm{OH}+2 \mathrm{H}_2 \mathrm{O}\)
Hence, for the reduction of each NO3 ion into an NH2OH molecule, 6 electrons are required.
Question 17. Identify the redox reactions among the following:
1. 2CuSO4 + 4KI→2CuI + I2 + 2K2SO4
2. BaCl2 + Na2SO4→BaSO4 + 2NaCl
3. 2NaBr + Cl2→2NaCl + Br2
4. NH4NO2→+N2 + 2H2O
5. CuSO4 + 4NH3→[Cu(NH3)4]SO4
6. 3I2 + 6NaOH→NaIO3 + 5NaI + 3H2O
Answer:
In this reaction, the oxidation number of Cu decreases (+ 2 → +1) and the oxidation number of I4 increases (-1→ 0) i.e. reduction of CuSO4 and oxidation of KI take place. Thus, it is a redox reaction.
1. \(\stackrel{+2}{\mathrm{CuSO}_4}+\stackrel{-1}{\mathrm{KI}} \rightarrow \stackrel{+1}{\mathrm{CuI}}+\stackrel{0}{\mathrm{I}} 2+\mathrm{K}_2 \mathrm{SO}_4\)
This reaction does not involve any change in the oxidation number of any element i.e., in this reaction, oxidation or reduction does not take place. Hence, it is not a redox reaction
2. \(\stackrel{+2}{\mathrm{BaCl}_2}+\stackrel{+1}{\mathrm{Na}_2} \mathrm{SO}_4 \rightarrow \stackrel{+2}{\mathrm{BaSO}}{ }_4+2 \mathrm{NaCl}^{-1}\)
In this reaction, the oxidation number of bromine increases from -1 to 0 and the oxidation number of chlorine decreases from 0 to -1. In this case, NaBr gets oxidized whereas Cl2 gets reduced. Hence, this reaction is a redox reaction.
3. \(2 \mathrm{NaBr}+\stackrel{0}{\mathrm{C}} \mathrm{l}_2 \rightarrow 2 \mathrm{Na}{ }^{-1} \mathrm{Cl}+\stackrel{0}{\mathrm{Br}}{ }_2\)
In NH4NO2, the oxidation number of N in NH4 increases from -3 to 0, while the oxidation number of N in NO2 decreases from +3 to 0, i.e., in this reaction, simultaneous oxidation of NH+ and reduction of NO2 in the compound occur. So, this reaction is a redox reaction.
4. \(\stackrel{-3}{\mathrm{NH}_4}{\stackrel{+3}{\mathrm{~N}} \mathrm{O}_2 \rightarrow \stackrel{0}{\mathrm{~N}}}_2+2 \mathrm{H}_2 \mathrm{O}\)
This reaction does not involve any change in the oxidation number of any element. So, it is not a redox reaction.
5. \(\left(\stackrel{+2}{\mathrm{CuSO}_4}+4 \mathrm{NH}_3\right) \rightarrow\left[\stackrel{+2}{\mathrm{Cu}}\left(\mathrm{NH}_3\right)_4\right] \mathrm{SO}_4\)
In the given reaction, the oxidation number of iodine increases from 0 to +5 and decreases from 0 to -1 i.e., both oxidation and reduction occur in this reaction. Thus, it is a redox reaction.
6. \(3 \stackrel{0}{\mathrm{I}}_2+6 \mathrm{NaOH} \rightarrow \mathrm{NaIO}_3^{+5}+5 \mathrm{NaI}^{-1}+3 \mathrm{H}_2 \mathrm{O}\)
Question 18. Which of the following reactions are disproportionation reactions and comproportionation reactions?
1. 4KClO3→KCl + 3KClO4
2. 3K2MnO4 + 2H2O→2KMnO4 + MnO2 + 4KOH
3. KIO3 + 5KI + 6HCI→3I2 + 6KCI + 3H2O
4. 2C6H5CHO + NaOH C6H5COONa + C6H5CH2OH
5. Ag2+ + Ag→2Ag+
Answer:
1.

In KClO3, the oxidation number of Cl = + 5. The oxidation numbers of Cl in KCl and KClO4 are -1 and + 7 respectively. So in this reaction, KClO3 undergoes simultaneous oxidation and reduction producing KClO4 and KCl respectively. Hence, it is a disproportionation reaction.
2.

The oxidation number of Mn in K2MnO4 = + 6. On the other hand, the oxidation numbers of Mn in the products KMnO4 and MnOa are + 7 and + 4 respectively. Therefore, in the reaction, K2MnO4 is oxidized and reduced at the same time forming KMnO4 and MnO4. Thus, it is a disproportionation reaction.
3.

The oxidation numbers of iodine in KIO3 and KI are + 5 and -1 respectively and the oxidation number of iodine in 12 is zero. This oxidation number lies between + 5 and -1, which is an intermediate oxidation state. So, it is a comproportionation reaction.
4.

In this reaction, the oxidation number of carbon in the benzene ring does not change. However, the oxidation number of carbon atoms in the —CHO group (the oxidation number of carbon in —CHO is +1 ) changes to a higher oxidation number of +3 in the —COONa group and a lower oxidation number of -1 in the —CH2OH group. Here, —the CHO group gets simultaneously oxidized & reduced. Hence, it is a disproportionation reaction.
5.

The oxidation numbers of the reactants Ag2+ and Ag are + 2 and O respectively and the oxidation number of the product is +1. This oxidation number is intermediate between the oxidation numbers +2 and O . so it is a comproportionation reaction.
Question 19. Identify the redox reaction(s) and also the oxidants B and the reductants from the following reaction(s).
1. \(2 \mathrm{MnO}_4^{-}+5 \mathrm{SO}_2+6 \mathrm{H}_2 \mathrm{O}\) → \( 5 \mathrm{SO}_4^{2-}+2 \mathrm{Mn}^{2+}+4 \mathrm{H}_3 \mathrm{O}^{+} \)
2. \(\mathrm{NH}_4^{+}+\mathrm{PO}_4^{3-} \longrightarrow \mathrm{NH}_3+\mathrm{HPO}_4^{2-} \)
3. \( \mathrm{HClO}+\mathrm{H}_2 \mathrm{~S} \longrightarrow \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{Cl}^{-}+\mathrm{S}\)
Answer:
In this reaction, the oxidation number of Mn decreases from (+7→+2) and the oxidation number of S increases from (+4→+6). So this reaction causes a reduction of MnO4 and oxidation of SO2. Hence, the reaction is a redox reaction. Here Mnt)ÿ acts as an oxidant and SO2 as a reductant.
This reaction does not involve any change in the oxidation number of any element. So it is, not a redox reaction.
This reaction involves an increase in the oxidation number of S from -2 to 0, and a decrease in the oxidation number of, Cl from +1 to -1. So it is a redox reaction. Here HCIO is an oxidising agent and H2S is a reducing agent.
Question 20. Determine the equivalent masses of Na2S2O3.5H2O +2 and KBrO3 In the following reactions.
⇒ \(2 \mathrm{~S}_2 \mathrm{O}_3^{2-}+\mathrm{I}_2 \rightarrow \mathrm{S}_4 \mathrm{O}_6^{2-}+2 \mathrm{I}^{-}\)
⇒ \(\mathrm{BrO}_3^{-}+6 \mathrm{H}^{+}+6 e \longrightarrow \mathrm{Br}^{-}+3 \mathrm{H}_2 \mathrm{O}\)
Answer:
In this reaction, two \(\mathrm{S}_2 \mathrm{O}_3^{2-}\) ions are oxidised by losing 2 electrons. So, for the oxidation of one \(\mathrm{S}_2 \mathrm{O}_3^{2-}\) ion, one electron is given up
Equivalent mass of Na2S2O3.5H2O
⇒ \(=\frac{\text { Molecular mass of } \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3 \cdot 5 \mathrm{H}_2 \mathrm{O}}{1}=248\)
This reaction produces Br- from BrO3. In the reduction of each molecule of KBr03, 6 electrons are accepted.
In the given reaction, the equivalent mass of KBrO3
⇒ \(\frac{\text { Molecular mass of } \mathrm{KBrO}_3}{6}=\frac{167}{6}=27.8\)
NCERT Solutions Class 11 Chemistry Chapter 8 Long Questions & Answers
Question 21. Determine the equivalent weights of the underlined compounds in the following two reactions:
1. \(\mathrm{FeSO}_4+\mathrm{KMnO}_4+\mathrm{H}_2 \mathrm{SO}_4\)
→ \(\mathrm{K}_2 \mathrm{SO}_4+\mathrm{MnSO}_4+\mathrm{Fe}_2\left(\mathrm{SO}_4\right)_3+\mathrm{H}_2 \mathrm{O}\)
2. MnO2+HCl → MnCl2+ Cl2+H2O
K = 39, Mn = 55, O = 16
Answer:
In this reaction, the decrease in oxidation number of Mn =(7-2) = 5 units. Equivalent weight of Mn.
1. \(\frac{\text { Molecular weight of } \mathrm{KMnO}_4}{\text { Change in oxidation number }}\)
= \(\frac{158}{5}\)
= 31.6
⇒ \(\stackrel{+4}{\mathrm{MnO}}{ }_2 \longrightarrow \stackrel{+2}{\mathrm{MnCl}}{ }_2\)
Here the oxidation number of Mn decreases by (4-2)
= 2 unit.
∴ Equivalent weight of MnO2
⇒ \(=\frac{\text { Molecular weight of } \mathrm{MnO}_2}{\text { Change in oxidation number }}=\frac{87}{2}=43.5\)
Question 22. Balance the following equation with the help of the oxidation number method.
⇒ \(\mathrm{Fe}_3 \mathrm{O}_4+\mathrm{CO} \rightarrow \mathrm{FeO}+\mathrm{CO}_2\)
Answer:
In Fe3O4, the oxidation number of each Fe -atom =+2.67. In the reaction, the oxidation number of each Fe -atom decreases from + 2.67 to 2. So, decrease in oxidation number. of eachFe -atom = +0.67
Therefore for three Fe -atoms, the total decrease in oxidation number 3 × (+ 0.67) =+ 2 unit.
On the other hand, the oxidation number of C increases from +2 to +4. Hence, an increase in the oxidation number of C= 2 units.
Therefore, in the reaction, Fe3O4 and CO will react with each other in the molar ratio of 2: 2 or 1: 1 .
Again 1 molecule of Fe3O4 will produce 3 molecules of F2O. Hence, the balance equation will be
Fe3O4 + CO → 3FeO + CO2.
Question 23. Balance by ion-electron method: MnO2 + HCl→+Mn2+ + Cl2 + H2O
Answer:

Or MnO2 + 4H+ + 4Cl→ Mn2+ + 2Cl + Cl2+ 2H2O
Therefore the balanced chemical equation is:
MnO2 + 4H + 4Cl → MnCl2 + Cl2 + 2H2O
Question 24. In a basic medium, balance the half-reactions below:
- \(\mathrm{Cr}(\mathrm{OH})_3 \rightarrow \mathrm{CrO}_4^{2-}\)
- \(\mathrm{Cl}_2 \mathrm{O}_7 \rightarrow 2 \mathrm{ClO}_2^{-}\)
Answer:
In order to equalize the number of O -atoms, one H2O molecule is added to the right side (because it has an excess O -atom) and for this 1 molecule of H2O, two OH Now, we get Cr(OH)2 + 2OH– ions are added to the left side. CrO2- +H2O. In this equation, five H -atoms are on the left side, and two H atoms are present on the right side. To equalize the number of H -atoms on both sides, three OH-(aq) and three H2O molecules are added to the left side and right side respectively. Finally, we get
⇒ \( \mathrm{Cr}(\mathrm{OH})_3+2 \mathrm{OH}^{-}+3 \mathrm{OH}^{-} \rightarrow \mathrm{CrO}_4^{2-}+\mathrm{H}_2 \mathrm{O}+3 \mathrm{H}_2 \mathrm{O}\)
Or, \(\mathrm{Cr}(\mathrm{OH})_3+5 \mathrm{OH}^{-} \longrightarrow \mathrm{CrO}_4^{2-}+4 \mathrm{H}_2 \mathrm{O}\)
For balancing the charge, 3 electrons are added to the right side. Therefore, the final half-reaction becomes,
⇒ \(\mathrm{Cr}(\mathrm{OH})_3+5 \mathrm{OH}^{-} \longrightarrow \mathrm{CrO}_4^{2-}+4 \mathrm{H}_2 \mathrm{O}+3 e\)
2. To balance the number of 0 -atoms on both sides, 3 molecules of H2O(Z) are added to the left side (because there are excess O -atoms on this side) and for these three H2O(l) molecules, six OH- (aq) ions are added to the right side. Then we get
⇒ \(\mathrm{Cl}_2 \mathrm{O}_7+3 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{ClO}_2^{-}+6 \mathrm{OH}^{-}\)
To balance the charge, electrons are added to the left side. Hence, the balanced equation is
⇒ \(\mathrm{Cl}_2 \mathrm{O}_7+3 \mathrm{H}_2 \mathrm{O}+8 e \longrightarrow 2 \mathrm{ClO}_2^{-}+6 \mathrm{OH}^{-}\)
Question 25. Balance the following reaction in acidic and alkaline medium: \(\mathrm{SO}_3^{2-}(a q) \longrightarrow \mathrm{SO}_4^{2-}(a q)\)
Answer:
Addlemedium:
⇒ \(\mathrm{SO}_3^{2-}(a q) \longrightarrow \mathrm{SO}_4^{2-}(a q)\)
In Tills reaction, this left side of the equation is deficient in one () -atom. So the number of O -atoms on both sides is equalized by adding one H2O(aq) molecule to the left side.
⇒ \(\mathrm{SO}_3^{2-}(a q)+\mathrm{H}_2 \mathrm{O}(l) \longrightarrow \mathrm{SO}_4^{2-}(a q)\)
To equalize the number of H -atoms on both sides, two H+ ions are added to the right side. So we get—
⇒ \(\mathrm{SO}_3^{2-}(a q)+\mathrm{H}_2 \mathrm{O}(l) \longrightarrow \mathrm{SO}_4^{2-}(a q)+2 \mathrm{H}^{+}(a q)\)
To balance the charge on both sides, 2 electrons are added to the right side, we get—
⇒ \(\mathrm{SO}_3^{2-}(a q)+\mathrm{H}_2 \mathrm{O}(l) \longrightarrow \mathrm{SO}_4^{2-}(a q)+2 \mathrm{H}^{+}(a q)+2 e\)
Basic medium: \(\mathrm{SO}_3^{2-}(a q) \longrightarrow \mathrm{SO}_4^{2-}(a q)\)
To balance the number of O -atoms on both sides, two OH”(a<7) ions and one H20(Z) molecule are added to the left side and right side respectively. This gives—
⇒ \(\mathrm{SO}_3^{2-}(a q)+2 \mathrm{OH}^{-}(a q) \longrightarrow \mathrm{SO}_4^{2-}(a q)+\mathrm{H}_2 \mathrm{O}(l)\)
To balance the change on both sides, we add 2 electrons to the right side. Thus, the balance equation is—
⇒ \(\mathrm{SO}_3^{2-}(a q)+2 \mathrm{OH}^{-}(a q) \longrightarrow \mathrm{SO}_4^{2-}(a q)+\mathrm{H}_2 \mathrm{O}(l)+2 e\)
Question 26. Determine the values of x and y in the following balanced equation:
⇒ \(5 \mathrm{H}_2 \mathrm{O}_2+x \mathrm{ClO}_2+2 \mathrm{OH}^{-} \longrightarrow x \mathrm{Cl}^{-}+y \mathrm{O}_2+6 \mathrm{H}_2 \mathrm{O}\)
Answer:
Oxidation half-reaction:
⇒ \(\mathrm{H}_2 \mathrm{O}_2+2 \mathrm{OH}^{-} \longrightarrow \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}+2 e\)……………………….(1)
Reduction half-reaction:
⇒\(\mathrm{ClO}_2+2 \mathrm{H}_2 \mathrm{O}+5 e \longrightarrow \mathrm{Cl}^{-}+4 \mathrm{OH}^{-}\) ……………………….(2)
Multiplying equations (1) and (2) by 5 and 2 respectively and adding the equations, we get—
⇒ \(5 \mathrm{H}_2 \mathrm{O}_2+10 \mathrm{OH}^{-}+2 \mathrm{ClO}_2+4 \mathrm{H}_2 \mathrm{O}\)→ \(2 \mathrm{Cl}^{-}+5 \mathrm{O}_2+10 \mathrm{H}_2 \mathrm{O}+8 \mathrm{OH}^{-}\)
∴ 5H2O2 + 2ClO2 + 2OH–→2Cl–+ 5HO2 + 6H2O ……………………….(3)
Comparing equation (3) with the given equation, we get x = 2 and y – 5.
Question 27. In the given reaction determine the equivalent weight of AS2S3:
Answer:
⇒ \(\mathrm{As}_2 \mathrm{~S}_3+7 \mathrm{ClO}_3^{-}+7 \mathrm{OH}^{-}-7\) → \(2 \mathrm{AsO}_4^{3-}+7 \mathrm{ClO}^{-}+3 \mathrm{SO}_4^{2-}+6 \mathrm{H}_2 \mathrm{O}\)
The increase in oxidation number of each As -atom =5-3 = 2 units.
So, the total increase in oxidation number of two As -atoms = 2 ×2
= 4 units.
Two increases in the oxidation number of each S-atom =+6-(-2)
Therefore, the total Increase in oxidation number of three S -atoms = 3 × 8
= 24 units.
Hence, the total Increase In oxidation number for each As 2S,t molecule In Its oxidation Is a (24 + 4) = 20 unit.
Equivalent weight of AS2S3 In the given reaction
⇒ \(\frac{\text { Molecular weight of } \mathrm{As}_2 \mathrm{~S}_3}{\text { Increase in oxidation number }}=\frac{M}{28}\)
Redox Reactions Chapter 8 Long Answer Solutions Class 11
Question 28. Determine the equivalent mass of Fe2O4 in the given Mn = 6-4 = 2 units. reaction: FeO4 + KMn04↓Fe2O3 + MnO2 (Assume that the molecular mass of Fe3O4 =M
Answer:
⇒ \(\stackrel{+\mathrm{F} / 3}{\mathrm{Fe}} \mathrm{e}_3 \mathrm{O}_4 \rightarrow \stackrel{+3}{\mathrm{~F}} \mathrm{e}_2 \mathrm{O}_3\stackrel{+\mathrm{F} / 3}{\mathrm{Fe}} \mathrm{e}_3 \mathrm{O}_4 \rightarrow \stackrel{+3}{\mathrm{~F}} \mathrm{e}_2 \mathrm{O}_3\)
In this reaction, the increase in oxidation number for each Pc -atom \(=3-\frac{8}{3}=\frac{1}{3} \text {. }\)
So, the total increase in oxidation number for 3 Fe -atoms
⇒ \(=3 \times \frac{1}{3}=1 \text { unit. }\)
Therefore, the equivalent mass of Fe3O4
⇒ \(=\frac{\text { Molecular mass of } \mathrm{Fe}_3 \mathrm{O}_4}{\text { Increase in oxidation number }}=\frac{M}{1}=M\)
Question 29. What is the ratio of equivalent weights of MnO4 in acidic, basic & neutral mediums?
Answer:
The reactions that the MnO4 ion undergoes in acidic, basic, and neutral mediums are as follows. Acidic Medium
⇒ \(\mathrm{MnO}_4^{-}(a q)+8 \mathrm{H}^{+}(a q)+5 e \rightarrow \mathrm{Mn}^{2+}(a q)+4 \mathrm{H}_2 \mathrm{O}(l)\)
- Equivalent weight \(E_1=\frac{M}{5}\) where M = KMn04.
- Basic Medium: MnO4 (aq) + e→MnO4 (aq)
- Equivalent weight \(E_2=\frac{M}{1}\)
Neutral Medium:
⇒ \(\mathrm{MnO}_4^{-}(a q)+2 \mathrm{H}_2 \mathrm{O}(l)+3 e \rightarrow \mathrm{MnO}_2(s)+4 \mathrm{OH}^{-}(a q)\)
Therefore \(E_1: E_2: E_2=\frac{1}{5}: 1: \frac{1}{3}=3: 15: 5\)
Question 30. MnO4 reacts with Ax+ to form AO3– , Mn2+, and O2. One mole of MnO4– oxidizes 1.25 moles of Ax+ to AO3–. What is the value of x?
Answer:
⇒ \(\mathrm{MnO}_4^{-}+\mathrm{A}^{x+} \rightarrow \mathrm{AO}_3^{-}+\mathrm{Mn}^{2+}+\mathrm{O}_2\)
The change in oxidation number of = 5 unit (+7→+2) and that of A = (5- x) unit [+x→+5 in AO3-3]
1 mole of MnO3 reacts completely with 1.25 mole of Ax+
Therefore, 1×5 = 1.25(5 -x)
Solving for x gives x = +1
Thus, the value of x = +1
Question 31. 20 mL solution of 0.1 (M) FeSO2 as completely oxidized using a suitable oxidizing agent. What is the number of electrons exchanged?
Answer:
20 mL of 0.1(M) FeSO4
=\(\frac{0.1}{1000} \times 20\)
=\(2 \times 10^{-3} \mathrm{~mol} \text { of } \mathrm{Fe}^{2+}\)
Fe2+ is oxidized to Fe3+, leaving 1 electron.
Hence, the number of electrons exchanged by 2 x 10-3 mol of Fe2+ is
2 × 10-3× 6.022 × 1023
= 1.2044 × 1021 electrons
Question 32. What are the oxidation numbers of the underlined elements in each of the following and how do you rationalise your results?
- Kl3
- CH3COOH
Answer: The chemical structure of KI3 is K+[1-1→1]¯
In Kl3-1 ion forms a coordinate Bond with I2. The Osditaion Number Of Each 1 atom in 12 molecules is zero, and the oxidation number of K+ id +1, therefore from the oxidation number of one I- will be -1

2. The C-1 atom is linked to one O-atom and one OH group, For O -atom and OH -group, the oxidation numbers are 2 and I respectively, As C-1 and C-2 are the atoms of the name element, the covalent linkage between them makes no change In oxidation number of either atom.
So, the oxidation number of C- 1 would be +3 since the total oxidation number of one O-atom and one Oil -group is -3. The total oxidation number of three OH-atoms linked to the C-2 atom Is +3. So, the oxidation number of C-2 would be -3.
Question 33. Justify the following reactions are redox reactions:
1. \(\mathrm{CuO}(s)+\mathrm{H}_2(g) \rightarrow \mathrm{Cu}(s)+\mathrm{H}_2 \mathrm{O}(g)\)
2. \( \mathrm{Fe}_2 \mathrm{O}_3(s)+3 \mathrm{CO}(g) \rightarrow 2 \mathrm{Fe}(s)+3 \mathrm{CO}_2(g)\)
3. \(4 \mathrm{BCl}_3(g)+3 \mathrm{LiAlH}_4(s)\) → \(2 \mathrm{~B}_2 \mathrm{H}_6(g)+3 \mathrm{LiCl}(s)+3 \mathrm{AlCl}_3(s)\)
4. \(2 \mathrm{~K}(s)+\mathrm{P}_2(g) \rightarrow 2 \mathrm{~K}^{+} \mathrm{F}^{-}(s)\)
5. \( 4 \mathrm{NH}_3(g)+5 \mathrm{O}_2(\mathrm{~g}) \rightarrow 4 \mathrm{NO}(g)+6 \mathrm{H}_2 \mathrm{O}(g)\)
Answer:
1. 
In the reaction, the oxidation number of Cu decreases (+2→0) indicating the reduction of CuO, and the oxidation number of 2 increases (0 →+1), indicating the oxidation of H2. Hence, it is a redox reaction.
2. 
In the reaction, the oxidation number of Fe decreases (+3→+0) and that of C increases
→+4). Therefore, the reaction involves the reduction of Fe2O3 and the oxidation of CO. Hence, it is a redox reaction.
3.

The reaction involves the reduction of BC13 because the oxidation number of B decreases from +3 to -3 and the oxidation of LiAH4 as the oxidation number increases from -1 to +1. So, it is a redox reaction.
4.

In the reaction, the increase in the oxidation number of K (0→+1) and the decrease in the oxidation number of F (0 to -1) indicate that the former undergoes oxidation and the latter reduces. Hence, the given reaction is a redox reaction.
5.

In the reaction, NH3 undergoes oxidation because the oxidation number of N-atom increases (-3 to +2), while O2 undergoes reduction, as is evident from the decrease in the oxidation number of O (0→-2). So, it is a redox reaction.
Redox Reactions Long Question Answers for Class 11 Chemistry
Question 34. Calculate the oxidation number of sulfur, chromium and nitrogen in \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) & NO3–. Suggest the structure of these compounds. Count for the
Answer:
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\): If the oxidation number of Cr in \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\)
Be x, then 2(x)+ 7(-2)
=-2
∴ x = +6
The structure of \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) is

Therefore, there is no fallacy.
NO3–:
According to a conventional method, the oxidation number of N in
NO3–: x+ 3(-2) = -1 or, x = +5
Where x = +3 -1. oxidation number of N in NO3–)
However, according to the chemical bonding method, the structure of NO3– is

If the oxidation number of N is -0 = 0 the above structure is x,
Then, x+ (-1) + (-2) + (-2)
⇒ \(\begin{gathered}
x+(-1)+(-2)+(-2)=0 \\
\quad\left(\text { for } \mathrm{O}^{-}\right)(\text {for }=0)(\text { for } \rightarrow 0)
\end{gathered}\)
or, x = +5
So, in both conventional and chemical bonding methods. the oxidation number of N in NO-3 is +5. Therefore, there is no fallacy.
Question 35. Suggest a list of the substances where carbon can exhibit oxidation states from -4 to +4 and nitrogen from -3 to +5
Answer:
Carbon (c):

Nitrogen (N):

Question 36. While sulfur dioxide and hydrogen peroxide can act as oxidizing as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. why?
Answer:
A species can act as both oxidant and reductant am one of its constituent atoms has an intermediate value of oxidation number. So, in a reaction the atom can increase or decrease its oxidation number i.e., it can act as an oxidant as well as a; reductant.
1. In SO2, the oxidation number of S is +4. The highest and lowest oxidation numbers of S are +6 and -2 respectively. Therefore, the S-atom in SO2 can increase its oxidation number in a reaction in which SO2 acts as a reductant and decrease its oxidation number in a reaction in which SO2 plays the role of an oxidant. Hence, SO2 can act as an oxidant as well as a reductant.
2. In H2O2, the oxidation number of O is -1 The highest and lowest oxidation numbers of oxygen are -2 and O respectively. Therefore, the oxygen atom in H2O2 is capable of increasing or decreasing its oxidation number. In the reaction in which H2O2 acts as an oxidant, the oxidation number of oxygen decreases from -1 to -2 and in the reaction in which it acts as a reductant, the oxidation number of oxygen increases from -1 to 0. Hence, H2O2 can act both as an oxidant and a reductant.
3. In O3, the oxidation number of oxygen is zero. Oxygen can show two oxidation numbers, -1 and -2. So, the oxidation number of oxygen O3 can reduce to -1 or -2, but it can never increase. Hence, O3 can act only as an oxidant.
4. In HNO3, the oxidation number of nitrogen is +5. It is the maximum oxidation number that nitrogen can exhibit. So, the only opportunity for nitrogen in HNO3 is to decrease its oxidation number. Hence, HNO3 can act only as an oxidant.
Question 37. Consider the reactions:
1. 6CO2(g) + 6H2O(l)→ C6H12O6(aq) + 6O2(g)
2. O3(g) + H2O2(l) → H2O(Z) + 2O2(g)
Why it is more appropriate to write these reactions
1. 6CO2(g) + 12H2O(l) → C6H12O6(aq) + 6H2O(l) +6O2(g)
2. O3(g) + H2O2(l) → H2O(l) +O2(g) + O2(g)
Also, suggest a technique to investigate the path of the above (1) and (2) redox reactions
Answer:
The reaction shown by the equation 1 takes place in the photosynthesis process. From the equation 1, it may seem that the reaction involves only the consumption of H2O. However, if we look at the steps that are supposed to be involved in the photosynthesis reaction, it becomes evident that consumption as well as formation of H2O takes place in the photosynthesis reaction. The proposed steps of the photosynthesis reaction are
1. Decomposition of H2O into H2 and O2
12H2O(Z)→12H2(g) + 6O2(g) …………………………(1)
2. Formation of C6H120O6 and H2O due to reduction of CO2(g) by H2(g) produced in step.
6CO2(g) + 12H2(g)→C6HI2O6(s) + 6H2O(l)…………………………(2)
Combining equations (1) and (2) gives the complete reaction for the photosynthesis process.
6CO2(g) + 12H2O(l) → C6H12O6(s) + 6O2(g) +6H2O(l)…………………………(3)
Thus, equation (3) will be more appropriate for representing the photosynthesis reaction because it gives the actual stoichiometry of the reactants and the products involved in the given reaction.
From the equation 2, the source of O2 formed in the reaction is not obvious. One may think O2 is formed from O3 or H2O2 or both O3 and H2O2. The detailed steps of this reaction as shown below reveal that O2 is formed from both O3 and H2O2.
 ……………..(1)
……………..(1)
Therefore, it is appropriate to represent the reaction by equation (1).
To investigate the paths of the reaction 1 and 2, we adopt the tracer technique method. In this method, we use H2O18 instead of H2O for reaction 1 and H2O218 instead of H2O2 for reaction 2.
Question 38. Whenever a reaction between an oxidizing agent and a reducing agent is carried out, a compound of a lower oxidation state is formed if the reducing agent is in excess, and a compound of a higher oxidation state is formed if the oxidizing agent is in excess. Justify this statement by giving three illustrations.
Answer:
1. The given statement can be justified from the examples of j reactions mentioned below.
The reaction of C (reductant) with O2 (oxidant) may result in CO or CO2 or a mixture of CO and CO2. However, if this reaction is initiated with the excess amount of C, the only product that forms is CO. On the other hand, if O2 is taken in excess in the reaction, only CO2 is formed. In CO, the oxidation state of C is +2, and in CO2, it is +4.
Thus, we see that taking an excess amount of reductant leads to the formation of a compound lower oxidation state. Conversely, a compound of a higher oxidation state is formed when the oxidant is taken in excess.

The reaction of P4 (reductant) with Cl2 (oxidant) results in PCl3 when P4 is taken in excess, while it results in PCl5 when Cl2 is taken in excess.

The oxidation state of PCl3 is +3 and that in PC15 is +5. Thus, an excess amount of reductant produces an oil compound lower oxidation state and an excess amount of oxidant produces a compound with a higher oxidation state.
The same thing happens when Na (reductant) is reacted with O2 (oxidant).In the presence of excess Na, the resulting compound is Na2O, in which the oxidation state of oxygen is -2 in the presence of excess O2, the resulting compound is Na202, in which the oxidation state of oxygen is -1. → ↑

[In this reaction, the mass of Na is 46g and that of oxygen is 64 g]
NCERT Class 11 Chemistry Redox Reactions Long Answer Solutions
Question 39. How do you count for the following observations? Although alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet In the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write a balanced redox equation for the reaction.
When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colorless pungent-smelling gas HC1, but if the mixture contains bromide then we get red vapour of bromine. Why?
Answer:
The reaction in acid medium:

Multiplying equation (1) by 6 and equation (2) by 5 and then adding them together, we have

The reaction in alkaline or neutral medium:

Multiply equation (3) by 2 and then adding it to equation (4), we have

Even though toluene oxidizes to benzoic acid in the presence of acidic or alkaline KMnO4, the manufacture of benzoic acid from toluene is usually carried out by using alcoholic KMnO4 as an oxidant.
This is because ofthe following advantages:
The use of alcoholic KMnO4 is cost-effective because carrying out the reaction in the presence of it does not require adding either acid or alkali in the reaction medium. In a neutral medium, OH- ions are produced during the reaction.
Both KMnO4 and toluene are soluble in alcohol and they form a homogeneous mixture. This facilitates the reaction and contributes towards speeding up the reaction.
When concentrated H2SO4 is added to an inorganic mixture containing chloride, HC1, which has a pungent smell, is produced.
Question 40. Identify the substance oxidized, reduced, oxidizing agent, and reducing agent for each of the following reactions
1. \(2 \mathrm{AgBr}(s)+\mathrm{C}_6 \mathrm{H}_6 \mathrm{O}_2(a q) \rightarrow 2 \mathrm{Ag}(s)+2 \mathrm{HBr}(a q)+\mathrm{C}_6 \mathrm{H}_4 \mathrm{O}_2(a q)\)
2. \(\mathrm{HCHO}(l)+2\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right]^{+}(a q)+3 \mathrm{OH}^{-}(a q)\)
→ \(2 \mathrm{Ag}(s)+\mathrm{HCOO}^{-}(a q)+4 \mathrm{NH}_3(a q)+2 \mathrm{H}_2 \mathrm{O}(l)\)
3. \(\mathrm{HCHO}(l)+2 \mathrm{Cu}^{2+}(a q)+5 \mathrm{OH}^{-}(a q) \rightarrow \mathrm{Cu}_2 \mathrm{O}(s)+\mathrm{HCOO}^{-}(a q)+3 \mathrm{H}_2 \mathrm{O}(l)\)
4. \(\mathrm{N}_2 \mathrm{H}_4(l)+2 \mathrm{H}_2 \mathrm{O}_2(l) \rightarrow \mathrm{N}_2(g)+4 \mathrm{H}_2 \mathrm{O}(l)\)
5.\(\mathrm{Pb}(s)+\mathrm{PbO}_2(s)+2 \mathrm{H}_2 \mathrm{SO}_4(a q) \rightarrow 2 \mathrm{PbSO}_4(s)+2 \mathrm{H}_2 \mathrm{O}(l)\)
Answer:

Question 41. Consider the reaction:
1. 2S2O3(aq) + I2(s) →S4O62-aq) + 2I–(aq)
2. S4O32-(aq) + 2Br2(Z) + 5H2O(l) → 2SO42-(aq) + 4Br–(aq)+10H+(aq)
Answer:
The standard reduction potential for Br2/2Br– system is greater than that for I2/2I- system
⇒ \(E_{\mathrm{Br}_2 / 2 \mathrm{Br}}^0=1.09 \mathrm{~V}\)
And \(E_{1_2 / 21^{-}}^0=0.54 \mathrm{~V}\)
This indicates that Br2 is a stronger oxidizing agent than I. The average oxidation number of S in
⇒ \(\mathrm{S}_2 \mathrm{O}_3^{2-}\) is +2. and that in \(\mathrm{S}_4 \mathrm{O}_6^{2-}\) is 2.5, while the oxidation number of S in SO2– is +6.
The oxidation number per S-atom changes by 0.5 units in the reaction
⇒ \(\mathrm{S}_2 \mathrm{O}_3^{2-} \rightarrow \mathrm{S}_4 \mathrm{O}_6^{2-}\) while in the reaction \(\mathrm{S}_2 \mathrm{O}_3^{2-} \rightarrow \mathrm{SO}_4^{2-}\) This change occurs by 4 units.
Being a stronger oxidizing agent, Br2 is capable of increasing the oxidation number of S in
⇒ \(\mathrm{S}_2 \mathrm{O}_3^{2-}\) To the maximum oxidation number of 6, thereby leading to the formation of S2O32-ion.
On the other hand, I2, being a weaker oxidizing agent, increases the oxidation number of Sin S2O32- to an oxidation number of 2.5 and results in the formation of
⇒ \(\mathrm{S}_4 \mathrm{O}_6^{2-}\) ion.
Question 42. Justify giving reactions that among halogens, fluorine is the best oxidant, and among hydrohalic compounds, hydroiodic acid is the best reductant
Answer:
The standard redox potentials (or standard reduction potentials) ofthe redox couples formed by halogens are

Are doxcouple consists of a reduced form and an oxidised form. The larger the value of E° for a redox couple, the greater the tendency of its oxidized form to get reduced and the smaller the tendency of its reduced form to oxidize. The reverse is true when the value of E° for a redox couple is small.
Combining this idea with standard electrode potentials of the redox couples given, we can infer that the tendency of oxidized forms (i.e., F2, Cl2, Br2, and I2) to get reduced or the strength of oxidizing power of the oxidized forms follows the order: F2 > Cl2 > Br2 > I2, and the tendency of reduced forms {i.e., F-, Cl-, Br- and I-) to get oxidized or the strength of reducing power ofthe reduced forms follows the order:
I¯> Br¯> Cl¯ > F¯
As the oxidizing power of F2 is the highest among the halogens, it is capable of oxidizing other halides to the corresponding halogens. No other halogen except F2, has this ability
⇒ \(\mathrm{F}_2(g)+2 \mathrm{Cl}^{-}(a q) \rightarrow 2 \mathrm{~F}^{-}(a q)+\mathrm{Cl}_2(g)\)
⇒ \(\mathrm{F}_2(g)+2 \mathrm{Br}^{-}(a q) \rightarrow 2 \mathrm{~F}^{-}(a q)+\mathrm{Br}_2(l)\)
⇒ \(\mathrm{F}_2(g)+2 \mathrm{I}^{-}(a q) \rightarrow 2 \mathrm{~F}^{-}(a q)+\mathrm{I}_2(s)\)
⇒ \(\mathrm{Cl}_2(g)+2 \mathrm{Br}^{-}(a q) \rightarrow 2 \mathrm{Cl}^{-}(a q)+\mathrm{Br}_2(l)\)
⇒ \(\mathrm{Cl}_2(g)+2 \mathrm{I}^{-}(a q) \rightarrow 2 \mathrm{Cl}^{-}(a q)+\mathrm{I}_2(s)\)
⇒ \(\mathrm{Br}_2(l)+2 \mathrm{I}^{-}(a q) \rightarrow 2 \mathrm{Br}^{-}(a q)+\mathrm{I}_2(s)\)
Therefore, F2 has the strongest oxidizing power among the halogens. The oxidation of a hydrohalic acid produces its halogen. The tendency of a hydrohalic acid to get oxidized or the reducing power of a hydrohalic acid is high when the halide ion of the hydrohalic acid exhibits a greater tendency to get oxidized. As the tendency ofhalide ions to get oxidized follows the order I¯ > Br¯ > Cl¯ > F-, the reducing power of hydrohalic acids will follow the order HI>HBr>HCl>HF.
The following reactions confirm this:
HI or HBr can reduce H2SO2 to SO2, but HC1 or HF cannot reduce it.
⇒ \(2 \mathrm{HI}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{I}_2+\mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(2 \mathrm{HBr}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Br}_2+\mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}\)
I– can reduce Cu2+ to Cu+, but Br– cannot.
⇒ \(2 \mathrm{Cu}^{2+}(a q)+4 \mathrm{I}^{-}(a q) \rightarrow \mathrm{Cu}_2 \mathrm{I}_2(s)+\mathrm{I}_2(s)\)
⇒ \(\mathrm{Cu}^{2+}(a q)+2 \mathrm{Br}^{-}(a q) \rightarrow \text { No reaction }\)
Therefore, we can conclude that HI is the strongest reducing agent among the hydrohalic acids.
Question 43. Why does the following reaction occur? XeO6-4 (aq) + 2F–(aq) + 6H+(aq) → XeO3(g) + F2(g) + 3H2O(l), What conclusion about the compound Na4XeO6 (of which XeO3 is a part) can be drawn from the reaction?
Answer:

The oxidation numbers of Xe in XeO6-4 and XeO3 are +8 and +6 respectively. Thus, in the reaction the oxidation number of Xe decreases, and hence XeO6-4– undergoes reduction and acts as an oxidising agent. On the other hand, the oxidation number of F increases from -1 to 0.
Therefore, in the reaction fluorine undergoes oxidation and hence it acts as a reductant. As the reaction is spontaneous and XeO6-4 oxidizes F–, it can be concluded that Na4XeO6 has stronger oxidizing power than F2.
Class 11 Chemistry Chapter 8 Long Questions Redox Reactions
Question 44. Consider the reactions:
1. H3PO2(aq) + 4AgNO(aq) + 2H2O(l) → H3PO2(aq) + 4Ag(s) + 4HNO3(aq)
2. H3PO2(aq) + 2CuSO4(aq) + 2H2O(Z)→ H3PO4(aq) + 2Cu(s) + H2SO4(aq)
3. C6H5CHO(l) + 2[Ag(NH3)2]+(aq) + 3OH–(aq) → C6H5COO–(aq) + 2Ag(s) + 4NH3(aq) + 2H2O(l)
4. C6H5CHO(l) + 2Cu2+(aq) + 5OH– →(aq) No change observed
What inference do you draw about the behavior of Ag+ and Cu2+ from these reactions?
Answer:
1. The reaction involves the reduction of the Ag+ ion to Ag and the oxidation of H3PO2 to H3PO4. Thus, in this reaction, the Ag+ ion behaves as an oxidant. It oxidizes H3PO2 to H3PO4.
2. The reaction involves the reduction of Cu2+ ion to Cu and the oxidation of H3PO2 to H3PO4. Thus, in this reaction, the Cu2+ ion behaves as an oxidant. It oxidizes H3PO2 to H3PO4.
3.The reaction involves the oxidation of C6H5CHO to C6H5COOH and the reduction of [Ag(NH3)2]+ to Ag. Thus, in this reaction, the Ag+ ion acts as an oxidant. It oxidizes C6H5CHO to C6H5COOH.
This reaction indicates that the Cu2+ ion is not capable of oxidizing C6H5CHO. This explains why the Cu2+ ion is a weaker oxidizing agent than the Ag+ ion.
Question 45. What sorts of information can you draw from the following reaction?
Answer:
Let us first analyze whether (CN)2 in the reaction gets oxidized or reduced or simultaneously oxidized as well as reduced. To know this the knowledge of the oxidation states of C in (CN)2, CN, and CNO- are required.
The oxidation state of C in (CN)2 is
⇒ \(+3\left[(\stackrel{+3-3}{(\mathrm{CN}})_2\right]\) is +2 \(\mathrm{CN}^{-}\left[\mathrm{CN}^{-}\right] \text {and }+4 \text { in } \mathrm{CNO}^{-}\left[\mathrm{C}^{+3-32} \mathrm{CN}^{-}\right]\)
Now let us consider the given equation
⇒ \((\stackrel{+3}{\mathrm{C}} \mathrm{N})_2(g)+2 \mathrm{OH}^{-}(a q) \xrightarrow[+2]{\mathrm{C}} \mathrm{N}^{-}(a q)+\stackrel{+4}{\mathrm{C}} \mathrm{NO}^{-}(a q)+\mathrm{H}_2 \mathrm{O}(l)\)
From this equation, we see that (CN)2 reduced to the oxidation number of C reduces (+3→+2) in this change, and It gets oxidized to CNO– because the oxidation number of C Increases (+3→+4) In this change. Thus, from this reaction, we have the following information: CD In an alkaline medium cyanogen gas dissociates into cyanide Ion (ON-) and cyanate Ion (CNO–).
It Is a redox reaction. More particularly, it is a disproportionation reaction because (CN)2 undergoes oxidation and reduction simultaneously. Cyanogen is a pseudohalogen. It acts as a halogen on reacting with alkalis.
Question 46. The Mn3+ ion Is unstable In solution and undergoes disproportionation to give Mn2+, MnO2, and H+ ions. Write a balanced ionic equation for the reaction.
Answer:
Mn3+ is unstable in an aqueous medium and undergoes a disproportionation reaction, forming Mn3+, MnO2 and H+. So, the reaction is—
Here the oxidation reaction is
⇒ \(\mathrm{Mn}^{3+}(a q)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{MnO}_2(s)+4 \mathrm{H}^{+}(a q)+e \quad \cdots[1]\)
Adding equation (1) and equation (2), we have
⇒ \(2 \mathrm{Mn}^{3+}(a q)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{MnO}_2(s)+\mathrm{Mn}^{2+}(a q)+4 \mathrm{H}^{+}(a q)\)
This is the balanced equation for the disproportionation reaction that the Mn3+ ion undergoes in an aqueous medium.
Question 47. Consider the elements: Cs, Ne, I, and F
- Identify the element that exhibits only a negative oxidation state.
- Identify the element that exhibits only a positive oxidation state.
- Identify the element that exhibits both positive and negative oxidation states.
- Identify the element that exhibits neither the negative nor the positive oxidation state
Answer:
1. Being an element of the highest electronegativity, F always shows a negative oxidation state. It exhibits only a -1 oxidation state
2. Cs is an alkali metal and shows a strong electropositive character. As a result, it always shows a positive oxidation state. It exhibits only a +1 oxidation state.
3. Like other halogens, I also show a -1 oxidation state. In addition, it shows positive oxidation states, +1, +3, +5, and +7 when it forms compounds with more electronegative elements.
4. Ne is an inert element and does not tend to gain or lose electrons. As a result, it shows neither a positive oxidation state nor a negative oxidation state.
Question 48. Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess chlorine is removed by treating it with sulfur dioxide. Present a balanced equation for this redox change taking place in water.
Answer:
The reaction that occurs when SO2 is used to remove excess Cl2 in drinking water is
⇒ \(\mathrm{Cl}_2(a q)+\mathrm{SO}_2(a q) \rightarrow 2 \mathrm{Cl}^{-}(a q)+\mathrm{SO}_4^{2-}(a q)\)
Oxidation reaction: \(\stackrel{+4}{\mathrm{SO}_2}(a q) \xrightarrow{+6} \mathrm{SO}_4^{2-}(a q)\)
To balance O-atoms, we add 2H2O to the left-hand side and 4H+ to the right-hand side.
⇒ \(\mathrm{SO}_2(a q)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{SO}_4^{2-}(a q)+4 \mathrm{H}^{+}(a q)\) ……………….(1)
Balancing charges on both sides, we have
Reduction reaction: Cl2(aq) + 2e→2Cl–(aq)………………..(2)
Adding equation (1) to equation (2), we have
⇒ \(\mathrm{Cl}_2(a q)+\mathrm{SO}_2(a q)+ 2 \mathrm{H}_2 \mathrm{O}(l)\) → \(\mathrm{SO}_4^{2-}(a q)+2 \mathrm{Cl}^{-}(a q)+4 \mathrm{H}^{+}(a q)\)
This is the balanced equation for the reaction.
Question 49. Refer to the periodic table given in your book and now answer the following questions:
- Select the possible non-metals that can show a disproportionation reaction.
- Select three metals that can show a disproportionation reaction.
Answer:
1. Non-metals such as P4(s), Cl2(g), and Br2(Z) undergo disproportionation reaction.
⇒ \(\mathrm{P}_4(s)+3 \mathrm{NaOH}(a q)+3 \mathrm{H}_2 \mathrm{O}(l)\) → \(\mathrm{PH}_3(g)+3 \mathrm{NaH}_2 \mathrm{PO}_2(a q)\)
⇒ \(\mathrm{Cl}_2(g)+6 \mathrm{NaOH}(a q)(\mathrm{Hot})\) → \(5 \mathrm{NaCl}(a q)+\mathrm{NaClO}_3(a q)+3 \mathrm{H}_2 \mathrm{O}(l)
\)
⇒ \(\mathrm{Br}_2(l)+6 \mathrm{NaOH}(a q)(\mathrm{Hot})\)→ \(5 \mathrm{NaBr}(a q)+\mathrm{NaBrO}_3(a q)+3 \mathrm{H}_2 \mathrm{O}(l)\)
Three metals that can show disproportionation reactions are copper, gallium and manganese.
⇒ \(2 \stackrel{+1}{\mathrm{C}} u^{+}(a q)\rightarrow \stackrel{0}{\mathrm{Cu}}(s)+\stackrel{+2}{\mathrm{Cu}^{2+}}(a q)\)
⇒ \(3 \mathrm{Ha}^{+}(a q)\rightarrow \stackrel{+3}{\mathrm{Ga}}^{3+}(a q)+2 \stackrel{\ominus}{\mathrm{Ga}}(s)\)
⇒ \(2 \mathrm{Mn}^{3+}(a q)+2 \mathrm{H}_2 \mathrm{O}(l)\) → \(\mathrm{MnO}_2(s)+\mathrm{Mn}^{2+}(a q)+4 \mathrm{H}^{+}(a q)\)
Redox Reactions Chapter 8 NCERT Long Answer Questions Class 11
Question 50. In Ostwald’s process for the manufacture of nitric acid, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.00 g of ammonia and 20.00 g of oxygen?
Answer:
Reaction:
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
4 × 17 g = 68 g 5 × 32 g
= 160 g 4 × 30 g = 120 g
Therefore, 160 g O2 is required to oxidize 68 g NH3.
Therefore, 160 g O2 is required to oxidize 68 g NH3.
20g O2 is required to oxidise \(\frac{68}{160} \times 20 \mathrm{~g}\)
= 8.5 g of NH3
So, here O2 is the limiting reagent. The amount of nitric oxide produced depends upon the amount of oxygen taken and not on the amount of NH3 taken
According to the above equation,
160 g O2 produces 120 g NO
⇒ \(20 \mathrm{~g} \mathrm{O}_2 \text { produces } \frac{120}{160} \times 20 \mathrm{~g} \mathrm{NO}=15 \mathrm{~g} \mathrm{NO}\)
Thus the reaction between 10 g NH3 and 20gO2 produces a maximum amount of 15 g NO.









 CaCl2 +B In this reaction, B is
CaCl2 +B In this reaction, B is
















