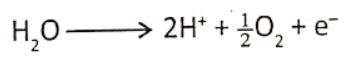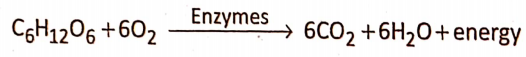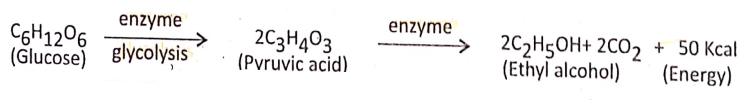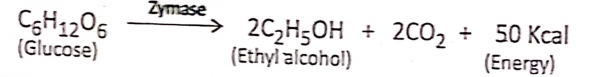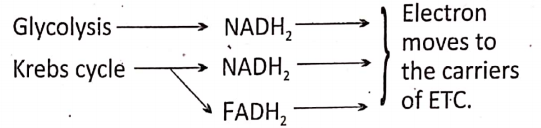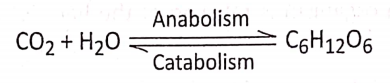Physiological Processes Of Life
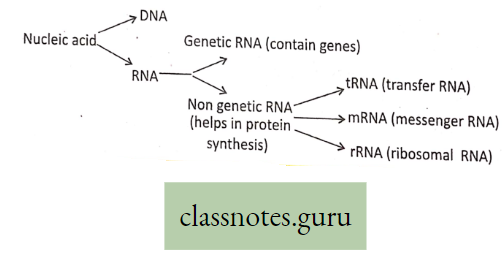
Photosynthesis
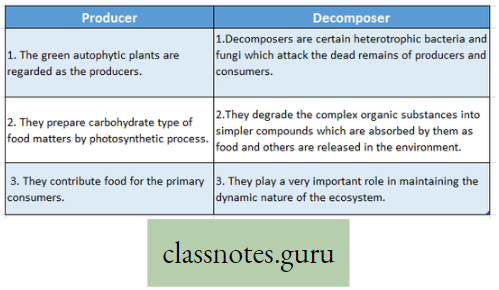
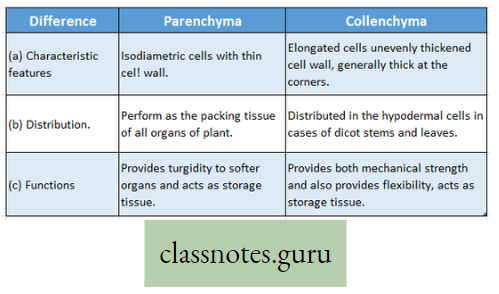
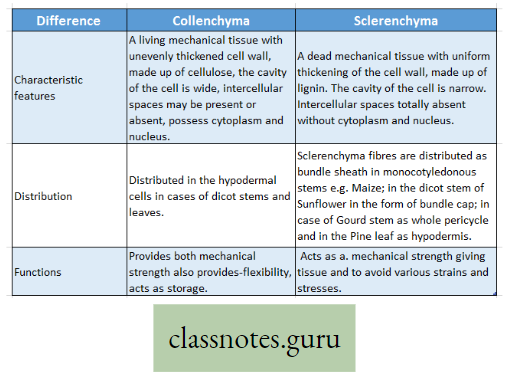
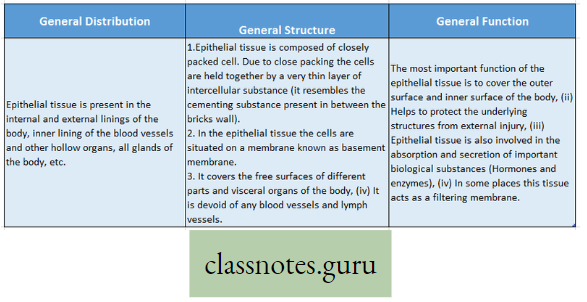
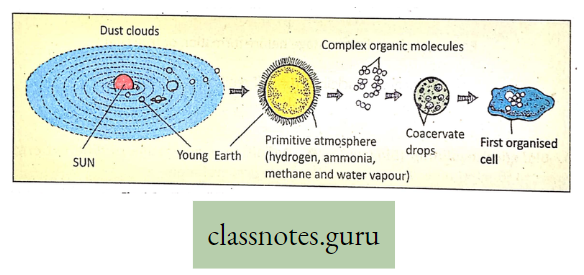
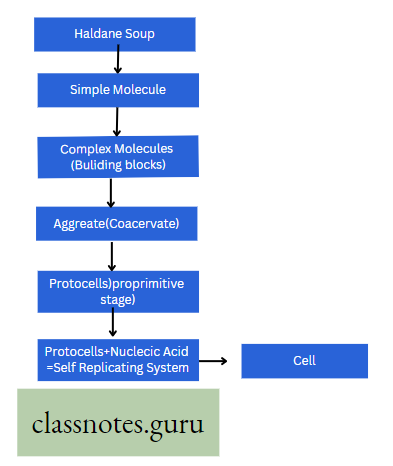
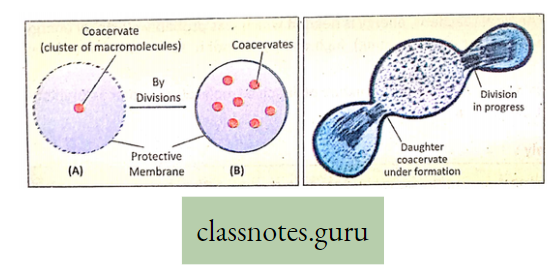
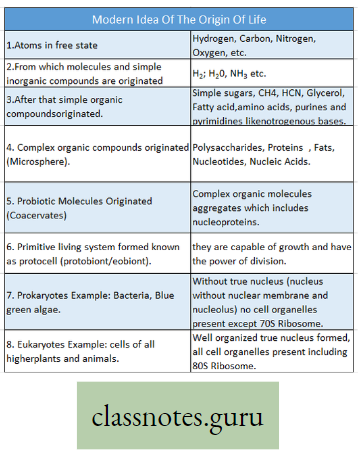
Photosynthesis Introduction: All living organisms need energy to perform their different normal metabolic functions. Food is the source of potential energy or stored energy.
- During oxidation it is converted to kinetic energy, this is called Respiration. From this kinetic energy, the necessary energy is supplied to the living body.
- The green organisms using a special physiological function produce food.
- During this physiological function, the solar energy is stored within the food as potential energy and this special physiological function (process) is called Photosynthesis.
Concept of Photosynthesis
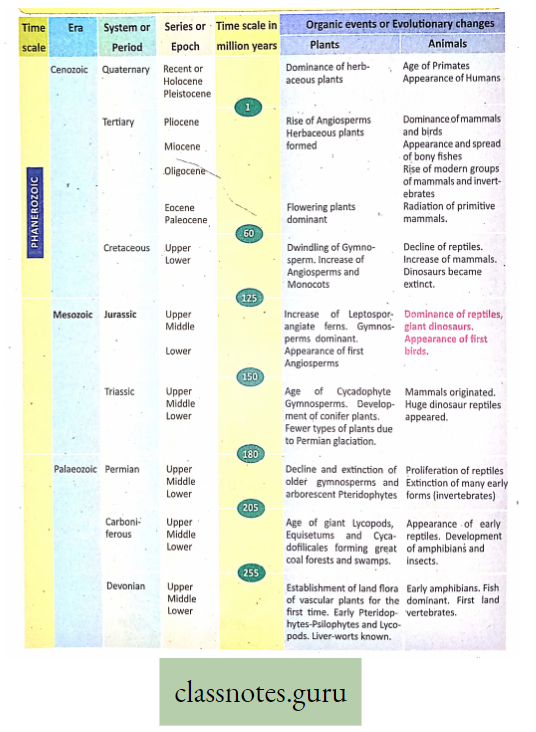
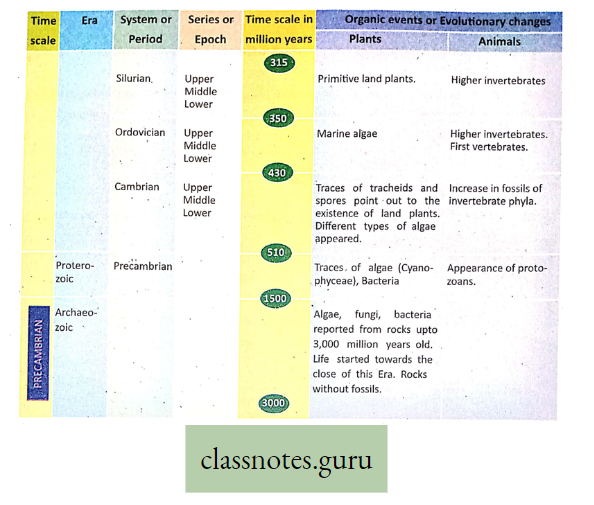
The term ‘photosynthesis’ (Photos, light: synthesis, building up) was coined by Barnes in the year 1898.
- The green cells synthesize enormous amounts of food materials with the help of light energy, preferably available from the sun.
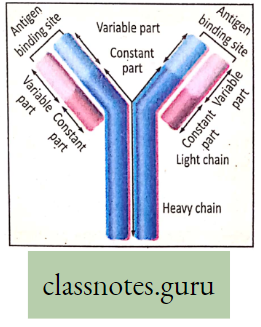
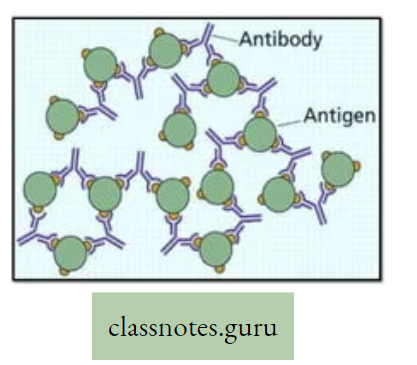
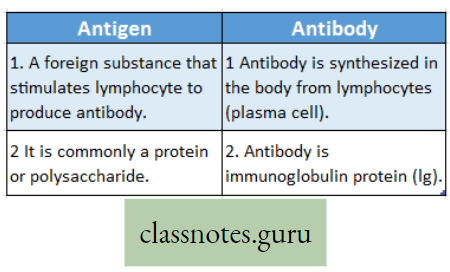
- Carbohydrate (starch) which is produced as a result of photosynthesis acts as the basic raw material because this carbohydrate is directly or indirectly converted to all types of organic compounds, which are needed for the entire living world.
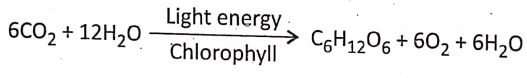
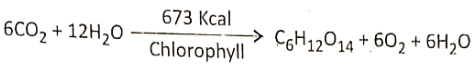
Concept Of Photosynthesis Definition: Photosynthesis is the photochemical, biochemical, anabolic, and oxidoreductive process by which green plant cells can prepare carbohydrate food material in the presence of sunlight, M20, CO2, and chlorophyll, producing O2 as a by-product.
Read and Learn More Class 9 Life Science
Explanation And Overall Reactions :
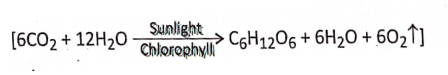
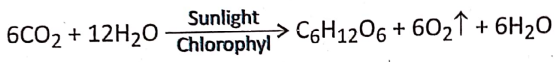
- During photosynthesis 6 molecules of carbon dioxide react with 12 molecules of water forming 1 molecule of glucose, 6 molecules of water, and 6 molecules of oxygen.
- The reaction occurs in the presence of sunlight and chlorophyll.
- The number of molecules of O2 evolved during this process equals the number of molecules of CO2 taking part in the process.
- The end products formed as a result of photosynthesis are water, oxygen, and glucose.
Characteristics Of Photosynthesis:
- It is a photochemical process because this chemical reaction occurs only in the presence of photon particles of sunlight.
- It is a biochemical process because this chemical process occurs only in the living plant cell.
- It is an anabolic process because, during this constructive process, glucose is synthesized causing an increase in the dry weight of the cell.
- It is an oxidoreductive process because during this process H2O is oxidized by losing Hf whereas CO2 is reduced by gaining H+.
- By this process, green plant cells can prepare carbohydrate food material in the presence of sunlight, H2O, CO2, and chlorophyll.
- During this process, CO2 is absorbed and O2 is evolved which is just the reverse of aerobic respiration.
- This is an endergonic process since solar energy is absorbed in this process.
- This process plays a very significant role in maintaining the O2-CO2 balance.
Chemical Transformation Or Transfer During Photosynthesis :
- C and O of CO2 enter into c6H12O6
- H of H2O enters into C6H12O6
- O6of H20 evolves as free O2.
Important Facts On Photosynthesis
- Photosynthesis an anabolic process: Photosynthesis is an anabolic process due to the following reasons—
- Photosynthesis is a constructive process, resulting in the formation of new cellular
materials. - It increases the dry weight of the organism.
- Energy is stored in the form of potential energy in glucose molecules. 1 mol of glucose contains 686 kcal of energy.
- Complex substance (glucose) is produced from simpler substances water, carbon dioxide, etc.
- By this process, micromolecules combine to form macromolecules. For example, water (H2O) and carbon dioxide (CO2) are micromolecules, which combine to form glucose (C6H12O6), which is a macromolecule. The sources of carbon and oxygen for glucose is CO2
Know The Facts:
- Main Organ of Photosynthesis Leaf,
- Organelle of Photosynthesis The Chloroplastid.
- Photosynthetic pigment Chlorophyll.
Photosynthetic unit-the Quantasomes: Presently quantosome is known as antenna 1 complex. The ‘photosynthetic units’ or ‘quanta somes’ represent an aggregation of several chlorophyll molecules.
- These are the main photosynthetic pigments along with accessory pigments, like carotenoid (carotene, xanthophyll) and proteins or phycobilins [phycocyanin (blue), phycoerythrin (red)].
- They are capable of capturing and converting solar energy into chemical energy in the form of ATP, during the light phase.
- Photosynthesis may also take place in the presence of artificial light (if the light intensity is sufficient) at a very slow rate.
Site Of Photosynthesis:
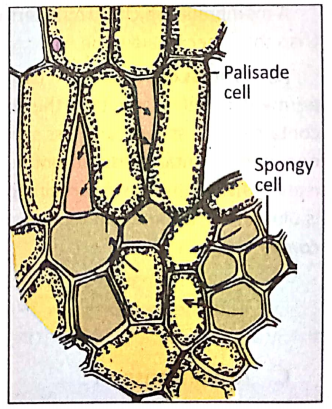
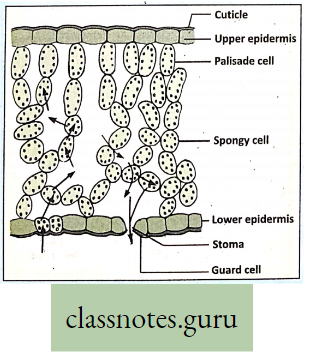
Main site of photosynthesis: Photosynthesis takes place within the chloroplast containing mesophyll tissue palisade parenchyma cells and spongy parenchyma cells, present between the upper and lower epidermis of the dorsiventral leaf.
- However, all chlorophyll-containing plant parts have the power of photosynthesis.
- Chloroplast containing chlorophyll is present in abundance in the mesophyll tissue r of the leaf.
- So the cells of the mesophyll tissue are the main site of photosynthesis
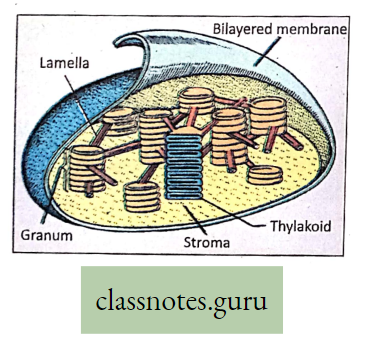
- To some extent, photosynthesis occurs in the green part of a growing stem Example, Opunti (B. Phanimanasha), and Logenaria (B. Lau).
- Photosynthesis also takes place in the thalamus of the flower which is a modified stem, in the roots, and the sepals of the green calyx of the flower.
Velamen: Epiphytic root of orchid, can perform photosynthesis. They are hanging roots and are green in color. These epiphytic roots do not reach to soil.
- So they have the problem of absorbing water.
- At the tip of the epiphytic root, there is a soft spongy structure called f velamen that can absorb atmospheric moisture.
- This moisture serves the purpose of water.
Things To Remember
Non-Photosynthetic plants:
- Plants without chlorophyll cannot photosynthesize. Examples Some bacteria and fungi.
- Roots cannot photosynthesize, due to a lack of photosynthetic pigment chlorophyll and
- Non-availability of sunlight, as it grows under the surface of the soil.
- Exceptions-Aerial roots of Orchid (Epiphytic roots of Vanda Rasna) and assimilatory roots of Tinospora-B.
- B-Gulancha can perform photosynthesis due to the presence of chlorophyll.
Brief Outline Of The Roles Of Different Components Of Photosynthesis
CO2 H2oSunlight And Pigment Chlorophyll And Carotenoids:
Carbon dioxide: The carbon element of the gaseous compound CO2 is directly taken 3y green plants from the atmosphere.
Role: In terrestrial plants, CO2 enters the mesophyll tissue cells by diffusion through the stomata or lenticel.
In submerged aquatic plants, CO2 enters the plant body by diffusion through the body surface of the plants. ‘
- During dark reactions within the stroma of the chloroplastids, carbon of CO2 is accepted by a five-carbon compound RuBP (Ribulose 1-5 bisphosphate).
- The CO2 is reduced to glucose through successive steps of enzymatic reaction.
- The carbon and oxygen of CO2 form the carbon and oxygen of glucose (CgHOg).
Water: Water is one of the chief components of photosynthesis.
Role: In terrestrial plants, absorption of water from soil takes place through the unicellular root hairs, by the process of diffusion and osmosis.
- Water diffuses into the mesophyll cells and from there into the chloroplasts. About 1% of the total amount of water absorbed by plants is required for photosynthesis.
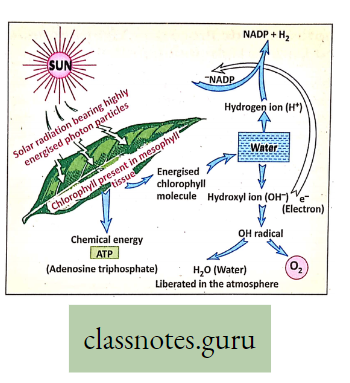
- Water molecule breaks up into H+ (hydrogen ion) and OH” (hydroxyl ion) by light-activated
chlorophyll molecule. - Oxygen formed from hydroxyl ions is liberated through stomata.
- The hydrogen ion is accepted by NADP+ and it gets converted to NADPH2. Water is produced from the hydroxyl ion.
- Hydrogen in water reduces CO2 to glucose.
H2O→H+OH,OH-e(Electron)=OH radical
4OH=2H2O+2
The hydrogen of water forms hydrogen.of glucose. H2O is the source of liberated oxygen during photosynthesis.
Sunlight: Solar radiation composed of highly energized invisible solar particles called quanta or photons is required for photosynthesis.
Role:
- 0-1% to 2% of total light solar radiation is absorbed by chlorophyll molecules.
- The highly energized photon particles of light activate the chlorophyll molecules liberating electron which then causes photolysis of water,
- Light causes photophosphorylation

ATP acts as the chief source of energy currency: The rate of photosynthesis is influenced by the intensity, quality, and duration of light.
- Strong light may even stop the photosynthetic process, or slow down its rate, a phenomenon called solarization.
- About 2400 kcal light energy is required for the synthesis of 1 mole of glucose.
Pigments: Chlorophyll and Carotenoid’s main photosynthetic pigment is chlorophyll, which is present in the thylakoid of grana in the chloroplast.
Role :
- When a photon particle strikes the chlorophyll molecule, an electron goes out of chlorophyll. Soothe chlorophyll becomes positively charged.
- This positively charged. chlorophyll is known as activated chlorophyll where different events of light reaction can start.
- In the activated chlorophyll in the presence of sunlight, H2O breaks down into H and OH known as photolysis.
- In the activated chlorophyll, ATP is produced by photophosphorylation.
Plant pigments: Plants may contain two types of pigments, green-colored chlorophyll, and yellowish-orange-colored carotenoid.
Chlorophylls are of two types
- Chlorophyll a (C55H72O5N4Mg) and
- Chlorophyll b (C55H70O6N4 Mg), Chlorophyll c, d, and e are also present.
For reference only :
Chlorophyll atoms are arranged in a circle with the help of four pyrrole rings with Mg atoms at the center. Along with the pyrrole ring is attached phytol (a type of alcohol).
Carotenoids are of two types
- Carotene (C4OH56)Orange orange-colored and Xathophyll (C40H56O2) is the yellow-colored pigment.
- Phycocyanin blue colored and Phycoerythrin the lavender red-colored pigments.
Carotenoids: Carotenoids are yellow, brown, or orange pigments found in close association with chlorophylls in all photosynthesizing cells.
- They occur in thylakoids and act as accessory pigments of photosynthesis.
- They are present in nearly all higher plants, algae, and some microorganisms.
For reference only: Goodwin (1960) suggested that the chlorophylls and carotenoids may be attached to ‘ the same protein, forming a complex known as photosynthesis.
Carotenoids Are Of Two Types:
- Carotenes: They are orange to yellow and are unsaturated hydrocarbons (C4OH56). They are insoluble in water but readily soluble in chloroform, ether, and carbon disulfide. They absorb blue and green lights and transmit yellow and red light. Four isometric forms of carotenes are now recognized a, 3, y, and 8 of which (3-carotene is most common in all green plants).
- Xanthophylls or Carotenols: They are yellow with empirical formula C4OH56O2.ln normal green leaves, proportionately there is more xanthophyll than carotene. The most common
xanthophyll in green leaves insulator or lutein. (Tomato is dark red due to lycopene pigment).
Apart from their role in the absorption of light energy and its transfer to chlorophyll, carotenoids play a very important role in protecting chlorophyll molecules from photooxidation in scorching sunlight.
What Will Happen
- When a portion of the grassland chosen is. covered with an earthenware pot. Small stones are placed between the edge of the pot and the ground. What is observed when the pot is removed after a few days?
- Two healthy potted plants are selected and kept in an airy place under light. An adequate amount of water is given to the seedling of one pot and water is not provided to the seedling of the other pot. What will be observed in the potted seedlings after seven days?
Explanation of absorption and action spectra
Absorption Spectrum :
Absorption Spectrum Definition: The absorption spectrum is the pattern of absorption of light at different wavelengths by the object.
- Colored pigments absorb only visible light.
- Spectrophotometric analysis shows that chlorophyll and chlorophyll can absorb maximum blue color (429 mp 453 mp. wavelength) and second maximum red color (642 mp 660 mp, wavelength).
- That’s why maximum photosynthesis occurs in the blue and red colors of the visible spectrum.
- The carotenoid pigments play a role in photosynthesis by absorbing light and passing it to chlorophyll where it is used in the photosynthetic process.
- Absorption spectra are one of the properties of chlorophyll, during which they can absorb a certain wavelength of light.
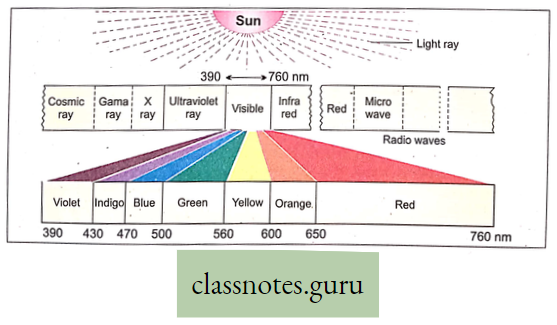
Do you know that minimum action and absorption occur in green light? That why minimum photosynthesis occurs in the green color of the visible spectrum.
Photosynthetic Process Light-Dependent Phases And Dark Or Light-Independent Phase
Photosynthesis is a complex photochemical reaction involving a series of reactions occurring in light as well as in darkness, leading to the formation of glucose.
- The light phase triggered by light energy was discovered by Robert Hill (1940), hence it is termed the Hill reaction.
- The dark phase, independent of light, was discovered by F. F. Blackman (1905) and is called Blackman’s reaction or chemical reaction.
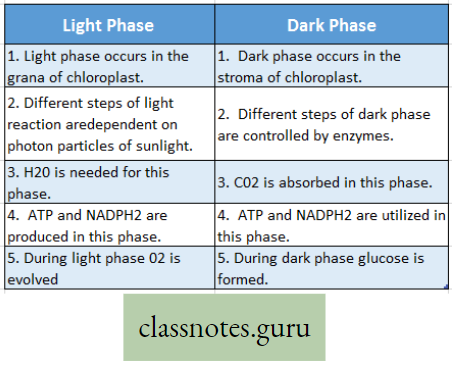
- The light phase occurs granum (pi. grana) of the chloroplast and the dark phase occurs within the stroma of the chloroplast.
- Light phase: The reductive reactions taking place in the presence of light within the grana are called the light phase.
- Light phase Definition: During photosynthesis, various oxidoreductive reactions that occur in the grana of chloroplast in the presence of solar energy (photon) are together called a light reaction or light phase.
- Occurrence: The light phase occurs in the grana of chloroplast.
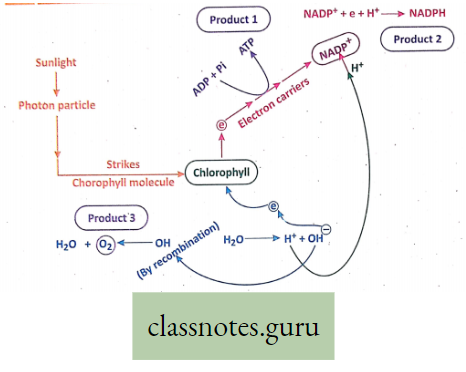
- Requirements: For light reactions, essential requirements are photon particles of sunlight, chlorophyll, and H2O.
- Products: After different steps of light reaction, the end products are ATP, NADPH (reduced NADpj, H2O, and O2.
Different steps of light reaction: Activation of chlorophyll, Photolysis, Evolution of O2, Formation of NADPH (Reduced NADP), and Photophosphorylation. These steps can be explained as follows:
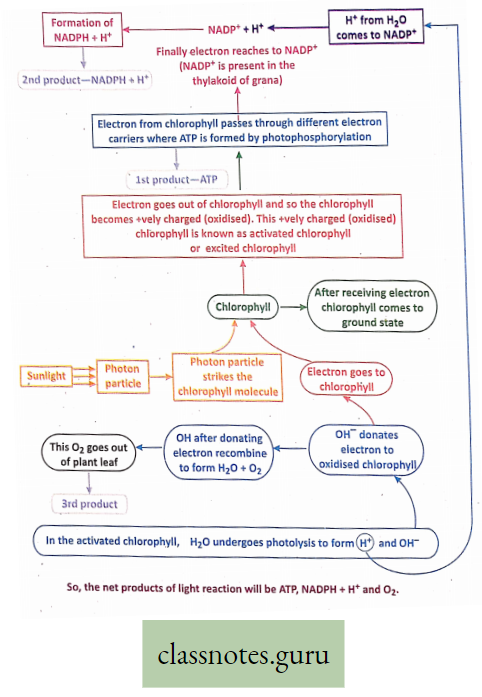
Activation of chlorophyll: When a photon particle strikes the chlorophyll molecule, an electron goes out of chlorophyll so the chlorophyll becomes +vely charged. This +vely charged chlorophyll is called activated or excited chlorophyll and this phenomenon is called activation or excitation of chlorophyll.

In this activated chlorophyll, other events of light reaction start.
Photolysis: In the activated chlorophyll in the presence of sunlight, H2O. breaks down into H+ and OH–. This is called photolysis.

Evolution of O2: OH- produced by photolysis donates electrons to chlorophyll and becomes OH. Several OHs combine to form H2O and O2. This O2 goes out of plant leaf. Hence the O2 evolved during photosynthesis comes from H2O and not from CO2.
Formation of NADPH (Reduced NADP): NADP is known as a Hill reagent. NADP+ receives electrons from OH” and H+ from H20 and thus forms NADPH (reduced NADP).
NADPH produced in the light reaction is utilized in the dark reaction.
Photophosphorylation: In the activated chlorophyll in the presence of sunlight, ADP and Pi combine to form ATP. This is called photophosphorylation.
For reference only: When a photon particle strikes the chlorophyll molecule, an electron goes out of chlorophyll.
- This electron while passing through different electron carriers generates energy (redox potential).
- This energy helps in the combination of ADP and Pi to form ATP.
For reference only: Photophosphorylation can be of two types Cyclic photophosphorylation and non-cyclic photophosphorylation.
Different Steps Of Light Reaction :
Summary Of Light Reaction :
Sequence of Light-dependent phase :
Entrapping of sunlight→ activation of chlorophylls photolysis of waters formation of the end product of light-dependent phase NADPH + H+,O2 and ATP, H2O also formed.
Know The Fact
A new concept for the photolysis of water : As oxygen does not exist in atomic form oxygen produced by splitting one molecule of water is written a O2. Two water molecules produce one molecule of oxygen which is later released into the atmosphere.
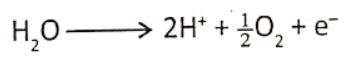
The breaking of water by light takes place indirectly by oxidizing P680 molecules.
Outline steps of dark reaction :
The dark reaction occurs in the stroma of the chloroplast. It is purely an enzymatic process. The most important enzyme is known as RuBisCO (Ribulose Bisphosphate Carboxylase Oxygenase).
- here. are many steps of dark reaction. Initial steps were discovered by Blackman but details of dark reactions were discovered by Calvin, Benson, and Bassham for which they were awarded the Nobel Prize.
- Hence the detailed steps of dark reaction are also called Calvin’s cycle.
- Dark reaction is fully dependent on light reaction. In the daytime in the presence of sunlight, a light reaction occurs in the grana of the chloroplast.
- The main products of the light phase ATP and NADPH are immediately utilized in dark reaction (Since ATP and NADPH cannot be stored in plant cel1).
- At night, in the absence of sunlight, the light reaction stops—so production of ATP and NADPH will be also stopped and hence dark reaction cannot continue.
- That’s why, a dark reaction does not occur in darkness.
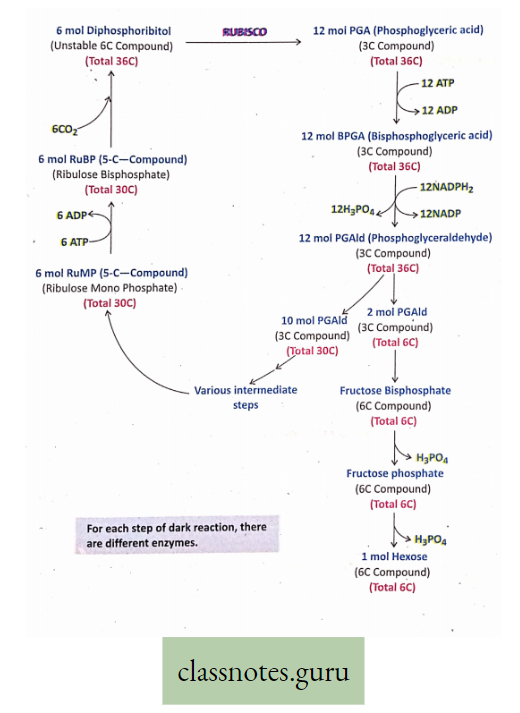
Different Steps Of Dark Reaction Are As Follows :
- 6 mol RuMP (Ribulose Mono Phosphate), a 5-C compound, is concerted into 6 mol RuBP (Ribulose Bisphosphate) (Total no. of carbon 6×5 = 30) in the presence of 6 ATP.
- 6 mol RuBP bind with 6CO2 to form 6 mol Diphosphoribitol, (a. 6-C compound, which is very unstable) (Total no. of carbon 6×6 = 36).
- 6 mol Diphosphoribitol quickly breaks into 12 mol PGA (Phosphoglyceric acid), a 3-C compound (Total no. of carbon 12 x 3 = 36). PGA is the first stable product in photosynthesis.
- 12 mol PGA is converted into 12 mol DPGA (Diphosphoglyceric acid) (No. of carbon 12 x 3 = 36) with the utilization of 12 mol ATP.
- 12 mol DPGA is transformed into 12 mol PGAId (Phosphoglyceraldehyde) (No. of carbon 12 x 3 = 36) with the utilization of 12 mol NADPH.
- 12 mol PGAId are divided into two groups. In one group 2 mol PGAId (2×3 = 6 carbon) produces glucose and other carbohydrates through different enzymatic steps.
- In the other group, 10 mol of PGAId (10 x 3 = 30 carbon) undergoes different enzymatic steps to resynthesize 6 mol RuMP (6 x 5 = 30 carbon). Then the whole cycle is completed with a net production of 1 mol hexose (glucose or fructose).
For completion of one total round of Calvin’s cycle and to produce 1 mol glucose, 18 ATP and 12 NADPH are required. So, ATP and NADPH, produced in light reactions are utilized in dark reactions
For Reference only: Have You heard about C3 and C4 plants?
Carbon Assimilation: During the dark reaction of photosynthesis carbon of CO2 is absorbed and assimilated into glucose. So the process of dark reaction may be called carbon assimilation.
For Reference only: CO2 is absorbed by RuBP to form PGA. This reaction is catalyzed by the enzyme RuBisCO.
Summary of pathway of Dark Reaction (C3—Pathway):
Sequence of Light-independent phase (Dark phase):

Relation Between Light Reaction And Dark Reaction :
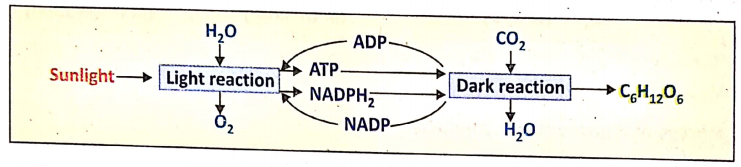
- ATP and NADPH2 produced in light reactions are utilized in dark reactions immediately.
- They are not stored. At night in the absence of sunlight, the light reaction stops so the production of ATP NADPH2 will be also stopped – hence dark reaction can not continue.
- Sp dark reaction is fully dependent on light reaction. That’s why a dark reaction does not occur in darkness.
Difference Between Light Phase And Dark Phase
However, both the light phase and the dark phase occur in the time only. The dark phase does not occur in darkness or at night.
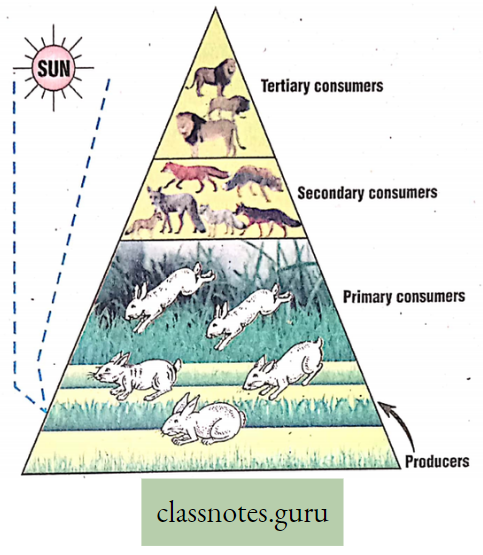
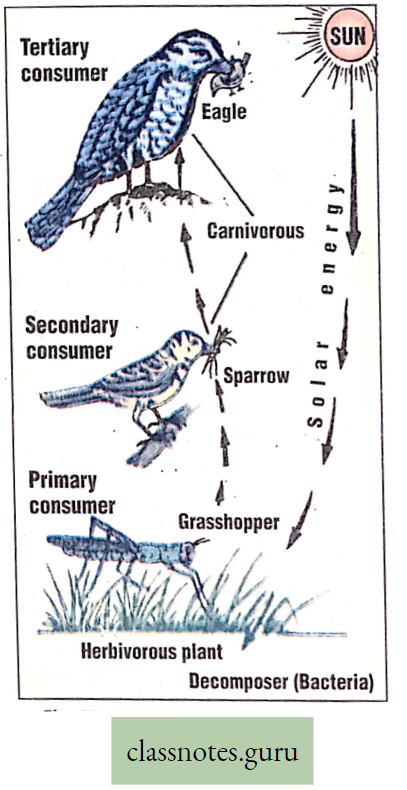
Significance of Photosynthesis
Entrapping of solar energy and conversion of solar energy into potential energy in
The radiant energy of the sun travels as electromagnetic radiation. This radiation is composed of small packets of highly energized invisible solar particles called ‘quanta’ or ‘photons’.
- When a photon particle strikes the chlorophyll molecule, different steps of light reaction start producing ATP.
- In ATP, solar energy is stored as chemical energy. When this ATP is utilized in a dark reaction producing c6H12°6′ solar energy is indirectly transferred into glucose and stored as potential chemical energy.
- This glucose is modified into different forms of food that are the sources of energy.
- In this way, solar energy is indirectly converted into potential chemical energy in different food materials. During respiration, food materials are oxidized when potential energy is released in the form of heat energy.
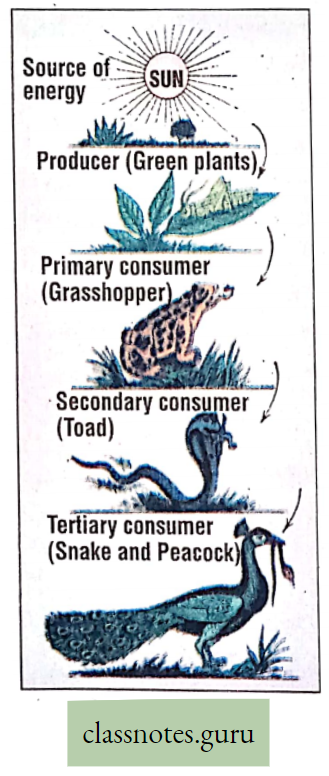
Solar energy →Chemical energy (in ATP)→ Potential energy (in Glucose) →Kinetic energy (in respiration) Food (storing energy), produced in plants by photosynthesis, is transferred to animals through the food chain and food web.
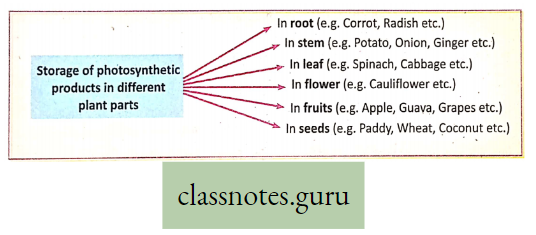
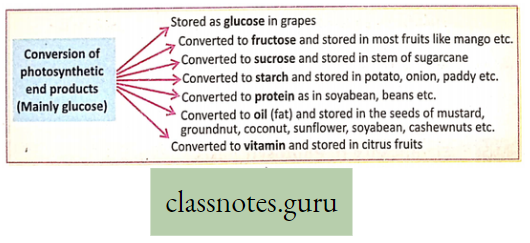
Storage of Photosynthetic products : In different plants, photosynthetic products are stored in various parts of the plant body.
This Can Be Illustrated As Follows:
Again, in different plants, the photosynthetic end product (mainly glucose) is transformed into various substances by enzymatic reactions and stored in many parts of the plant body.
This Can Be Illustrated As Follows :
O2 – CO2Balance In The Atmosphere :
The normal percentage of O2 and CO2 in the air is 21% and 0-03% respectively. Oxygen used during respiration and combustion causes oxygen deficit in the atmosphere which is balanced by the O2 liberated during photosynthesis by green plants. CO2is liberated during respiration, and
combustion which is absorbed during photosynthesis by green plants. Thus O2 — CO2 balance is maintained. The average percentage of dissolved O2 in water is nearly 0-7%.
For reference only:
- Compensation point: The compensation point is the light intensity where the rate of photosynthesis exactly matches the rate of respiration.
- At this point, the amount of C02 evolved in respiration in mitochondria is utilized by the chloroplast for photosynthesis and the amount of O2 evolved by photosynthesis in the chloroplast is utilized in mitochondria for respiration.
- So, there is no net exchange of O2 and CO2 between the leaf and the atmosphere.
- Time factor: The rate of photosynthesis is directly dependent on the intensity of sunlight. At different times of the day intensity of light varies so the rate of photosynthesis also varies. For example, at dawn or dusk, the intensity of light is low, so the rate of photosynthesis is also slow.
- But as the day progresses, the intensity of light increases, rate of photosynthesis also rises.
- This variable rate of photosynthesis at different times of the day is called as time factor.
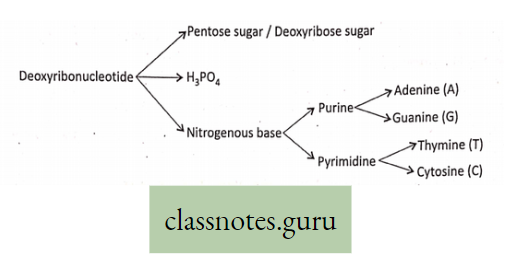
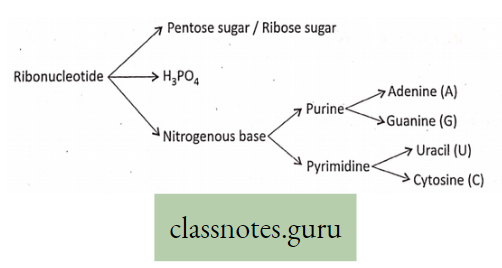
Mineral Nutrition
Mineral Nutrition Introduction: All living organisms require food. It is needed for growth, movement, reproduction, etc. A living organism either synthesizes or collects its necessary food from the environment.
- The food may be utilized directly or indirectly. The process involved in the conversion of complex food into simpler products or products is termed digestion.
- The digested product (or products) is absorbed within the body and is transformed into constituent(s) of protoplasm by the process of assimilation.
- In addition to energy-yielding food like carbohydrates, proteins ‘ and fats, minerals, vitamins, and water are also required for various life processes.
- All these essential substances are collectively called nutrients. The process that involves ingestion and ‘ digestion of food materials and after that absorption and finally assimilation of absorbed food is called nutrition.
What Are Nutrients?
Nutrient Definition: The organic and inorganic materials that the living organism collects from nature to perform all the fundamental activities of the body are called nutrients.
- All nutrients that are collected by living organisms from their surroundings are not considered food.
- Nutrients do not require digestion. The essential substances like minerals, Vitamins, and water are collectively called nutrients.
What is nutrition?
Nutrition Definition: “Nutrition is the combination of processes by which the living organism receives and utilizes the materials necessary for the maintenance of its functions and for the growth and the renewal of its components – [Turner D. F. (1959)].
Significance or Importance of Nutrition:
- Promote growth, repair wear and tear of the damaged tissues, and gain energy to control the different metabolic processes are the main functions of nutrition.
- The potential energy stored within food is transformed into usable energy through nutrition. The different physiological functions of the living body like movement, locomotion, excretion, reproduction, etc. are controlled by utilizing this energy.
- Through nutrition, the disease-resistant power (immunity) of the living body is developed.
- Through nutrition, future food matters are stored within the living body. From those stored food (in the plant body mainly as starch and in animals as glycogen and fat) the future energy is produced during food shortage.
- Nutrition plays a special role in the production of heat energy in the animal body to meet the caloric demands of an individual.
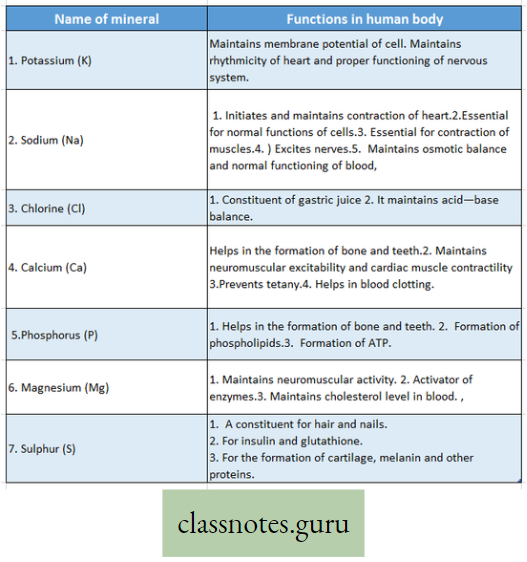
Concepts of Macro and Micro-nutrients with examples:
Macro-element Definition: The inorganic ions (essential elements) that are required in relatively large amounts for normal growth and other physiological functions of plants are called macro-elements.
Examples of macro-elements: Macro-elements are, Carbon (C), Hydrogen (H), Oxygen (0), Phosphorus (P), Sulphur (S), Potassium (K), Nitrogen (N), Calcium (Ca), Iron (Fe), and Magnesium (Mg) respectively. In the absence of any of these elements, the normal growth of the plant is disturbed, and different deficiency symptoms are exhibited by the plants.
Difference between Macro-elements and Micro-elements:
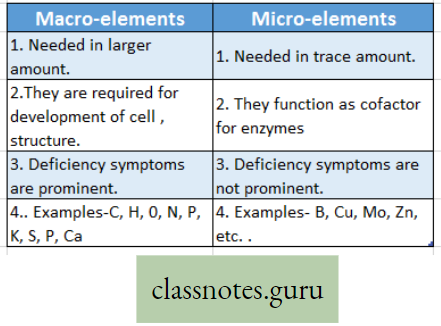
Micro-element:
Micro-element Definition: The essential elements that are required in trace amounts are called trace elements or micro-elements.
Examples are Manganese (Mn), Boron (B), Zinc (Zn), Copper (Cu), Molybdenum (Mo) sometimes Sodium (Na), Iodine (I), Silicon (Si) and Aluminium (Al), etc.
Listing Of Macro-Elements In Plants

Potassium (K): In plants
- Activates the enzymes and also takes part in carbohydrate and protein synthesis,
- It helps in the permeability of the cell membrane.
Phosphorus (P): In plants
- This is necessary to manufacture nucleic acids, phospholipids, coenzymes like NAD or DPN (Nicotinamide Adenine Dinucleotide) or (Diphosphopyridine Nucleotide), NADP or TPN (Nicotinamide Adenine Dinucleotide Phosphate) or (Triphosphopyridine Nucleotide), FAD (Flavin Adenine Dinucleotide).
- Photosynthesis, respiration, protein, and lipid synthesis, Phosphorus helps in the synthesis of chlorophyll pigment.
Calcium (Ca): In plants
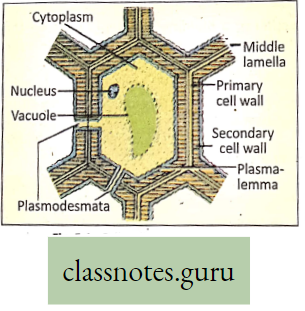
- It plays an important role in cell wall formation and cell division,
- Calcium acts as an activator (cofactor) of many enzymes. It occurs in the middle lamella of the cell wall.
Magnesium (Mg): In plants
- It acts as an activator of enzymes involved in protein synthesis, nucleic acid synthesis,
- Magnesium is one of the constituents of chlorophylls. Due to a deficiency of Mg, chlorosis occurs in plants.
Sulphur (S) r In plants
- A component of protein
- Components of vitamins like thiamine, biotin
- Helps in the formation of coenzyme A.
Nitrogen (N2): In plants
- Components of amino acids and proteins,
- Helps in the formation of ATP and coenzymes,
- A component of chlorophylls.
Iron (Fe) : (nowadays considered as a microelement) In plants
Takes part in chlorophyll synthesis and respiration, iron is needed in the case of respiratory enzymes and Cytochromes.
Listing Of Micro-Elements In Plants
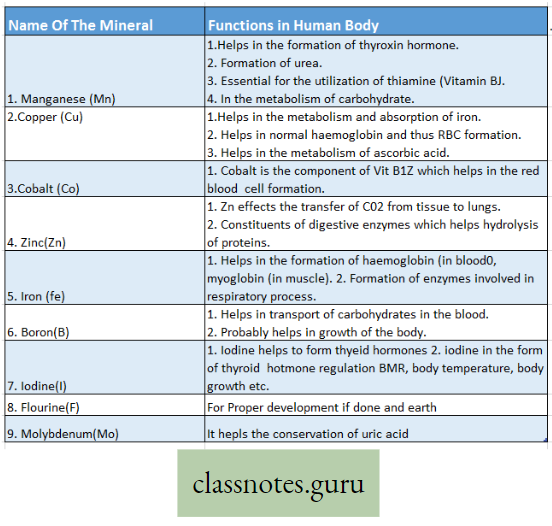
Manganese (Mn): In plants
- Helps in enzyme activation;
- Acts as a catalyst and
- Acts as an electron carrier.
Copper (Cu): In plants
- Helps in the reduction of nitrate,
- Acts as an electron carrier,
- Component of certain enzymes.
Chlorine (Cl): In plants
- It influences plant development,
- Regulates osmotic pressure.
Cobalt (Co): In plants
- Acts as a growth promoter and is present in Vitamin B12.
- It activates the plant enzymes like peptidases,
- In blue-green algae, it is related to nitrogen fixation.
Molybdenum (Mo): In plants: Takes part in nitrogen fixation, nitrate reduction, etc. Acts as an activator of enzymes.
Zinc (Zn): In plants: It is involved in IAA synthesis acts as an activator of many enzymes and helps in protein synthesis.
Boron (B): In plants
- The growth and development of plants are influenced when it is present in minute amounts,
- Helps in carbohydrate translocation and
- Prevents phenolic acid storage toxicity.
Iodine (I): In plants: Helps in plant metabolism, without it the growth of the plant is disturbed.
Sodium (Na): In plants:
- Essential only for certain species of green algae,
- Helps in the vigorous development of varieties of plants by its presence in the soil.
Fluorine (F): In plants: Not known.
General Functions Of Essential Mineral Nutrients
The general functions of essential mineral nutrients are
- Formation of protoplasm: The formation of a major part of the protoplasm in a living cell needs mineral elements like carbon, hydrogen, and oxygen. Nitrogen, sulfur, and phosphorus also take part in the formation of protoplasm.
- Structure of enzyme: Some mineral elements like magnesium, manganese, cobalt, etc. perform the functions of activators or inhibitors in the system of enzymes. Phosphorus helps in the formation of coenzymes like NAD, NADP, FAD, ATP, etc. Mg, S, and K act as an activator of enzymes.
- Copper is a component of certain enzymes (i.e. phenolases, laccase, and ascorbic acid oxidases sulfur contains coenzyme A. Calcium is an essential part of amylase, an enzyme that helps in starch digestion. Iron is noted in enzymes like peroxidases and catalases. Manganese helps in the formation of the enzymes decarboxylase and oxidase.
- Oxidation-reduction reaction: Oxidation is simply regarded as a chemical reaction with oxygen (loss of electrons). The reverse process of loss of oxygen is called reduction. Reaction with hydrogen is also regarded as reduction (gain of electrons).
- Osmotic balance: Certain mineral nutrients counteract the poisonous effect of some other elements by maintaining the osmotic balance (an ionic balance). The behavior of one ion in reversing the normal effect of another ion is called antagonism. To this group of elements lies Ca, Mg, and K. They are termed balancing elements, whereby osmotic balance is maintained.
- Formation of Chlorophyll: Magnesium and nitrogen plays a great role in the formation of chlorophyll, the green pigments in plants. Iron takes part in chlorophyll synthesis.
- Buffer effect: The effect produced when a solution resists change in pH when an acid or alkali is added or when the solution is diluted is called buffer effect (Acid-Base balance).
- The mineral nutrients play a great role in the pH of all sap. Some of the vital buffer systems in an organism’s body are the carbonate-bicarbonate system and phosphate buffer.
Transpiration
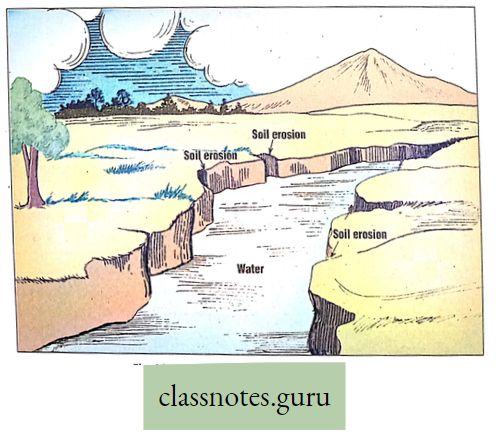
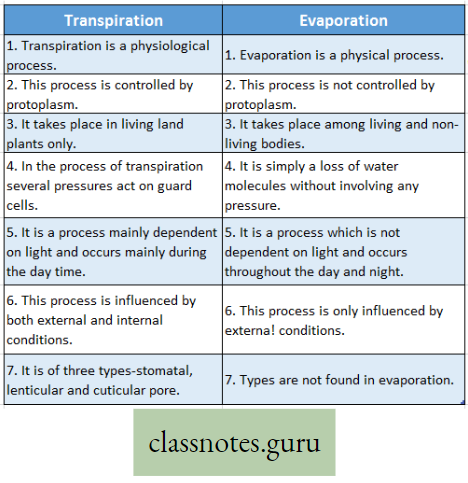
Explanation: Plants absorb large amounts of water from the soil, of which only a small fraction is used for different metabolic functions taking place within their body. The remaining water is liberated in the form of vapor through the leaf, stem, and lenticel using a physiological process termed transpiration.
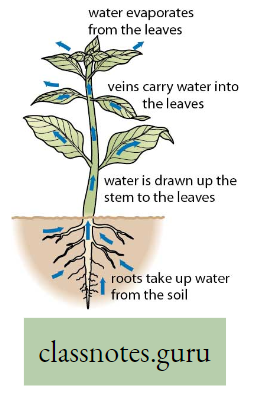
Transpiration Definition: The elimination of non-utilized excess water in the form of vapor from the plant body, under the influence of sunlight and controlled to some extent by protoplasm is called transpiration.
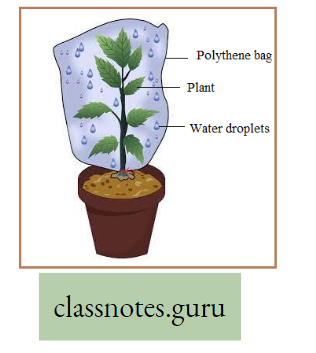
But evaporation is a physical process in which water changes from a liquid to a gaseous form unsaturated atmosphere from the free exposed surfaces of the living and non-living bodies.
Transpiration is a modified process of evaporation controlled by the protoplasm.
Difference Between Transpiration And Evaporation :
Sites Of Transpiration
There are three types of transpiration based on the organs that are involved in the process stomatal, lenticular, and cuticular pore.
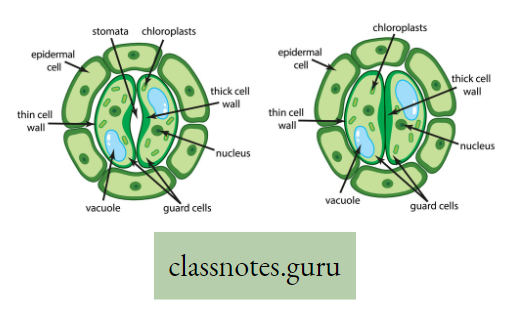
Stomatal transpiration: The loss of water vapor through the openings of stomata is called stomatal transpiration. A major portion (80-90%) of water in the form of vapor occurs through the stomata of leaves.
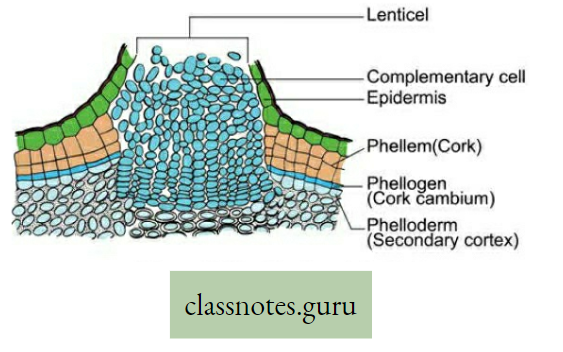
Lenticular transpiration: The loss of water vapor that takes place through the lenticels (formed during secondary growth on woody stems and fruits) is called lenticular transpiration. The lens-shaped structure lenticel always remains open and helps for the escape of water vapor through the loose mass of complimentary cells.
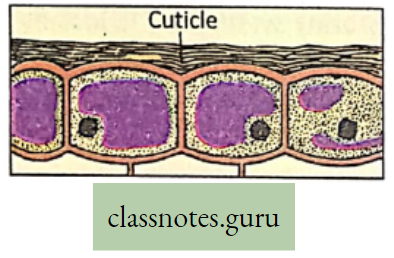
Cuticular transpiration: The water vapor which is lost through the pore of the cuticle of the leaves directly, is called cuticular transpiration. Some amount (10-20%) of water vapor is lost by direct evaporation from the epidermal cells through the cuticle of the leaves.
Physiological Processes Of Life Difference Between Transpiration And EvaportionTranspiration is affected by several external and internal factors.
For reference only: Using absorptive paper saturated with cobalt thiocyanate transpiration can be experimentally demonstrated. When free from water cobalt thiocyanate paper is blue but when combined with water (moisture) it takes a pink color.
Observe The Fact
Put some amount of water on a dish and observe after a certain period. What you will observe? Can you name that phenomenon?
External Factors:
- Light: Transpiration increases in the presence of light and decreases in the absence of light, as stomata open during the day and close during the night. Light influences transpiration by increasing the temperature of the leaf.
- Humidity of air: Under conditions of high humidity, air remains saturated with water vapor, hence it receives less water vapor, thereby reducing the transpiration rate.
- Temperature: Temperature indirectly affects the transpiration rate by regulating humidity. High temperature lowers humidity, and increases the rate of transpiration, whereas low temperature, increases humidity thereby decreasing the rate of transpiration.
- Wind Velocity: Wind velocity directly regulates the rate of transpiration. With high wind velocity, the rate of transpiration is high and with low wind velocity, the rate of transpiration is low.
- Availability of soil water: Transpiration rate depends on water absorbed by the roots, .ess absorption of water causes less transpiration.
- Atmospheric pressure: Atmospheric pressure affects transpiration. At high atmospheric pressure, the transpiration rate is low and when the atmospheric pressure is low, the transpiration rate is high.
For reference only:
Wilting: Due to inadequate water supply or excessive transpiration in scorching heat, there may be a fall in turgor pressure, so nonwoody parts of the plant (like a leaf, young soft parts, etc.) may dry out, droop, and wither called willing.
Internal Factors:
- Structure of leaf: The structure of the leaf plays a vital role in storing water that is lost by
transpiration. Leaf surface area, the total number of stomata, the amount of cuticularisation, the position and number of stomata, the nature of mesophyll cells, and their compactness play a vital role in influencing the transpiration rate. - Efficiency of roots: The efficiency of the root system in water absorption also regulates the transpiration rate.
- Hormonal influence: Cytokinin, Abscisic acid, influences the opening of stomata thereby indirectly controlling the rate of transpiration. The rate of transpiration can be measured by Ganong’s photometer.
Relation between transpiration and ascent of sap: Through the process of transpiration, water goes out of the plant leaf in the form of water vapor through the stomata. Thus volume of water in the leaf decreases and a partial vacuum is created in the plant leaf.
- This vacuum creates a suction pull (suction pressure) that pulls the water column and sap upwards through the xylem vessel from the root through the stem to the leaves.
- This is called transpiration pull. Thus transpiration indirectly helps in the ascent of sap.
Why is transpiration called a ‘necessary evil’: This physiological process is considered to be, “a necessary evil” (Curtis). It is evil as it involves the loss of huge amounts of water, often leading to water deficit in plants, bringing about a reduction in photosynthesis, growth, premature leaf fall, and above all it may also result in dedication and finally the death of a plant.
- These are the harmful effects of transpiration.
- On the other hand, the loss is accepted because the beneficial effect of transpiration (such as osmoregulation, thermoregulation, ascent of sap, etc.) is much more than the harmful effect.
- Hence transpiration is called a necessary evil.
Significance (beneficial effects) of transpiration:
- Transpiration helps keep the temperature of a plant low (thermoregulation) even when it is exposed to bright sunlight for a long time. It thus prevents the damage of delicate cells,
- It helps in the distribution of water throughout the plant,
- Transpiration helps in the translocation of water and minerals through the xylem.
- Transpiration develops a suction force which is helpful in the ascent of sap and absorption of water from the soil through roots,
- It helps in giving out excess water (osmoregulation) absorbed by plants from the soil which is not utilized by the plant,
- It helps in maintaining turgor pressure in the cells and keeps the plant body cool.
- Transpiration, therefore plays an important role in a plant’s life.
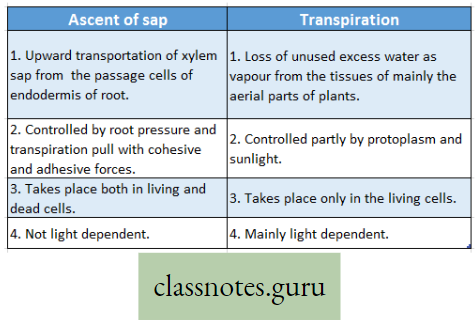
Difference Between Ascent Of Sap And Transpiration :
For reference only:
Guttation: It is the process where water and dissolved minerals are removed from the leaf margin of plants through water pores or hydathodes in the form of liquid droplets.
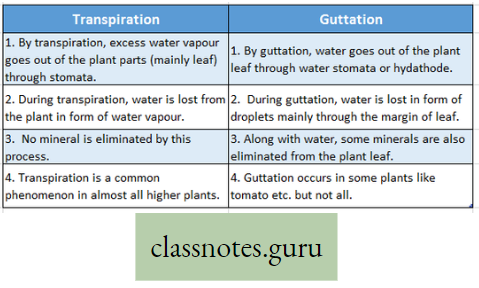
Difference between Transpiration and Guttation:
Movement Of Water, Minerals, Food And Gases
Introduction: For performing several functions like growth, respiration, excretion, etc. a free movement of oxygen, carbon dioxide, enzymes, vitamins, hormones nutrients, etc., throughout the living body is essential. It is also essential to excrete the toxic substances formed as a result of metabolic activities.
- For all these, a suitable liquid medium is required. There are various circulating media found in the organism, such as water (in plants), blood, and lymph (in animals). Movement of various substances dissolved in the liquid medium throughout the living body is called circulation.
- A living plant body is made up of cells. To survive, all these cells require food, oxygen, water, etc. Substances absorbed or made in one part of the body of the organism are carried to other parts of its body. For this, transport systems are necessary.
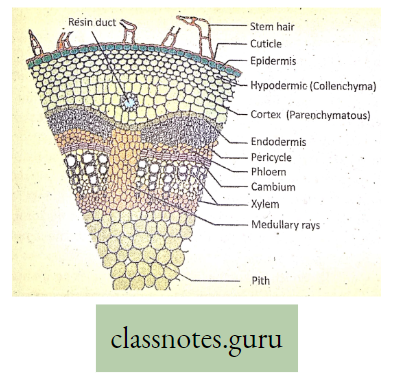
- Special tissues or organs are needed. Oxygen for respiration and carbon dioxide for photosynthesis are directly taken by plants from the air by the process of diffusion. The transport system is hence necessary for circulating (conducting) food, water, and minerals.
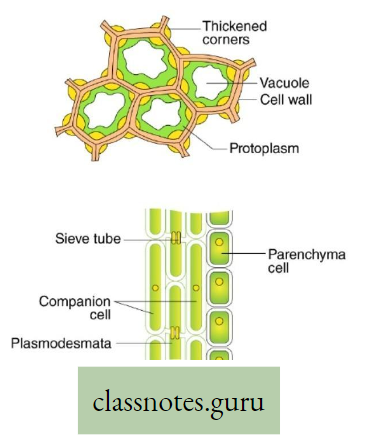
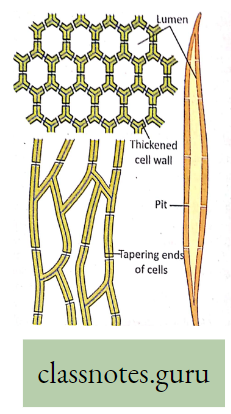
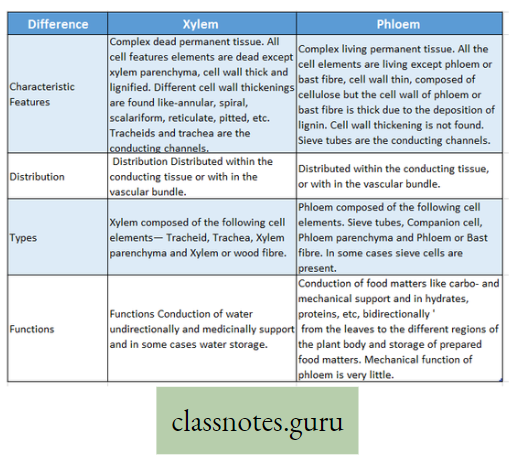
- The plants have two conducting tissues xylem and phloem. Xylem carries water and minerals, whereas phloem carries food, prepared by the plant itself. In lower aquatic plants water circulation takes place by cell-to-cell osmosis.
Definition of Plant circulation: The process by which the movement of food, water, minerals, hormones, enzymes, and other substances takes place through the liquid medium of the living body by special conducting tissues xylem, and the phloem, is called plant circulation.
The different substances are transported in the plant body using passive transport (diffusion and osmosis) and Active transport.
Medium of transport: Water: It is the chief medium of transport in the plants. Water is absorbed from the soil by the roots.
- In aquatic plants, water is absorbed throughout the body surface.
- The various processes involved in the transportation of different substances in plant bodies are stated below
Passive Transport Features of Diffusion And Osmosis
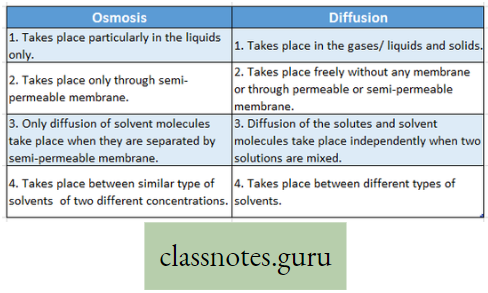
Diffusion:
Diffusion Definition: The spontaneous spread of molecules of any substance from a region of great abundance i.e. from higher concentration to a region of lesser concentration or nil abundance is called diffusion. It does not need any energy (ATP) for its function, hence this type of transport is passive.
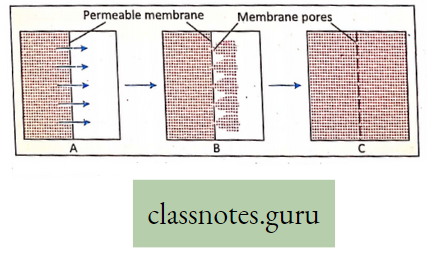
Characteristics of diffusion:
- Diffusion takes place from higher concentration to lower concentration.
- During diffusion, both solute and solvent can flow.
- Diffusion takes place without any membrane or if there is any membrane, it must be a permeable membrane.
- Diffusion can take place between two liquids, two gases, liquid and gases, solids and gases.
Some common examples of diffusion :
- Between two liquids: One drop of ink/eosin in a glass of water.
- Between two gases: One incense stick burns at one corner of the room and the gas molecules spread all over the air of the room.
- Between liquid and gas: Spraying of perfume and its smell spreads all over.
- Between solid and gas: The smell of naphthalene spreads all over the box through the air.
For reference only:
- DP (Diffusion Pressure): Pressure exerted by the diffusible molecules of higher concentration is called Diffusion pressure.
- DP0 (Diffusion Pressure Deficit): The difference of DP (Diffusion Pressure) of two- solutions having different concentrations is called the Diffusion Pressure Deficit of lower concentrated solution.
- WP (Wafl Pressure): The pressure exerted on the contents of a plant cell by the cell, the wall that is equal in force and opposite in direction to the turgor pressure is called the wall pressure.
- HP (Hydrostatic Pressure): The pressure exerted by water molecules of a cell on its cell wall at a given point is called hydrostatic pressure.
- TP (Turgor Pressure): When a plant cell is fully saturated with water, it is called a turgid condition and the maximum hydrostatic pressure in a turgid condition is called turgor pressure.
Osmosis:
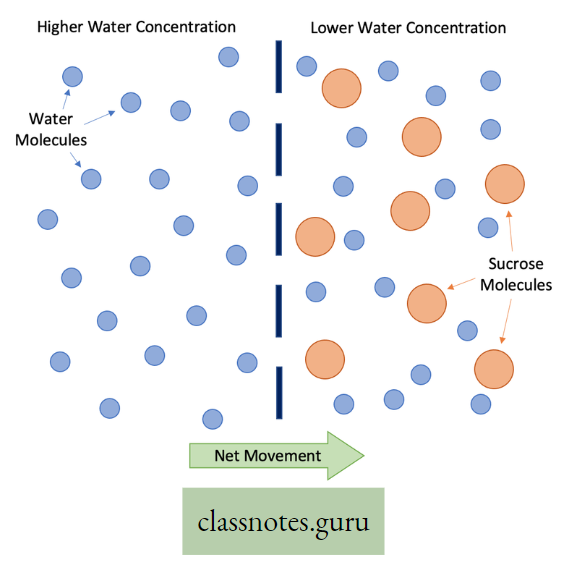
Osmosis Definition: The movement of solvent molecules through a semi-permeable membrane from a region of higher concentration to a region of lower concentration is called osmosis.
- Osmosis is a special kind of diffusion of a solvent through a semipermeable membrane. Water is known as the universal solvent.
- With the help of osmosis, only solvent molecules can pass through semipermeable membranes from a region of higher solvent concentration to of lower solvent concentration.
- For this reason, osmosis is known as the diffusion of solvent molecules.
- if plain water and sugar solution are kept separated by a semipermeable membrane, water molecules From the plain compartment will pass to the sugar solution.
Types of Osmosis: About any living cell, osmosis may be of two types
- Endosmosis: The process by which the solvent molecules from outside enter into h – is known as endosmosis.
- Exosmosis: The reverse process of endosmosis which involves, the exit of solvent molecules from the cell, is known as exosmosis.
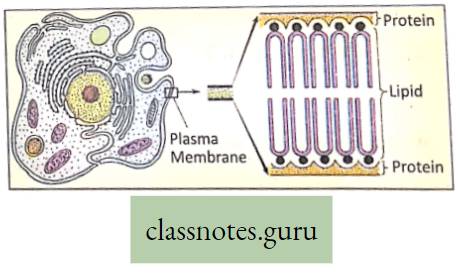
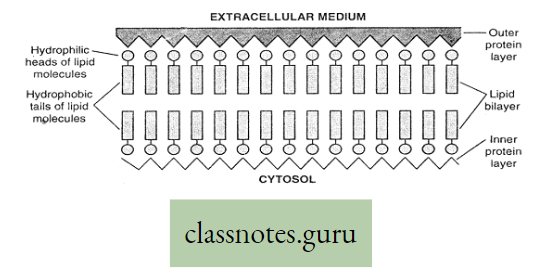
Nature of membranes: According to physical properties, the membranes are of three types
- Permeable membrane: Such membrane allows all the molecules or ions of a solution (both solute and solvent molecules) to pass through it, for example, filter paper, cell wall, etc.
- Semi-permeable membrane: Such membrane allows passage of water (solvent) molecules and positively charged hydrogen ions, but not the solute molecules nor the other positively charged and negatively charged particles, for example, air bladder of fish, egg’s membrane, etc.
- The semipermeable membrane can be better termed as differentially permeable, which allows passage of some solutes but, holds back others at different rates of diffusion,
- Impermeable membrane: It does not allow either the solvent molecule to pass example rubber sheet or, a plastic sheet.
- A membrane that permits certain solute and solvent substances to pass through more easily and the other is said to be selectively permeable.
Process of osmosis: If two solutions of different concentrations are separated by a semi- a permeable membrane, then the solvent molecules from the less concentrated solution (which contains more solvent and less solute) move through the membrane to the more concentrated one (which contains less solvent but more solute).
During such movement, the fluid level gradually rises in the compartment containing the more concentrated solution and a position of equilibrium is ultimately reached when the hydrostatic pressure prevents further entry of solvent into this Compartment.
Osmotic Pressure Definition: The molecules of the water inside the compartment of the cell create a pressure known as hydrostatic (HP). The hydrostatic pressure wb\h which develops during osmosis is known as osmotic pressure (OP).
Active transport: Active transport is the movement of lower to higher concentration, which involves carrier molecules and needs energy.
Role of active transport: The theory of transport across the cell membrane was first proposed by Hoagland and others in 1923 while working with Nitella. The mechanism of active transport was studied by Hoagland and Davis.
Active Transport Definition: The transport of matter in the form of ions or molecules across the cell membrane from lower concentration to higher concentration with the expenditure of metabolic energy is called active transport (uphill).
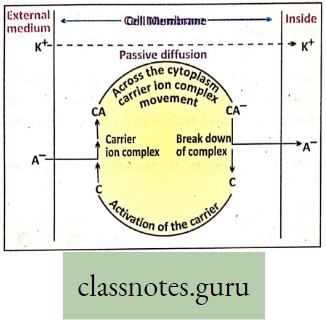
During active transport, the membrane proteins use energy to pump molecules of matter across the cell membrane against a concentration gradient, from low to high concentration. Salt absorption is related to the energy of the cell.
Process of active transport: The process of active transport through carriers involves a sequence of events which are stated as follows :
- On the outer surface of the cell membrane substrate i.e. ions or molecules bind to membrane protein (carrier),
- The carrier substrate complex thus formed moves across the cell membrane.
- Reaching the inner surface of the cell membrane, the carrier substrate complex breaks (dissociates).
- The molecules or ions are released and enter the cell, The carrier (membrane protein) returns to its original state and is free to accept another ion or molecule.
Cell To Cell Transport
D.Role of osmosis and diffusion in circulation :
- The unicellular root hairs absorb capillary water of the soil by osmosis.
- Water then enters the cortical cells of the root by cell-to-cell osmosis, finally carried to the xylem vessel.
- Minerals in the form of ions reach the cells from the soil by diffusion. Later, water and minerals are mixed up to form the xylem sap.
- Xylem sap moves upwardly and enters the cells of mesophyll tissue by osmosis and diffusion.
- Sieve tubes of phloem conduct prepared food formed in the mesophyll cells to different parts of the plant body by diffusion. Thus, the process of diffusion and osmosis plays an important role in the conduction of plants.
Mechanism of absorption and transport of water in plants:
- Water absorption by plants through unicellular root hairs is facilitated by the process of osmosis.
- Root cells have higher osmotic pressure than that of external soil solution. Water enters the root hair cells by endosmosis through the cell membrane acting as a semi-permeable membrane,
- With the entrance of water by endosmosis the root hair cells turn turgid,
- Osmosis is set up between the cells of the cortex of the root and root hairs,
- Water enters the cells of the cortex leaving the root hair cells flaccid. These flaccid cells turn turgid by reabsorbing capillary water,
- This process allows the continuous transfer of water from the soil to cortical cells Cell-to-cell osmosis brings about turgid conditions in all cells,
- Water is forced to enter the xylem vessels from where it is conducted to other parts of the plant body,
- The ability of entry of solvent (water) into the cell is determined by the difference between osmotic pressure (OP) and turgor pressure (TP). This is known as suction pressure (SP) or diffusion pressure deficit (DPD).
Difference between Osmosis and Diffusion :
Concept Of Ascent Of Sap Through Xylem (Role Root Pressure And Transpiration Pull)
- The upward movements of sap from the root region take place through the Xylem (mainly through the Xylem vessel). The upward movement of sap is called the conduction of water.
- It takes place due to the combined effect of root pressure. Adhesion-Cohesion force and active transpiration pull. For that reason, the water column moves through the xylem vessels which are in a state of cohesion and adhesion.
- The solution of water containing minerals called sap, commonly known as xylem sap.
- The process of upward transportation of sap against the force of gravity, from the passage cells through the xylem vessels to the leaves is called ascent of sap.
Factors Affecting the ascent of Sap:
Root Pressure: The pressure exerted by plant sap in the root of the plant is called root pressure. Plants absorb water and minerals with the help of root hairs from the soil. This water along with minerals (sap) reaches the cortex cells by the process of cell-to-cell osmosis and diffusion. Then water

Flows through endodermis, and pericycle and finally reaches xylem vessels. This active hydrostatic pressure created in the parenchymatous cortical cells of the roots is called the root pressure. The sap reaches the stem at a certain height through the xylem vessel.
Adhesion-Cohesion Force:
Adhesion: Adhesion is the intermolecular attraction among dissimilar molecules union between water molecules with the inner xylem vessel
Cohesion: Cohesion is the intermolecular attraction among similar molecules the union between water molecules.
- Water molecules form a continuous chain from the root to the leaf by cohesion pressure or cohesion force.
- Water molecules also exert a sideway pressure on the xylem vessel called adhesion pressure or adhesion force.
- This cohesion-hesion pressure or force keeps the water molecules in position in the xylem and prevents the water column from breaking up.
Transpiration Pull: During transpiration, water is lost from the leaf in the form of water vapor. Thus a partial vacuum is created in the plant leaf, that pulls the water column upwards, known as transpiration pull. This pull helps in the ascent of sap.
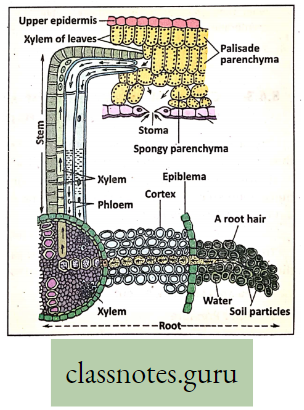
- Transpiration creates a pull from above and the continuity of the water column is maintained by the cohesion of water molecules and adhesion of water molecules with the inner xylem vessel wall which prevents the water column from breaking down. Root pressure also pushes the water column upwards.
- Hence ascent of sap is the combined effect of pushing force (root pressure, adhesive-cohesive force) and pulling force (transpiration pull).
Relation Between Absorption And Ascent Of Sap: Unicellular root hairs absorb water and minerals (capillary water and minerals) from the soil.
- This water enters the cortical cells by cell-to-cell osmosis and diffusion. Hydrostatic pressure exerted by these turgid parenchymatous cortical cells helps the water to enter the xylem vessels.
- The transpiration pull from the leaves and the root pressure from below accounts for the ascent of sap through the xylem vessels.
- The continuity of the water column is maintained by the cohesion of water molecules with each other and the adhesion of water molecules with the xylem vessel wall.
Characteristic features of Transportation of Food through Phloem
Plants synthesize carbohydrates, a type of food matter in the green parts and in the chlorophyll-bearing regions, mainly in leaves. Food prepared in these areas has to xylem phloem be transported to all the parts of the plants through the interconnected phloem tubes.
Translocation Definition: The transport of food through phloem tissue in plants is called translocation.
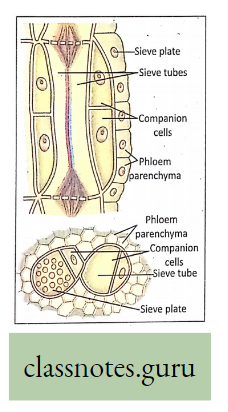
- Sieve tubes, the non-nucleated living cells of phloem, are responsible for carrying food by translocation,
- The companion cells with dense cytoplasm and prominent nucleus are also indirectly associated with the sieve tube for such conduction,
- The sieve plates (end walls of the sieve tubes) are perforated by sieve pores.
- The protoplasmic connections (Plasmodesmata) passing through the sieves take part in the conduction of food materials
- Food is also carried to the growing regions and storage regions like fruit, stem, root, etc.
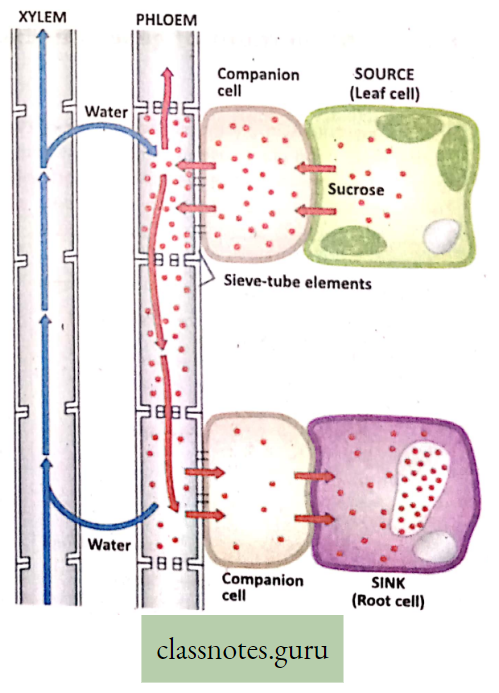
For reference only: During active growth or in times of energy requirement to run the different vital functions, the stored food is hydrolyzed for transport through the phloem to growing regions of the plant body.
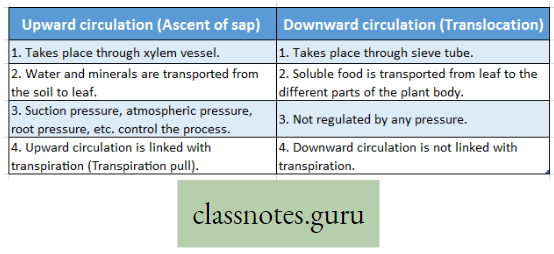
Difference Between Upward Circulation And Downward Circulation :
Movement of gases i.e Exchange in Plants
For reference only:
Photosynthesis in plants involves intake of carbon dioxide and disposal of oxygen. During respiration plants use oxygen and give out carbon dioxide. In plants, there are no respiratory organs.
- The elaborate liquid transport system cannot be used in the transport of gases.
- The leaves are well adapted to carry out gaseous exchange during photosynthesis.
- The rate of respiration in the roots, stems, and leaves of plants is lower than that of animals.
- In the leaf and stem of plants, the living cells are located close to the surface.
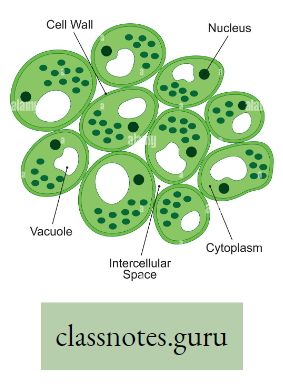
- The loosely arranged parenchyma cells with intercellular spaces in leaves, stems and roots provide an interconnecting system of air spaces.
- The diffusion of gases is faster through the air than through water.
- So diffusion of oxygen and carbon dioxide takes place rapidly through the interconnecting system of intercellular air spaces.
- These gases also pass through the cell wall and plasma membrane by a diffusion process.
- Aquaporin channels in the plasma membrane help in diffusion across the membrane.
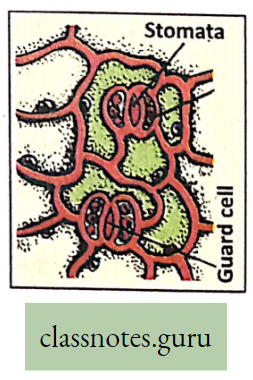
Absorption of gases by Leaves: Gaseous exchange in leaves takes place through stomata when light falls on a leaf. These openings remain open in the morning and close during the night.
- This happens due to a change in the turgor pressure of the guard cells. Guard cells possess a thick and elastic inner wall.
- When turgor pressure develops the outer thin wall of the guard cell extends out forcing the inner walls into a crescent shape, resulting in the opening of the stoma.
- Again, loss of turgor pressure causes inner walls to regain their original shape resulting in the closing of the stoma.
- During the day when osmotic pressure in the lower epidermal cells remains constant and the osmotic pressure of guard cells increases then the stomata open.
- In the evening stomata closes when the osmotic pressure of guard cells drops to nearly that of surrounding cells.
Absorption of gases by Roots and Stems: The dead cork cells present in mature roots and woody stems contain suberin (a waterproof substance) which makes the cork impervious to oxygen, carbon dioxide, and water.
Activity:
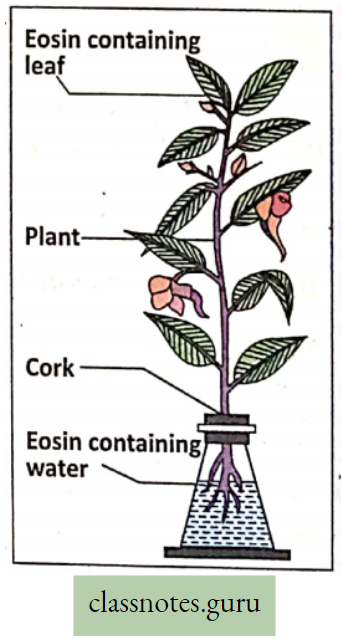
Take a fresh Balsam plant out of the soil with an intact root system. Fill 3/4th of the conical flask with water. Add a few drops of eosin dye into water.
- The water turns red. Place the Balsam plant in the conical flask in such a way that the roots only remain submerged in water.
- Keep the experimental setup in this position for an hour.
- Observe the setup after an hour and note down your observation.
- Cork of mature roots and woody stems contain non-suberized perforated pores called lenticels which allow entry to oxygen to reach
- The intercellular spaces of interior tissue and CO2 are released into the atmosphere.
- Stems of many plants are green in color and use stomata for gas exchange rather than lenticels.
Organ-level Respiration (Respiratory Organs)
Organism Definition: Respiratory organs are the specific type of organs that help the process of exchange of gases, O2, and C02 between the environment and the organism.
Characteristic features of respiratory organs :
- The respiratory membrane must be thin and permeable so that it is easily gaseous. exchange.
- The respiratory surface must be extensive to provide a greater surface area for gaseous exchange.
- The respiratory organ must be always kept moist to facilitate the diffusion of gases.
- In higher animals, the respiratory organ is highly vascularised so that blood can transport respiratory gases) from respiratory organs to different cells of the body and vice versa for CO2.
Now, can you explain why the skin of a toad acts as a respiratory organ but not the skin of a many)
Respiratory Sites of Plants
Stomata: These are microscopic apertures in the leaf epidermis of plants guarded by two semi-circular guard cells. Stomata are generally present on the ventral surface in monocot plants. Through the opening of stomata gaseous exchange takes place.
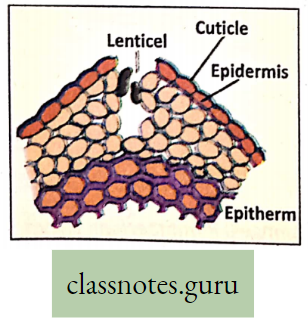
Lenticels: Lenticel is a porous tissue consisting of cells with large cells with intercellular spaces, generally on the bark of woody stems of dicot plants. It functions as a pore that provides a pathway for direct exchange of gases between the internal tissues and atmosphere through the bark. (Tough thick bark is otherwise impermeable to gases)
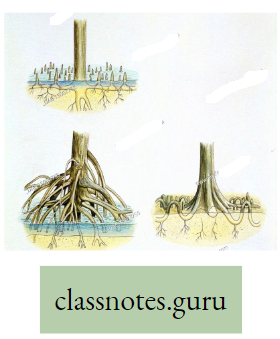
Pneumatophores: These are breathing roots, found in mangrove trees and they are negatively geotropic adventitious branches of the root. In fact in saline soil, the capillary space of the soil is almost blocked by a huge deposition of NaCI. So there is less capillary air hence the root suffers from O2 deficiency. So, some of the adventitious roots provided with pores bend upward and come above the soil surface to absorb O2 directly from the air. Example pneumatophores in Sundri Plant.
Respiratory Organs Of Animals
Introduction: Usually, respiration in different animals is performed by the definite organs called respiratory organs. In lower animals, the exchange of gases takes place through the body surface, whereas in higher animals, there are complex organs like trachea, lungs, etc. The following is a brief account of the different types of respiratory organs found in different animals.
Respiratory Organs Of Different Animals
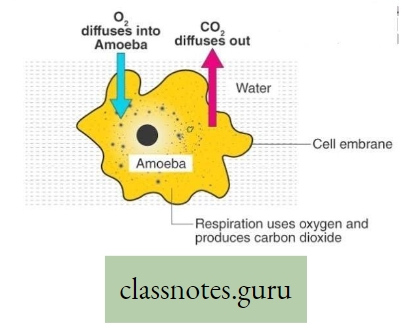
- Body surface i.e., cell membrane Amoeba, Sponge, Poramoecium, Hydro.
- Moist skin Earthworm, Leech.
- Skin, lungs, buccal cavity Toad
- Trachea Insect, Cockroach, Grasshopper.
- Gill Fish, Mollusca, Prawn, Crab.
- Labyrinthine organ, gills Koi fish.
- Respiratory tube, gills Singhi fish.
- Lungs Whale, Lizard, Crocodile, Mammal.
- External gills Tadpole.
- Book lungs Scorpion, Spider.
- Book gill King crab (Limulus)
- Lungs, 9 air sacs Pigeon
Body surface: In the case of aquatic animals like Amoeba, Sponges, Poramoecium, Hydro, etc. exchange of gases between cells and their environment takes place by simple diffusion through the cell membrane (body surface).
Skin: Terrestrial animals like earthworms, leeches, etc. respire partly or wholly through thin and highly blood-supplied moist skin.
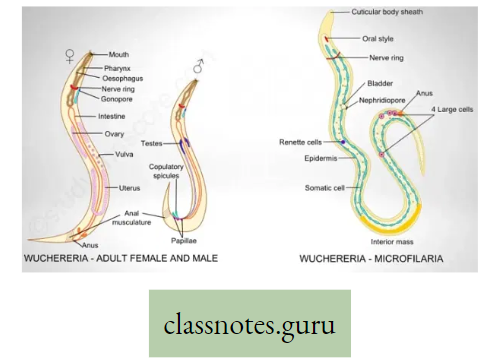
Do you know that in Toads or Frogs, there are three respiratory organs: Lungs (Pulmonary respiration) in normal conditions; Skin (cutaneous respiration); in hibernation as well as in normal conditions also; inner wall of the buccal cavity (buccopharyngeal respiration) mainly v during ingestion of food.
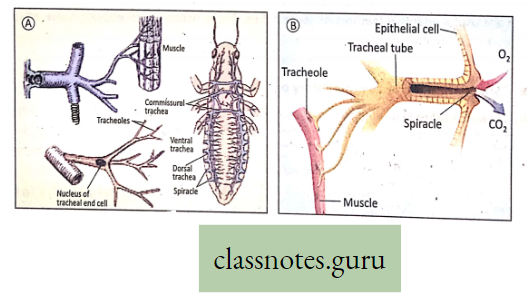
Trachea: Insects and varieties of Arthropods have elaborate networks of air-filled tubes called trachea which open onto the body surface through the small pores called spiracles or stigmata. (Do you know there are 10 pairs of spiracles in Cockroach ?) The trachea branches repeatedly into tubes called tracheoles through which gaseous exchange takes place.
Try to write the comparison between stomata and stigmata.
In insects, blood does not carry 02 because 02 is directly carried to different cells of the body through the trachea.
Gills: In aquatic animals like fish, external respiration takes place through gills. These are specialized structures of dark color, provided with thin walls and blood capillaries
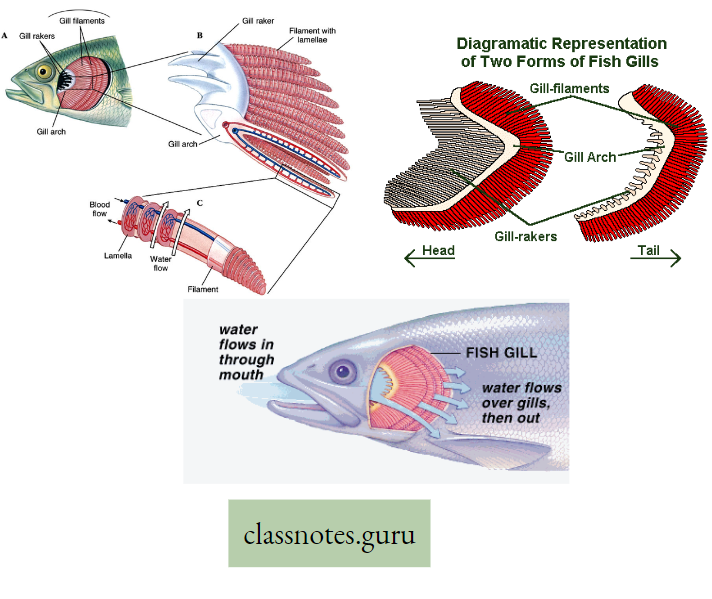
- which favors easy diffusion of gases between dissolved 02 in water and the circulating blood passing through them. The actual site of gaseous exchange in fish is the gill lamella of the gill filament.
- Do you know that besides fishes, so many other animals breathe by gills Example Snails (Mollusca), Prawns (Arthropoda), and so on?
- Try to find out in which animal the respiratory organ is book gill. What is the respiratory organ of spiders and scorpions?
Accessory respiratory organs Definition: The organs that partly accomplish respiration and are additional complementary respiratory structures are called accessory respiratory organs (other than gills). Some jewel fishes like Koi, Magur, and Singhi are provided with these organs.
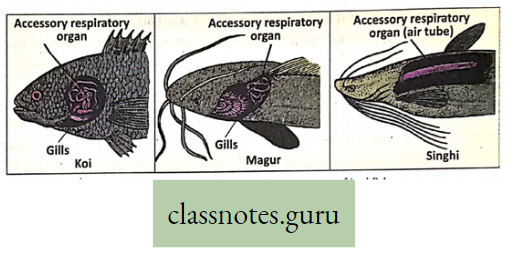
- Accessory respiratory .organs develop in addition to the normal pharyngeal gills, to help the fish live in aquatic environments with low oxygen
- concentration or to breathe oxygen directly from the air, aestivate (summer sleep) over prolonged droughts during summer, and meet the extra demand for oxygen.
- However, accessory respiratory organs in fishes can perform gaseous exchange so long as they remain moist.
- Gills are incapable of utilizing oxygen in the air, so accessory respiratory organs are useful adaptive features of some fishes.
- These fishes that possess these adaptive features are called jellyfish. Now you can understand why the jeol fishes can survive on land for a long time.
Structures Of Accessory Respiratory Organs :
- In Koi (Anabas testudineus): The presence of a labyrinthine organ located within the cavities of the gill chamber. It is rose-shaped and covered by epithelium having numerous blood vessels.
- In Magur (Clarius batrachus): Presence of tree-like dendritic or arborescent organ located within suprabranchial cavities of the gill chamber.
- In Singhi (Heteropneustes fossilis): The presence of long tubular dorsally situated respiratory tubes or air sacs arising from the gill chamber and extending up to the tail.
Lungs: Lungs are the specialized respiratory organs of all land vertebrates Example, birds (pigeons), reptiles (lizards, snakes), amphibians (frogs, toads), ‘and mammals (rats, cows). Aquatic mammals (For example whale, dolphins) are also provided with lungs.
- That is why some animals (Whales, dolphins, and Crocodiles) often come above the surface of the water for aerial respiration.
- The lungs of amphibians, reptiles, and mammals are paired sac-like spongy structures due to the presence of air-filled alveoli.
- Alveoli are provided with numerous blood vessels (capillaries) for gaseous exchange.
- The lungs of pigeons are more compact and are supplemented by thin-walled 9 air sacs for storage of air that also help to increase buoyancy.
- Air sacs can store air only but no gaseous exchange. Gaseous exchange occurs only in the lung alveoli of pigeons.
Do you know there are nine major air sacs and four minor (accessory) air sacs in pigeons?
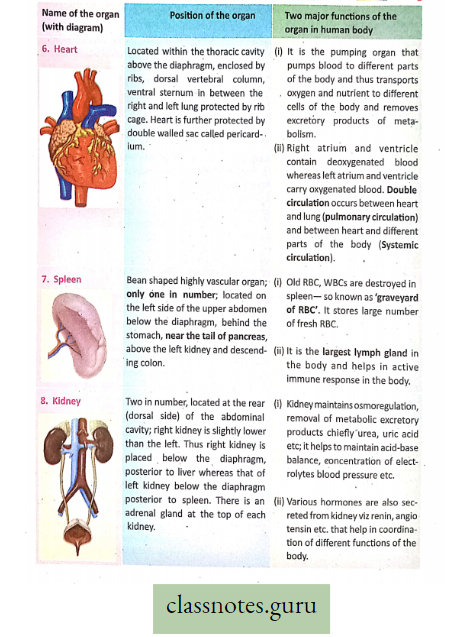
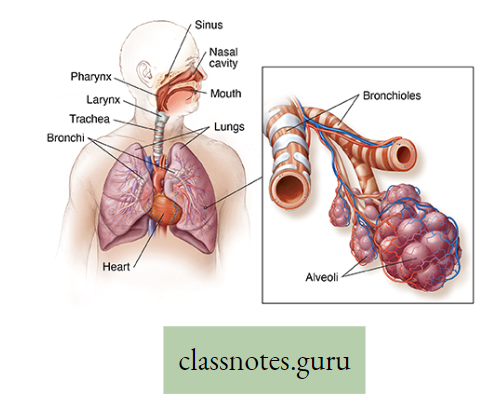
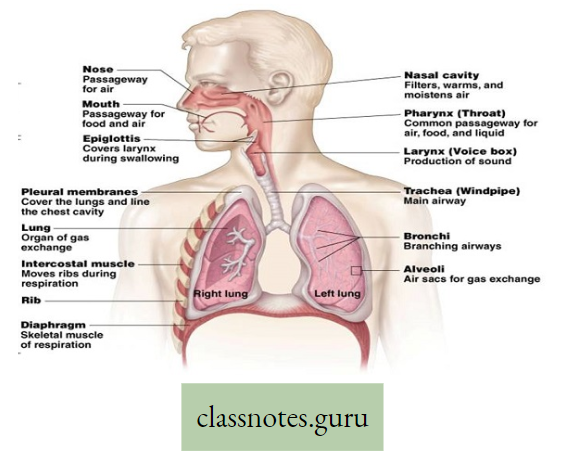
Lungs in man: Two reddish sponge-like lungs are situated in the thorax above the diaphragm. Each of the conical lungs is enclosed in a double-layered membranous sac, called pleura.
- A small amount of serous fluid (pleural fluid) is present in the closed pleural cavity.
- The right and left lungs are divided into 3 and 2 lobes, respectively. Inside each lung, the primary bronchi divide and subdivide into many terminal bronchioles.
- Each of them leads to several alveolar ducts.
- Each alveolar duct opens into many small, thin-walled sacs, called alveoli. Around each alveolus, blood capillaries form a network.
- The exchange of gases takes place between the blood in these capillaries and the air inside the alveoli.
Lungs and breathing in human
The process of breathing in man is completed in two phases Inspiration and Expiration.
Inspiration: The active process by which air from the atmosphere enters into the lungs is known as inspiration.
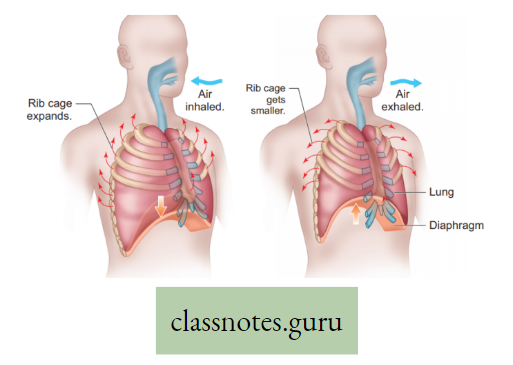
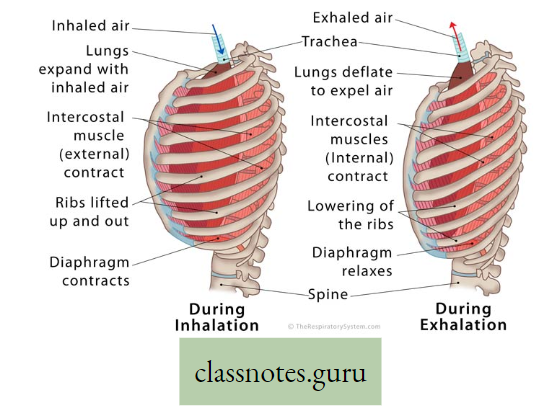
Expiration: The passive process by which air from the lungs is expelled into the atmosphere is called expiration. Organs involved in Inspiration and Expiration are mainly the diaphragm and intercostal muscles.
Diaphragm: It is a dome-shaped sheet of unpaired internal skeletal muscle that separates the thoracic cavity from the abdominal cavity.
Intercostal muscle: The muscles which remain obliquely in between ribs are known as intercostal muscles.
The process of breathing in man is accomplished by coordinated movement of diaphragm and intercostal muscle as follows :
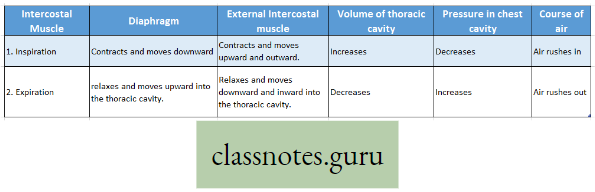
Try to discover what happens during forced inspiration and forced expiration.
Lungs And Healthy Life
Breathing exercise plays a very significant role in increasing lung volume. Decreased lung capacity can negatively affect quality of life.
- Athletes, singers, dancers, and other health-conscious individuals also practice various methods to improve lung capacity. Specific exercises can help to increase lung volume and its elasticity.
- A deep breathing technique for increasing lung capacity is called “Mother Breath”. This exercise can be done by slow inhaling for seven seconds.
- The breathing is to be held for a few seconds and then slowly exhaled for seven seconds. The sequence may be repeated. This is just an example. There are, however, many more techniques.
- There are different breathing exercises in “Pranayam”, which help to improve the function of the heart and lungs. However, someone must be cautioned not to do any breathing exercises on a full stomach i.e. after lunch or dinner. It should be practiced on an empty stomach preferably in the early morning after getting up from bed.
- You have heard of “Laughing Club” where so many people together practice lung exercises in the early morning. They have some definite guidelines for practice. Try to practice lung exercise regularly.
Cigarette Smoking Is Harmful For Respiratory System: Cigarette smoke contains innumerable constituents of which almost all of them are harmful, toxic, poisonous, and badly carcinogenic (that induces cancer) to the respiratory system.
- Some serious diseases of the respiratory system are associated with smoking like Cancer, Bronchitis, Emphysema as well as Cardiovascular disorders, and so on.
- Millions of smokers all over the world are dying of Cancer every year.
- Try to create awareness among people in your locality about the dangers of smoking.
- Have you heard the term “active smoking” and “passive smoking?
Cellular Respiration
Concept Of Cellular Respiration
What is Cellular Respiration: Definition: Cellular respiration is the oxidative, catabolic, enzymatic breakdown of organic substances when potential energy is released in the form of kinetic energy.

AH, organisms require energy to maintain the vital functions of the body. This energy is derived from the food they take.
- During photosynthesis, solar energy is fixed in food as potential chemical energy. This food is used as a cellular respiratory substrate which is oxidized to release energy.
- In the presence of O2 (aerobic respiration), there is complete oxidation of food that results in a greater amount of energy release whereas in the absence of O2 (anaerobic respiration), there is incomplete oxidation of food that yields less energy.
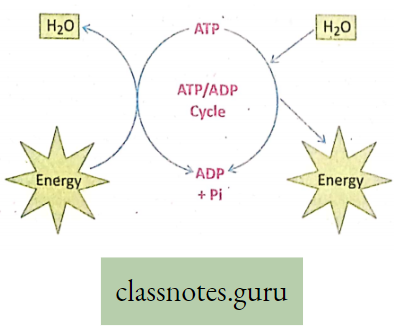
- However, the energy released by cellular respiration is stored temporarily in ATP (Adenosine Triphosphate). This ATP (energy currency) is broken to release energy for various activities of the body.
Respiration: A catabolic process: Respiration is called a catabolic process due to the following reasons
- Respiration is a destructive process, where respiratory substrates such as carbohydrates, proteins, fats, and organic acids are broken down into simpler products with the help of enzymes in different metabolic processes in the body.
- The process results in a decrease in the dry weight of an organism.
- The stored energy of food i.e., potential energy is converted to released energy, i.e. kinetic energy.
- Simpler substances (micromolecules) are formed from complex substances (macromolecules).
Cellular Respiratory substrate: Those protoplasmic substances which when oxidized liberate energy, are called the respiratory substrates. Although carbohydrates, proteins, fats, and organic acids are used as respiratory substrates, carbohydrates, particularly glucose are the chief energy-yielding substrate.
1 gram Carbohydrate Yields 4.0KCal energy
1 gram protein Yields 4.1KCal energy
1 gram fat Yield 9.3Kcal energy
Time of respiration: In every living cell the process of respiration takes place throughout the whole day and night.
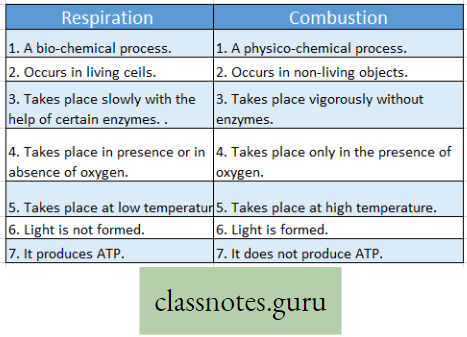
Respiration and Combustion :
- Respiration Definition: It is a biochemical process that takes place in each living cell either in the presence or in the absence of oxygen. During respiration mainly the glucose molecules are slowly oxidized step by step by the action of several enzymes. The energy thus released from the oxidation of sugars is transformed into the energy-rich compound called ATP. Thus respiration may be a controlled burning process (combustion) within the living cells, or controlled cellular combustion that is controlled by the action of various enzymes.
- Combustion Definition: It is a physio-chemical process that takes place in nonliving objects. Only in the presence of oxygen, these objects are burnt violently which produce generally light, heat, and ashes. No enzyme is required for this process. Thus combustion may be called a physico-chemical process by which any substance outside the body is oxidised in the absence of enzyme but in the presence of oxygen and produces heat and light.
Difference between Respiration and Combustion :
Do you know, that fireflies can produce light known as bioluminescence? Is it combustion or respiration?
Types Of Cellular Respiration (Aerobic Anaerobic And Fermentation)
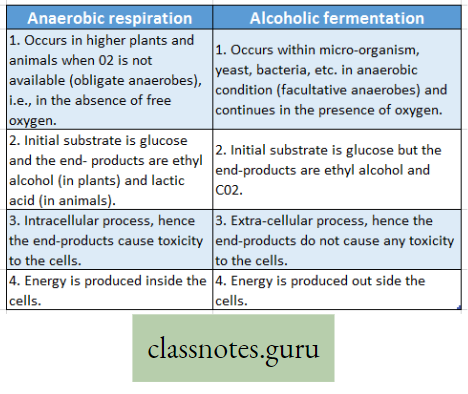
Respiration is mainly of two types, depending upon the nature of oxidation of the substrates i.e., aerobic respiration and anaerobic respiration. Another type of biochemical process occurs in the micro-organism which is called fermentation.
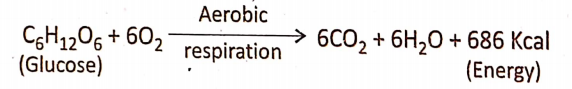
Aerobic Respiration Definition: Aerobic respiration is the process by which complete oxidation of the respiratory substrate (glucose) takes place in the presence of free oxygen, producing end products like C02 and water, with the generation of energy.
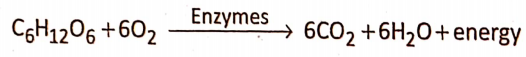
In the living cell oxidation of substrate takes place in two ways i.e., either by removal of hydrogen or by addition of oxygen. In aerobic respiration, the molecular or free oxygen is used as an electron or hydrogen acceptor in its oxidative process.
Occurrence: Aerobic respiration takes place in the living cells of all the aerobes (organisms that require oxygen).
Process Of Oxidation And Production Of Energy: In the living cell, aerobic respiration is completed in three stages Glycolysis the first stage, Krebs cycle the second stage, and Terminal oxidation (ETC) the third and final stage.
Can you explain why the rate of breathing increases while playing football?
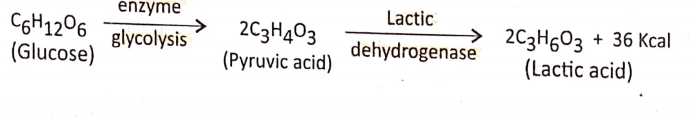
- Anaerobic respiration Definition: Anaerobic respiration is the process of incomplete oxidation of the respiratory substrate (mainly glucose) in the absence of free O2 or the presence of bound O2forming CO2 and ethyl alcohol (in plants) or lactic acid (in animals) with partial release of energy.
- Occurrence: Anaerobic respiration is a special method of respiration that generally takes place in anaerobic bacteria (For example Clostridium, Lactobacillus), unicellular fungi (Yeast), and in plant seeds during germination, parasitic animals like tapeworm [Taenia solium), roundworm [Ascaris lumbricoides), Monocystis, etc.), in the skeletal muscle fibers (cells) during vigorous exercise, etc.
- Process of oxidation and production of energy: Anaerobic respiration occurs in the anaerobes, an organism that can live without oxygen. Thus in the cytoplasm of anaerobes, one molecule of glucose (C2H12O6) is transformed into two molecules of pyruvic acid (C3H4O3).
- In some anaerobic bacteria like Thiobacillus, methane bacteria, etc. glucose [in the absence of free oxygen, but in the presence of bound oxygen present in chemical compounds like nitrate ‘ (NO3-), carbonates (CO3-), sulfates (SO4+)], gets incompletely oxidized to form carbon dioxide (CO2), water (H2O) and energy.
- The energy released in the process is less, due to the very short phase of terminal respiration. The overall chemical equation is stated below :
Glycolysis is the common phase of both aerobic and anaerobic respiration.
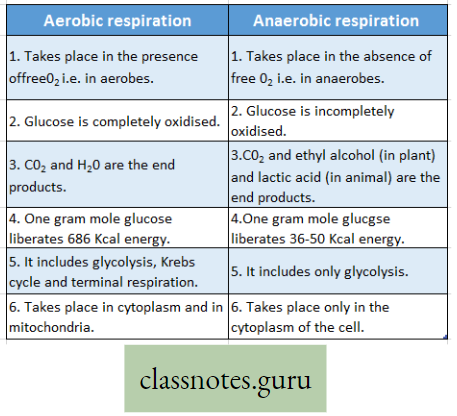
Difference between Aerobic and Anaerobic respiration :
Fermentation :
Fermentation Definition: Fermentation is the chemical process that involves decomposition of the complex organic substances into simpler ones and is brought about by the catalytic influence of the enzyme.
Occurrence: The process of fermentation takes place in the carbohydrate solution (glucose, sucrose, etc.). The organisms causing fermentation are yeast (fungus), Lactobacillus (a bacterium), germinating seeds, etc.
Process of oxidation and production of energy: Different types of fermentation are found in various organisms. Some common examples are mentioned here :
In animal cells: In the sarcoplasm (cytoplasm) of the muscle cells (sarcomere) the pyruvic acid is reduced to lactic acid and yields a small amount of energy in the presence of lactic Dehydrogenase(LHD) (reducing enzyme).
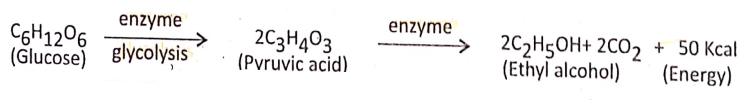
In the plant cell: The pyruvic acid in the presence of certain enzymes is partially oxidized into carbon dioxide, and ethyl alcohol and yields a small amount of energy.
In yeast cells (Alcoholic fermentation): Alcoholic fermentation is the extracellular anaerobic process that occurs with the help of the zymase enzyme of yeast, where glucose: is decomposed into ethyl alcohol and CO2 with the release of a certain amount of energy. Zymase
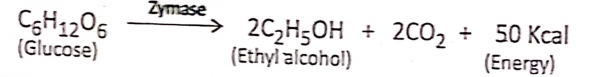
Economic importance of fermentation :
- Alcohol is useful in the wine industry, preparation of different medicines, tonics, biochemical; medicinal research, biological experiments, cosmetics, etc.
- Vinegar contains acetic acid which is produced by the acetic acid fermentation of Acetobacter bacteria.
- Curd contains lactic acid which is produced by lactic acid fermentation of lactobacillu bacteria.
- Lemon squash contains citric acid which is produced by citric acid fermentation of citrobacter bacteria.
For reference only:
Putrefaction and Fermentation
Putrefaction— It is the process of decomposition of organic materials, especially the anaerobic splitting of proteins by microorganisms. As a result, incompletely oxidized and ill-smelling compounds are produced.
Fermentation— It is the process of decomposition of complex organic compounds into simpler ones in the presence of microorganisms in living cells. It is brought about by the catalytic influence of the non-living highly complicated nitrogenous compounds known as enzymes. As a result, organic substances, waste gases, and heat are produced.
Difference between Anaerobic respiration and Alcoholic fermentation :
Steps Of Cellular Respiration And Cellular Sites Where They Occur
As mentioned earlier, there are three essential steps of cellular aerobic respiration: the first stage—Glycolysis, the second stage—Krebs cycle, and the third and final stage-Terminal Steps of glycolysis Respiration (ETC- Electron transport-chain).
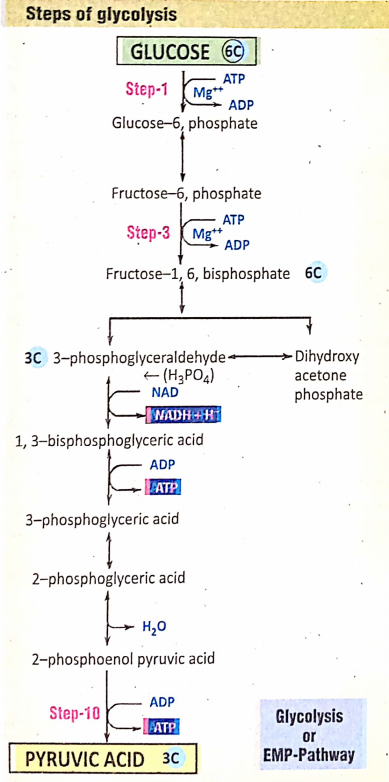
Stage 1. Glycolysis
Glycolysis Definition: Glycolysis is an anaerobic oxidative process by which the glucose, in the presence of certain enzymes in the cell cytoplasm, is converted into pyruvic acid.
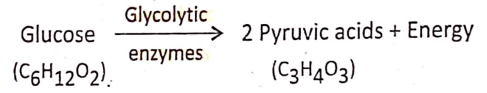
Different steps of glycolysis were discovered by three scientists Embden, Meyerhof, and Parnas. According to the first letter of their name, glycolysis is also known as the EMP pathway.
During glycolysis, no free O2 is needed so this is an anaerobic process. During glycolysis, there is a loss of Hydrogen so this is an oxidative process.
For reference only: Steps 1, and 3,10 of glycolysis are irreversible.
Site of glycolysis: Cytoplasm (outside mitochondria)
End products of glycolysis : 2 molecules of pyruvic acid + 2 molecules ATP + 2 molecules NADH2 (NAD = Nicotinamide Adenine Dinucleotide)
Stage 2. Krebs cycle
Krebs cycle Definition: It is the cyclic, aerobic, oxidative, biochemical pathway that occurs in mitochondria where the cycle starts from citric acid and through different enzymatic steps ends in oxaloacetic acid.
Site cfXrebs cycle: Mitochondria
End products of Krebs cycle: 2 molecules CO2 + 2 molecules H2O + 3 molecules NADH– – 1 molecule FADH- I molecule ATP.
- Alternate names of Krebs cycle: In 1937 the famous English biochemist Sir Hans Adolf Krebs first discovered this pathway.
- Thus according to his name, the cycle is called Krebs cycle. the first product of this cycle is Citric acid, thus Krebs cycle is also known as the citric acid cycle.
- The first formed acid (citric acid) contains three carboxyl – COOH; groups, hence it is also known as the tricart: acid cycle or in short TCA cycle.
For reference only:
In the Krebs cycle, there are three irreversible steps
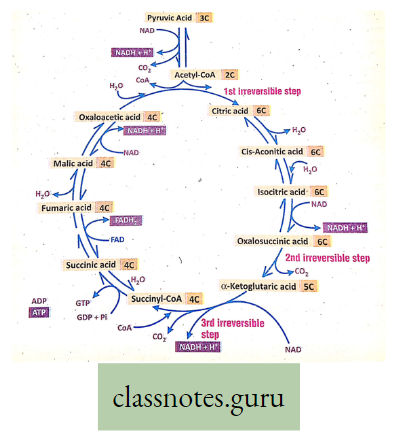
Stage 3: Terminal Respiration (ETC/ETS-Electron Transport chain or system) This is the last stage of aerobic respiration that follows the Krebs cycle.
- This process also occurs in mitochondria.
- This step consists of several electron carriers through which electron moves sequentially and ultimately to molecular oxygen at last.
- Electron comes from NADH2 and FADH2 formed in glycolysis and Krebs cycle.
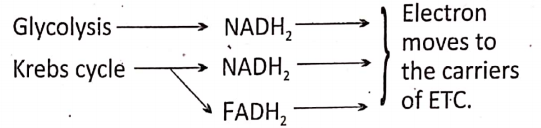
Now can you compare cellular respiration and breathing?
Significance Of Respiration
Release of Energy: Respiration is an energy-producing process. During photosynthesis, solar energy is stored as potential energy within the foodstuff. During respiration, this potential energy is converted into kinetic energy (i.e. released energy).
- Some amount of energy released during respiration is conserved and stored as ATP (a high energy-rich organic compound) in the mitochondria for future uses. This energy is used to perform various life processes in the living organism.
- The rest of the energy is released as heat energy to maintain the body temperature.
- Do you know—The light, which radiates from the glow-worm and some sea animals (bioluminescence) is a form of energy produced during respiration.
- Have you heard the term— Luciferin?
- The electricity which is produced from the electric ray fish is also a form of energy produced during respiration.
- The daily calorie requirement of an adult is 2500—3000 Kcal. It is obtained from ATP, produced during respiration.
Maintenance of oxygen (O2) and carbon dioxide (CO2) balance: The approximate normal percentage of O2 and CO2 in the air is 20-4% and 0-03% respectively. During photosynthesis, the plant body utilizes CO2 and releases O2.
- This may cause an increase in O2 and a decrease in CO2 in the atmosphere, but it does not happen.
- Because during respiration the living organism takes O2 and releases CO2.
- In this way, the process of respiration maintains an O2—CO2 balance in the environment.
Differences between Cellular Respiration and Breathing :
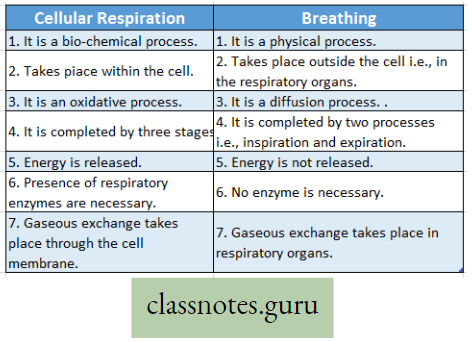
Differences between Photosynthesis and Respiration :
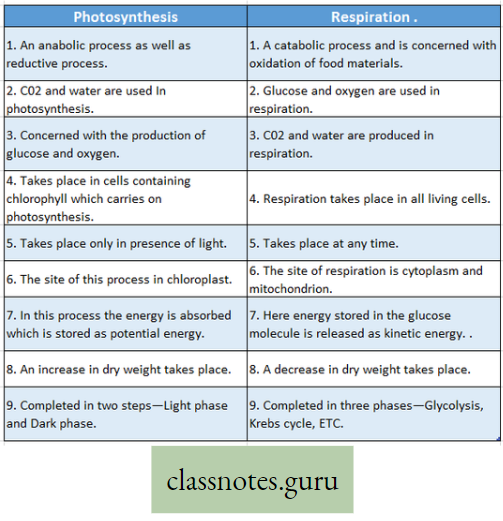
Concept of Nutrition
Introduction: All living organisms require food. II. Is needed for growth, movement, reproduction, energy production, healing and repair, disease resistance, and so on.
- A living organism either synthesizes or collects Its necessary food from the environment. The food may be utilized directly or indirectly.
- The process involved in the conversion of complex food into simpler products or products is termed digestion.
- The digested product (or products) is absorbed within the body and is transformed into constituent(s) of protoplasm by the process of assimilation.
- In addition to energy-yielding food like carbohydrates, proteins, and fats, minerals, vitamins, and water are also required for various life processes, All these essential substances are collectively called nutrients.
What is a nutrient?
The organic and inorganic materials that the living organism collects from nature to perform all the fundamental activities of the body are called nutrients, for protein, fat, carbohydrates, vitamins, minerals, and water.

Significance Or Importance of nutrition :
- Growth promotion, repairing wear and tear of the damaged tissues, and gaining energy to control the different metabolic processes are the main functions of nutrition.
- The potential energy stored within food is transformed into usable energy through nutrition.
- The different physiological functions of the living body like movement, locomotion, excretion, reproduction, etc. are controlled by utilizing this energy.
- Nutrition helps to develop the disease-resistant power of the living body.
- Nutrition helps to store food in the body for future use. From those stored foods (in the plant body mainly as starch and in animals as glycogen or animal starch and fat) the future energy is produced during a shortage of food.
- Nutrition plays a special role production of heat energy in the organisms to meet the caloric demand.
Can you explain the relation between malnutrition and frequent infection in the body? Explain in which tissues of the human body food is generally stored.
Malnutrition
A state of abnormal nutrition, that is caused by an insufficient or unbalanced intake of the basic nutrients or their impaired assimilation and utilization by the body is called malnutrition.
Types of Nutrition
Plant Nutrition: Plant nutrition is of two types Autotrophic plant nutrition and heterotrophic plant nutrition. The two phases of plant nutrition are synthesis and assimilation. The preparation of organic food matters is called synthesis and the incorporation of simple food matters within the protoplasm for different metabolic functions is called assimilation.
In autotrophic nutrition, plants obtain simple, inorganic food elements, in the form of liquid (H2O) and gases (CO2) and produce organic food matters, this type of nutrition is called holophytic nutrition.

Autotrophs and Heterotrophs:
Autotrophs Definition: Plants that possess chlorophyll and can prepare their food from CO2 and H2O in the presence of sunlight are called autotrophs.
Examples are green plants, Neem (Azadirachta indica), Mango [Mangifera indica), epiphytic plant Vanda (B. Rasna), etc.
Heterotrophs Definition: Plants that depend on other host plants or dead decaying organic matter for nutrition are called heterotrophs.
Examples—Fungi—Mucor, Agaricus.’
Different types of Heterotrophic plant nutrition: The nutrition in heterotrophic plants is called heterotrophic nutrition.
Parasitic Nutrition Definition: The process by which plants obtain their necessary nutrition from any other living plants or animals of different species (host) is called parasitic nutrition. Plants drawing in nourishment are called parasites and from which nutrients are drawn in are called hosts. Parasitic plants generally suck in the nutrients by haustoria or sucking roots from the host body.
Total Parasite or Holoparasite—Plants that draw their total nourishment from their respective hosts. Examples: Total stem parasite Example Cuscuta refiexa (dodder, swarnalata). Total root parasiteExample Balanophora dioica, Rafflesia Arnoldi (Largest flower in the world).
Partial parasite or Semi-parasite— Plants that can prepare food as they contain chlorophyll, but are dependent on the host plants for water and minerals. Examples Partial stem parasite Example Viscum album, Loran. thus longiflorus. Partial root parasite Example Santalum album (B. Chandan).
Some Notes On Parasitic Nutrition
Parasitism: Parasitism is a close association between two living organisms of different species which are beneficial to one (to the parasite) and harmful to the other (to the host). The parasite obtains food from the host and generally takes shelter.
Ectoparasites: The parasites that live on the outer surface of a host. Examples are Head lice, ticks, fleas, leeches, and phytophthora (fungus causing disease in potatoes).
Endoparasites: The parasites that live within the host. Examples are Plasmodium (a parasite that causes malaria), the tapeworm Taenia, and Swarnalata.
Saprophytic Nutrition Definition: The nutrition of certain non-green plants that draw their nourishment from the dead and decomposed organic substances formed as a result of the decay of plants and animals is termed saprophytic nutrition.
Total saprophytes: Saprophytes dependent fully on dead decaying organic substances for their nutrition are called total saprophytes. Examples: Total saprophyte Mucor, Penicillium, Agaricus, Monotropa uniflora (devoid of roots and chlorophylls).
Partial saprophytes: Green plants that depend partially on dead decaying organic matter for nutrition are called partial saprophytes. Examples: Partial saprophyte Pinus (they absorb organic substances with the help of certain fungus(mycorrhiza) growing on their roots.
Difference Between Parasite And Saprophyte :
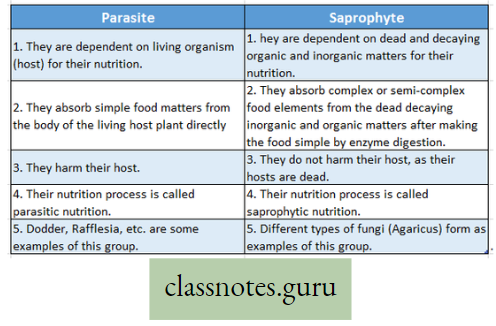
Differences Between Parasite And Symbiotic Nutrition:
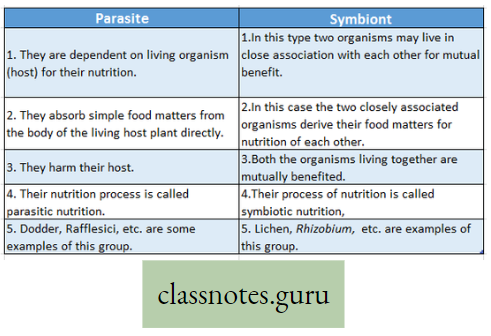
Symbiotic Nutrition: When two different species of organisms live in close association with each other for their mutual benefit in nutrition and shelter, the type of nutrition is called symbiotic nutrition. Each of the pair is called symbiont and the mode of their association is termed symbiosis.
- Examples: Symbiosis between bacteria and plant: Rhizobium leguminosarium residing in the root nodules of leguminous plants are capable of fixing atmospheric nitrogen forming nitrate and supplying it to the plant. In return, the plant provides carbohydrate food and shelter to the bacterium,
- Symbiotic association between plants and animals exists between Zoochlorella and Hydro, Lichen an association between an alga and a fungus (Symbiosis between plant and plant).
- The fungus protects the photosynthetic organism while algae prepare food by photosynthesis, which is shared by fungus. Thus both algae and fungal components are mutually beneficial.
Insectivory (Nutrition of insectivorous plants) (Carnivorous plant): Some green plants that are unable to draw nitrogenous nutrients from soil secure the same from insects. These plants are called insectivorous plants. The process of nutrition by which these plants take in insects and digest the protein part within the body with the help of certain enzymes is called insectivorous nutrition.
Examples: Pitcher plant (Nepenthes khasiana) Here the leaf blade is modified into a pitcher and the leaf apex into a lid. On the inner wall of the pitcher, some glands attract the insects. When the insect moves in, the lid.is closed, the insect is trapped and the body fluid of the insect is sucked in by the pitcher.
Imagine What Will Happen When The Pitcher Will Be Full Of Dead Bodies Of Insects:
Bladderwort (Utricularia stellaris): The leaf of this rootless aquatic plant is modified into a bladder-like structure and is supplied with a valve, that opens inwardly. The inner wall of the bladder bears digestive glands. It is a rootless aquatic insectivorous plant.
Sundew (Drosera indica): The upper surface of the leaf blade contains hair and glands secreting a sticky fluid to which insects get entangled.
Venus fly trap (Aldrovanda vesiculosa): A rootless aquatic plant that bears leaves with two halves. Sensitive trigger hair and digestive glands present on the upper surface of the leaf serve as a trap for insects.
Difference between Autophyte and Heterophyte :
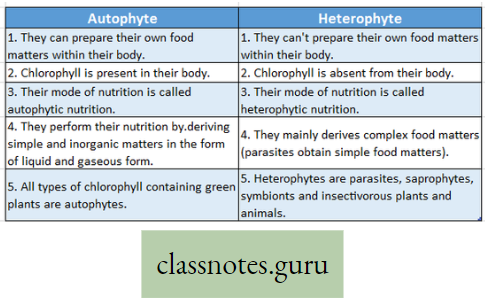
Animal Nutrition :
Animal Nutrition Definition: Animals are mostly heterotrophic and their nutrition is commonly known as holozoic nutrition where the animal ingests liquid or solid organic material.
Parasitic nutrition definition: An animal parasite is an animal that depends on the host body of different species for food and shelter causing harm to the host. This type, of nutrition, is called parasitic nutrition, Example Ascaris (Roundworm) is a parasite in the human intestine, Taenia solium (Tapeworm) is a parasite to the human intestine; Plasmodium (malarial parasite) is a parasite to human RBC and liver, etc.

Symbiotic nutrition (Mutualism) Definition: When two different species of organisms live together for the mutual benefit of nutrition it is called symbiotic nutrition and each of the species is called a symbiont.
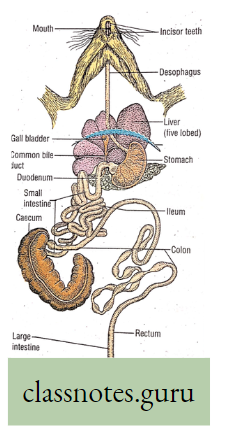
Examples:
- The symbiosis between animal and plant: Green Hydra and Zoochlorello (alga) living together where green Hydra provides protection and shelter to Zoochlorella. In turn, Zoochlorella performs photosynthesis and provides O2 to Hydra.
- Symbiosis between animal and protozoa: Termites feed on wood but they do not contain cellulase enzyme. In the digestive system of termites, there are a large number of flagellate protozoa named Trichonympha that can produce the enzyme cellulase which helps in the digestion of cellulose. Thus termites provide shelter to protozoa and protozoa provide cellulase to termites so both of them are mutually beneficial.
- Symbiosis between Ruminant mammals and bacteria: Ruminant mammals also need cellulase enzyme for digestion of cellulose which is produced by symbiotic Rumenococcus bacteria, who get food and shelter, in turn, from cattle.
- Saprozoic nutrition Definition: The nutrition where an animal gets its nutrients from decaying organic matter after digesting it with the help of exoenzyme is called saprozoic nutrition.Example.Housefly, Earthworm. Nematodes, etc.
- Coprophagy Definition: In rabbits, guinea pigs, etc., cellulose is digested in the large intestine where cellulase enzyme is released from the caecum (cellulase is synthesized by symbiotic cellulolytic bacteria in the caecum). This digested but unabsorbed cellulose goes out of the body through the rectum and anus, which is reingested by mouth. These digested cellulose droplets are softer and lighter having no smell and are directly eaten from the anus sometimes. (The actual fecal stool droplets are harder having a bad smell, that is egested from the body).
- Sanguinivory Definition: The animals who live on sucking blood from the body of other organisms are called sanguinivorous animals, Example. Mosquitoes are sanguinivorous as they suck blood from others; Leech sucks blood from cattle and other animals including man; vampire bat sucks blood (Haematophagy) of mammals.
Difference between Plant Nutrition and Animal Nutrition :
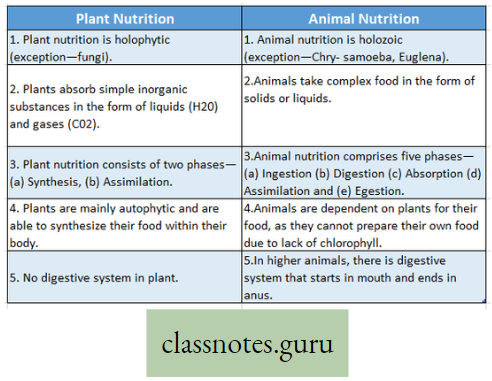
Do you know that Euglena is no longer considered an animal but is considered a Protista?
However, they contain chlorophyll and can perform photosynthesis.
Steps Of Holozoic Nutrition And Associated Parts Of The Alimentary Canal In Huma
Holozoic nutrition in man is completed in five stages and different organs are involved in each stage as follows:
Step 1: Ingestion: The process by which food is taken in the body of man is called ingestion. Associated organs Teeth, Tongue, Lips, and Hand.
Step 2: Digestion: The process by which insoluble, unabsorbable, macromolecule of food is broken into soluble, absorbable, micromolecule by the action of different enzymes is called digestion. Associated organs: Mouth, stomach, small intestine, and different digestive glands like salivary gland, gastric gland, pancreas, Intestinal gland, liver. The gland secretes digestive juice-containing enzymes.
Step 3: Absorption: The process by which soluble micromolecules of food are taken into the lymph or bloodstream of the body is called absorption. Associated organs Lymph vessels (Lacteals) and blood vessels of the villi of the small intestine mainly. Lacteals are the lymph capillaries in the intestinal villi.
Step 4: Assimilation: The process by which the absorbed food materials are incorporated into protoplasmic substances of body cells as well as some extracellular materials is called assimilation. Associated organs cells, tissues, blood, and extracellular fluid of the body.
Step 5 : Egestion: The process by which undigested and unabsorbed materials go out of the body is called an egestion. Associated organs Colon, Rectum, and Anus.
Do you know?
Sometimes, transportation is considered a step of holozoic nutrition. Transportation is more a part of circulation which is related to nutrition for the distribution of absorbed food materials all over the body. Can you explain why is the stool commonly yellowish in color?
Components Of Alimentary System And Their Role In Nutrition
Alimentary (Digestive) System Definition: The system concerned with the intake, digestion, and absorption of food and the elimination of unabsorbed food from the body is known as the digestive system.
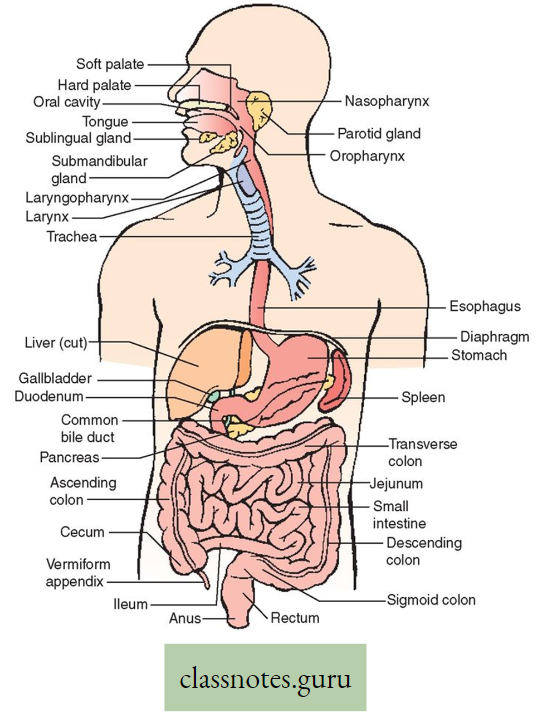
Structure: The alimentary system consists of the alimentary canal and several accessory or associated organs.
How can you compare the alimentary system to land digestive system?
Alimentary canal: Alimentary canal is a tube-like structure whose diameter is modified ( changed) in various parts of it. It is an open tube that begins at the mouth cavity and ends in the anus.
Mouth: It contains mainly tongue and teeth. The mouth is a cavity bounded by muscles other soft tissues and bones. It communicates with the exterior through the oral aperture between the lips and opens behind the pharynx.
Tongue: The muscular tongue, attached with hyoid and mandible bones. It is located on the floor of the mouth and bears many tiny projections known as papillae on its upper surface. Taste buds are present in some of these papillae.
Teeth: In each jaw bone of an adult, 16 teeth are lodged inside the tooth socket. These are called permanent teeth which consist of the following types 4 incisors, 2 canines, 4 premolars, and 6 molars. The child, on the other hand, may carry only up to 10 teeth in each jaw (a total of 20 teeth); these are called deciduous teeth or milk teeth and are later replaced by permanent teeth. Therefore dental formula in adult men is 12⁄2, C1/1, PM2/2, M3/3
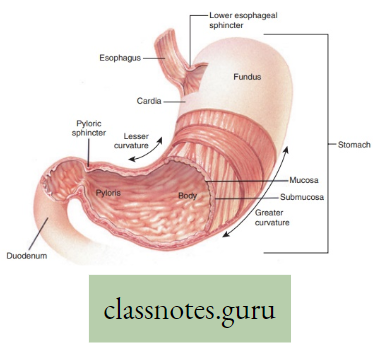
- Salivary glands: Three pairs of salivary glands are present near the mouth. They secrete salivary juice (saliva).
- Pharynx: Pharynx is a broad funnel-shaped cavity of about 10-12 cm long and lies behind the mouth. It serves as a common passage for both the alimentary tract and the respiratory tract. The mouth cavity, the nasal cavity, and the two eustachian tubes coming from the two middle ears, all open into the pharynx. The pharynx opens into the esophagus.
- Oesophagus: Oesophagus is a long narrow about 25 cm long tube. It is continuous with the pharynx above and passes through the thorax, running behind the trachea and the heart, and ultimately through the diaphragm and opens into the stomach.
- Stomach: The stomach is a dilated T-shaped sac-like structure of the alimentary canal situated in the abdominal cavity. The stomach consists of three parts The cardiac part Is the junctional part of the stomach and the esophagus. Here the smooth, thick circular band, called the cardiac sphincter, is present, the Pyloric part The other end of the stomach is called the pyloric part. To this end, the stomach becomes continuous with the small intestine. At the pyloric end pyloric sphincter is present middle part is known as the Body.
- Small Intestine: A small intestine is a coiled, narrow, muscular tube that is about 6 m long. It originates from the stomach and ends in the caecum. The first part of the small intestine is called the duodenum which is almost a ‘C’-shaped loop of about 20-25 cm long. Ducts of the pancreas and liver (gall bladder) open together into the duodenum to send their digestive juices. The second and last part of the small intestine are highly coiled tubes and are respectively known as jejunum (about 2-5 m long) and ileum (about 3-5 m long).
- Large Intestine: The large intestine is much shorter (about 1.5 m long) and broader than the small intestine. The caecum is the wide sac-like structure of the large intestine situated just around and below the ileocolic junction. It is only about 6-5 cm long, continuous with the colon above and blind below. A blind finger-like tube, called a vermiform appendix, opens into the caecum. The colon is a longer tube which is about 1-4 m in length. It ascends first from the upper end of the caecum to the ventral surface of the liver at the right side of the abdomen. It is known as the ascending colon. The colon then proceeding transversely towards the left side up to the spleen is known as the transverse colon. At last, the colon runs down along the left side of the abdominal cavity and is known as the descending colon ultimately forming a downward and slightly curved loop of the pelvic colon (Sigmoid colon), which continues into the rectum. The latter is the dilated and short last part of the colon and opens to the exterior through theanus.
Function: The large intestine absorbs mainly water from the food remnants, stores the remnants and afterward forms a stool. It is eliminated to the exterior through the anus.
Accessory Organs: Certain glands such as salivary glands, liver, and pancreas form the accessory organs.
Salivary glands: Three pairs of salivary glands are present in the mouth cavity.
Parotid gland: It is located at the corner of the mandible and ear lobe on each side of the face.
Submaxillary or Submandibular gland: It is surrounded by the mandible and Sublingual gland It is situated below the tongue. Salivary ducts, carrying the saliva, open into the mouth near the molar teeth and also on the floor of the mouth.
Function: Salivary glands secrete saliva.
Parotid is the largest salivary gland but maximum saliva is secreted from the submaxillary or (submandibular gland).
Liver: The liver is the largest gland of the body. It is situated below the diaphragm and above and on the right side of the stomach. It is divided mainly into the right and left lobes. The gall bladder is an elongated, pear-shaped, muscular elastic sac about 7-8 cm long, lying under the lower surface of the liver. Bile secreted by the liver comes out through the hepatic and cystic ducts and remains stored in the gall bladder. From the gall bladder, bile passes through the common bile duct into the duodenum.
Function:
- The liver secretes bile.
- It acts as an important metabolic as well as an excretory organ of the body.
Pancreas: The pancreas is a mixed gland that is about 20 m long and 4cm broad situated beneath the stomach within the ‘c’-shaped space of the duodenum.
Functions:
- It is composed of pancreatic alveoli or acini (exocrine part) which secretes pancreatic juice (digestive juice),
- The a and (5 cells of islet of Langerhans tissue of the pancreas (endocrine part) secretes two hormones in the blood, glucagon and insulin respectively.
General Functions of Alimentary System: The main functions of the alimentary system are
- Ingestion: Food is taken by the mouth cavity,
- Digestion: complex food is converted to simple food by the process of digestion in the stomach and small intestine. Different digestive glands take part in this process,
- Absorption: The digested simple food is absorbed by the villi of the small intestine of the alimentary canal,
- Egestion: Temporarily stored undigested food in the rectum is eliminated as stool.
Different Parts Of the Digestive System And Their Role In Nutrition :
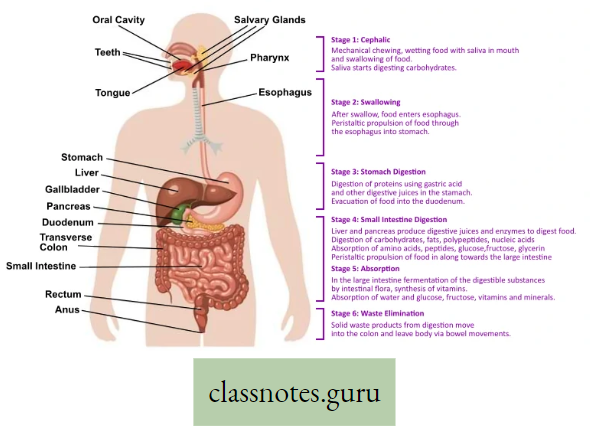
How can you explain that from the viewpoint of digestion, the pancreas is more important than the liver?
Overview of Digestion
The whole process of digestion is completed by two major events:
- Mechanical (physical)events
- Biochemical (Enzymatic)events.
1. Mechanical (Physical) Events during digestion:
- Chewing (Mastication): It is a physical process by which solid food breaks into small fragments by teeth. These smaller fragments of food mixed with saliva form bolus that are easily swallowed. The total surface area of fragmented food particles is increased so that digestive enzymes can act better.
- Swallowing (Deglutition): The physical process (movement) by which food bolus passes
from the mouth cavity into the stomach through the esophagus is called swallowing or deglutition. It is a complex inborn reflex process. - Peristalsis: It is the slow but lateral wavy movement of the alimentary canal consisting of waves of alternate contraction and relaxation by which food materials move downward in the alimentary canal. Peristalsis helps in digestion by proper mixing of food with digestive juice. It also helps in the absorption of digested food and the digestion of feces.
Peristalsis:
A slow but lateral progressive wave-like movement that occurs involuntarily in hollow tubes, like the esophagus of the alimentary canal. It consists of the contraction of muscles above the distention, with the relaxation of the region immediately below the distended region. This causes the contents of the tube to be forced down the tract. Movement of food in the form of bolus through the alimentary tract takes place by peristalsis. Can you explain why you feel abdominal pain if you run after a full lunch?
2. Biochemical (Enzymatic) events during digestion: Protein, fat, carbohydrates, and other macromolecules must be properly digested into soluble micromolecules so that they can be properly absorbed. During this digestion, there are profound chemical changes. Various digestive enzymes are responsible for biochemical reactions at different parts of the alimentary canal.
LHydrolysis: It means the breakdown of chemical bonds of macromolecules of food by the addition of water during enzymatic digestion.
Example: Sucrose(C12H22O11)+H2→Gluscose(C6H12O6)+Fructose(C6H12O6)
Now, do you understand why we should drink water after lunch or dinner?
Digestive Enzymes
Digestive Enzymes: Enzymes taking part in digestion are called digestive enzymes.
They are of three types
- Proteolytic enzymes or Protein-hydrolysing enzymes: These enzymes hydrolyze proteins into polypeptides and amino acids, for Example.Pepsin, Trypsin, Chymotrypsin, Erepsin, Renin.
- Lipolytic enzymes or Lipid hydrolyzing enzymes: These enzymes hydrolyze lipids (fats) into fatty acid and glycerol Example. Lipase (Gastric Lipase, Pancreatic Lipase, Intestinal lipase).
- Amylolytic enzymes or Carbohydrate hydrolyzing enzymes: These enzymes hydrolyze the carbohydrate into monosaccharides (glucose), for example, Amylase, Sucrase, Lactase, Maltase, etc.
Bile does not contain any digestive enzyme but it breaks down bigger molecules o smaller droplets to increase the surface area for the action of lipase. This is called emu s of fat.
Digestive enzymes with examples and their role in digestion
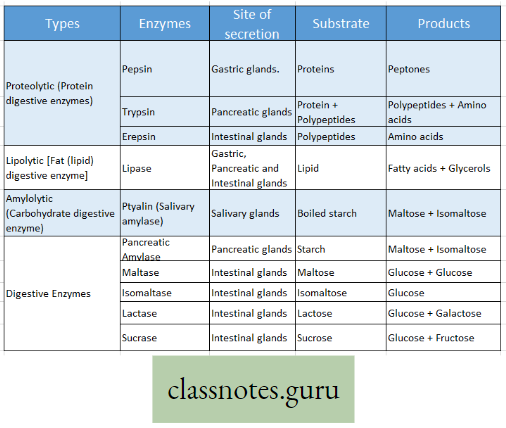
Try To Reflect:
- The pancreas is a mixed gland having exocrine and endocrine parts. All pancreatic enzymes are secreted from the exocrine part of the pancreas called acini. (Endocrine part secrete hormones).
Do you know the name of the endocrine part of the pancreas?
- Salivary gland enzymes react in the neutral medium; Gastric enzymes react in an acidic medium; Intestinal enzymes react in an alkaline medium. HCL is secreted from the stomach. Hence medium of the stomach is acidic. The alkaline substance is secreted from bile and the pancreas—so the intestinal medium is alkaline.
Try to explain why the ‘rice feeding ceremony’ (Annaprasan) is held after 6 months of a baby.
Difference between Chyme and Chyle :
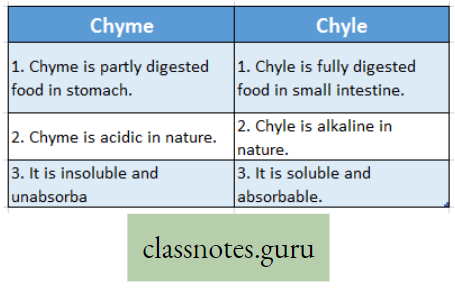
The action of different enzymes present in the digestive juices of the alimentary canal:
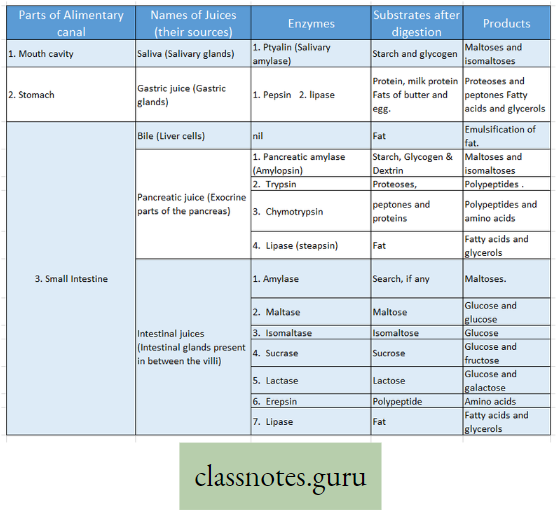
Overview of Absorption Assimilation and Egestion
- Absorption: The process by which the water-soluble digested end products of carbohydrates, proteins, fats, and other ingested food are transported from the alimentary canal into the lymph or bloodstream is called absorption.
- Site of absorption: Absorption occurs chiefly through the small intestinal epithelial cells present on the surface of the villi.
- Process of absorption: After absorption through epithelial cells of villi, molecules like amino acid, monosaccharides, vitamins, minerals, and H2O are transported by blood capillaries, whereas fatty acids, triglycerides, etc. are transported by lymph in lacteal vessels. The process of absorption may be active or passive. It may be also carrier-dependent or carrier-independent.
2. Assimilation: In this process, the absorbed food material is transformed into intracellular protoplasm as well as extracellular material.
- site of assimilation: This process occurs in all cells and tissues of the body.
- Process of assimilation: Suppose, you have eaten a certain amount of rice that contains starch. This starch is digested into glucose and is distributed all over the body by blood. In different cells of the body, the same glucose molecule may be utilized in different ways. For example, Glucose may be transformed into glycogen In the liver and muscle or may undergo glycolysis in neurons, WBC, or other cells, or may be converted to other biomolecules like amino acid, glycerol, or fatty acid in some other cells. This conversion is assimilation which depends on the needs of the cell.
3. Egestion: In this process, undigested food materials go out of the body as feces.
- Site of egestion: Colon, rectum, and anus.
- Process of egestion: As the rectum is gradually filled up with stool, there will be an autonomous reflex that results in the contraction of the rectal muscle. The anal aperture opens and the fecal matter is discharged outside the body. Can you write a few points of difference between excretion and egestion?
Metabolism
Metabolism Definition: Metabolism is the total of biochemical reactions in the living cell of an organism, which involves the synthesis of complex substances from simple ones with the utilization of energy and the breakdown of complex substances into simpler ones with the release of energy. Now try to compare digestion and metabolism.
Anabolism Definition: It is the constructive process by which complete molecules are formed into simpler units with the help of energy resulting in increases in the dry weight of the organism.
Examples: Formation of glycogen from glucose, formation of protein from amino acids, photosynthesis, etc.
Catabolism Definition: It is the destructive procedure by which complex molecules are broken into simpler units with the liberation of energy resulting in a decrease in the dry weight may the organism.
Example: By respiration, glucose is broken into CO2 and H2O with the release of energy.
Anabolism and catabolism are opposite processes of reversible chemical reactions which may be represented as follows:
Small Molecule → Large Molecules
Difference between Anabolism and Catabolism :
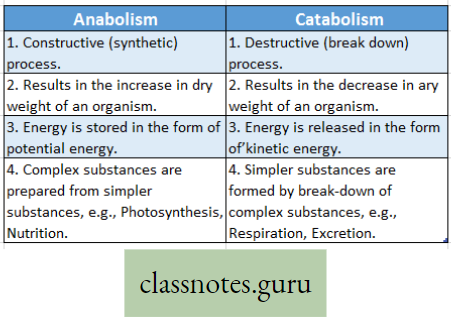
Significance Of Metabolism:
- The functional activities of the body depend on various metabolic reactions continuously taking place within the cell of an organism.
- Various anabolic reactions result in the formation of macromolecules like fat, glycogen, etc. that are stored in respective organs Example fat in adipose tissue, glycogen in the liver and muscle, etc.
- Many enzymes participate in all metabolic reactions resulting in intramolecular or intermolecular transformation.
The growth of an organism occurs when anabolism exceeds catabolism.
Difference between Digestion and Nutrition :
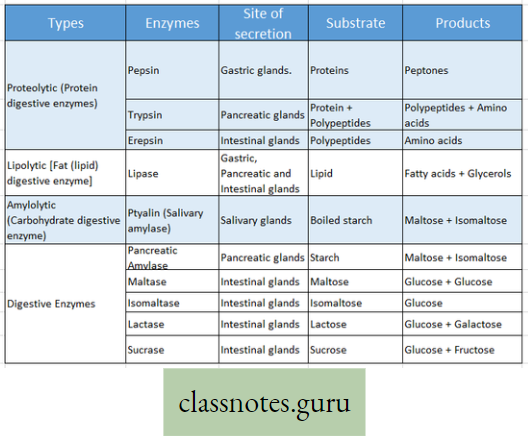
In a tabular form, can you mention what are the metabolic end product and digested end product of protein, fat, and carbohydrate?
Digested end product and Metabolic end product:
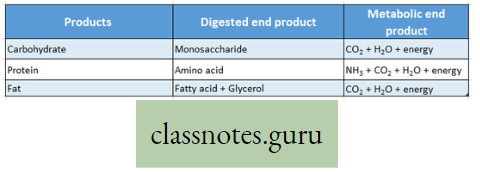
Dietary Food Intake, Energy Requirement, And Associated Problems
Concept of a Balanced Diet:
Balanced diet Definition: A diet that contains carbohydrates, fats, proteins, vitamins, and mineral salts in correct proportion to meet the energy requirements of the working capability of an organism is known as a balanced diet.
- The concept of a balanced diet varies from one group of animals to others, For Example, (Milk contains almost all important constituents except iron and vitamin C).
- Milk serves as a balanced diet for children but not for adults. The general ratio of a balanced diet of carbohydrates, protein, and fat is 4:1:1.
- A balanced diet depends on age, sex, height, weight, type of work, climate, and some other conditions.
Basal metabolic rate (B. M. R.) :
- Direction: The rate of the amount of heat required for performing the vital activities by
a subject who, though awake, is lying in a state of maximum physical and mental rest under comfortable conditions (of temperature, pressure, and humidity), 12-18 hours after meal is called basal metabolic rate. - Normal BMR: It is usually expressed as large calories (Kcal) per square meter (m2) body surface per hour.
- In adult male the BMR is40 Kcal/m2/hour
- In adult females, the BMR is 37 Kcal / m2 / hour.
BMR can be measured by an instrument named the Benedict Roth apparatus.
Factors affecting B. M. R.: Age, sex, body surface area, climate, diet, habit, hormones, etc.
Significance: BMR signifies the metabolic rate and function of different vital organs of the body.
Calculation of energy required daily for an adult human :
Calorie (cal) is the unit of energy and is defined as the amount of heat energy that can raise the temperature of 1 gm of water from 15°C to 16°C. In the biological system calorie is used as Kilocalorie (Kcal).
For daily calorie requirements of an adult healthy man, weighing about 60 70 kg and doing moderate work is about 2500 3000 Kcal. This amount of energy is derived from energy-yielding food like carbohydrates, proteins, and fats. On complete oxidation (combustion) these types of food yield the following amount of energy :
1 gram of carbohydrate → yields 4-0 Kcal
1 gram of protein → yields 4-1 Kcal
1 gram of fat →yields 9-3 Kcal
Therefore, a man (60-70 kg body weight) doing moderate work requires about 3000 kilocalories. Thus to fulfill this calorie requirement he should consume the following amount of carbohydrates, proteins, and fats in a ratio of approximately 4:1:1. (Carbohydrate: Protein: Fat) along with vitamins, minerals, and water to satisfy his or her daily calorie (energy) requirements.
Carbohydrates 415 gram = 415 x 4-0 = 1660 Kcal
Proteins 100 gram = 100 x 4-1 = 410 Kcal
Fats 100 gram = 100 x 9-3 = 930 Kcal
Total energy yields = 3000 Kcal
Metabolism-related problems in the human body: So many problems may develop in the body due to metabolic disorders. Some of the common examples are mentioned below:
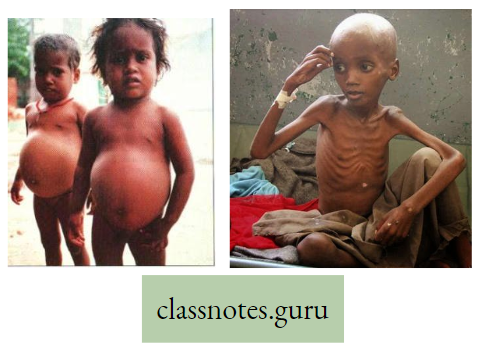
Obesity: This is defined as a body weight 10 to 20 percent above a desirable standard as the result of an excessive accumulation of fat. Obesity is hazardous to health and may cause— heart disease (cardiovascular disease), high blood pressure (Hypertension), Lung disease (Pulmonary disease), Diabetes, Arthritis, etc.
Kwashiorkor: It is Protein Calorie Malnutrition (PCM) when someone takes sufficient food but not a balanced diet, taking 2nd class protein (incomplete proteins). It may cause swelling of the abdomen, enlarged liver, lethargy, dry skin, loss of hair, and r sometimes mental retardation.
Marasmus: This is also Protein- Calorie-malnutrition (PCM). Here someone does not get sufficient food (inadequate intake of both protein and nonprotein food). This may result
in retarded growth, being underweight, dry r skin, and hair, etc. Fig. io.io: A-Kwashiorkor, B-Marasmus.
Phenylketonuria (PKU): It is an inheritable genetic disorder that results in the metabolic error of the amino acid phenylalanine. This may cause mental retardation.
Cystic Fibrosis (CF): This is an inherited disease of metabolic disorder of exocrine glands that affects the pancreas, respiratory passage, salivary glands, and sweat glands.
Circulation
Circulation Introduction: There are several essential physiological functions of the body like growth, nutrition, respiration, excretion, etc.
- For all these functions, free movement of respiratory gases (O2 and CO2), enzymes, vitamins, hormones, nutrients, etc. is urgently needed.
- It is also very much essential to remove excretory toxic substances (like urea, uric acid, etc.) produced by metabolism. For all these, a suitable liquid medium is required.
- The major circulating medium in animals is blood and lymph. Movement of various substances dissolved in the liquid medium throughout the body is called circulation.
What is Circulation: The process by which various necessary substances like nutrients, respiratory gases (O2 and CO2) hormones, enzymes, minerals as well as excretory products, etc. are transported to respective organs of the body is called circulation.
Concept of Circulation
1. Importance (Functions) of Circulation :
Circulation of blood acts mainly as a transport medium and helps the living body to perform several important functions as stated below:
- Transportation of nutrients—The digested food substances are absorbed from the digestive canal and carried by blood plasma or lymph to all living body cells to provide nutrition.
- Transportation of respiratory gases—The oxygen (O2) for respiration is carried from the lungs to the tissue cells and carbon dioxide (C02) produced in the tissue cells is carried by blood (RBC) to the respiratory organs from where it goes out.
CO + Hb → Carboxyhaemoglobin O2 + Hb →Oxyhaemoglobin ;
CO2 -t- Hb →Carbaminohaemoglobin.
- Transportation of enzymes and hormones—The enzymes, hormones, etc. are essential for the proper functioning of the living body. They are synthesized and liberated from several sources and are carried by blood (plasma) to the respective sites of action.
- Transportation of waste products—Various toxic metabolic waste products formed as a result of cellular metabolism are liberated from the body through circulation (plasma).
- Helps in storage—The essential substances or other organic matter formed in excess within the living body are carried by circulation (plasma) to the storage organs for future use.
- Maintenance of blood pressure—Blood circulation maintains blood pressure and thus regulates the exchange of fluids and ions between blood and tissues.
- Transportation of minerals—Various minerals are transported by circulation (plasma) from one part of the body to another. ‘
- Generation of heat—By circulation the cells receive food (respiratory substrate) and O2 and then the cells produce heat energy by respiration.
2. Components of the circulatory system of humans: The blood-vascular system of man consists of blood, blood vessels, and the heart. The heart is the pumping organ. The contraction of the heart is systole and that of expansion is diastole. Due to this pumping action, a pressure gradient is generated by which blood flows all over the body through blood vessels—arteries, veins,s and capillaries.
Are you aware of the fact that a parallel fluid (besides blood) flows all over the body?
Difference between Artery and Vein :
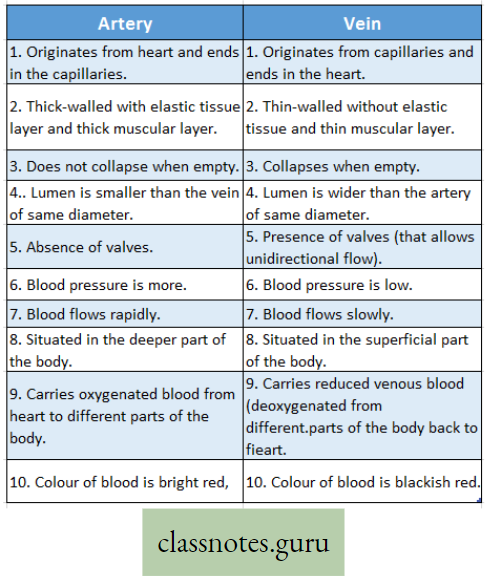
Difference between Pulmonary artery and Pulmonary vein :
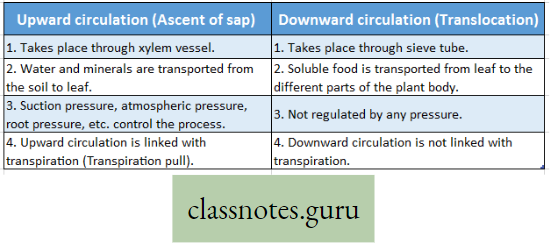
Types of Circulation (The basic idea of open and closed circulation
Two types of blood vascular systems are found in animals; such as—the open blood vascular system and the closed blood vascular system.
Open circulation in Cockroach Definition: The system in which blood from the heart passes through the blood vessels at first and then opens into the body cavity (hemocoel) is called open circulation.
The open circulatory system consists of the blood vessels (without capillaries) and the hemocoel or lacuna or sinus. In this type of circulation blood flows partly through the blood vessels and partly through the body cavity or hemocoel. As the blood flows out of the blood vessels into the lacunae or sinuses or hemocoel, it comes in direct contact with the tissue cells.
Examples: This type of circulation is found in many invertebrates like cockroaches, grasshoppers, prawns (Arthropoda) snails, union, etc. (Mollusca).
Closed circulation in Human Definition: The system in which the circulating blood always remains within the heart and blood vessels but never flows into the body cavity is called closed circulation.
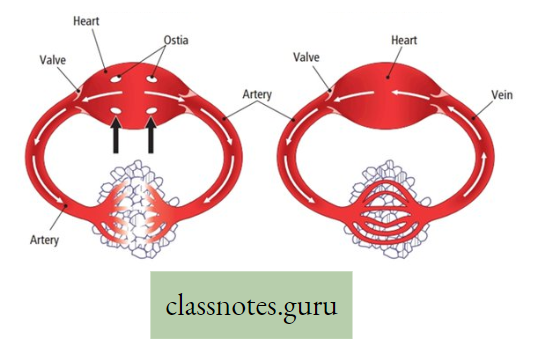
The closed blood vascular system consists of blood vessels and the heart. In this type of Open Circulation and Closed Circulation in man. circulation the blood is pumped out by the heart to arteries and then to capillaries present in the tissues. From the capillaries, blood returns to the heart again through the large veins. Thus blood never comes out of the blood vessels.
Examples: This type of circulation is found in invertebrates like earthworms and in all vertebrates like fish, Amphibia, Reptiles, Birds, and Mammals,
Difference between Closed and Open circulation :
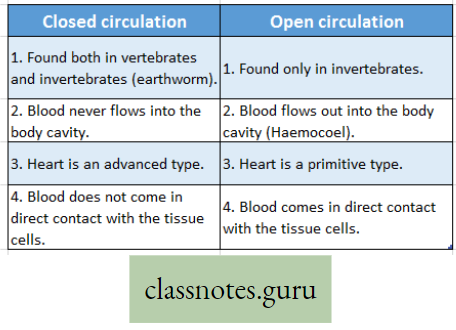
Can you explain the advantages and disadvantages of open and closed circulation?
Different Body Fluids Their Location And Function (Role)
Blood Definition: It is the fluid connective tissue and is the most important transporting medium of the body. It consists of plasma and blood corpuscles.
Location: Blood is present in the blood vessels and heart of the body.
Role (Function): It carries various materials from one part of the body to the other. (Details of the composition and function of blood will be explained later)
Lymph Definition: The alkaline clear, pale yellow color watery modified tissue fluid found in the lymphatic vessels and lymph glands (nodes) is called lymph.
Location: Lymph is present in lymph vessels and lymph glands of the body. The total amount of lymph in man is about 1-2 litres.
It contains 94% water and 6% solid substances. Solid substances are both organic (proteins, carbohydrates, fat, non-protein nitrogenous substances) and some inorganic substances.
Role (Function) of lymph:
- Acts as transport medium: Lymph transports nutrients and oxygen and supplies them to the tissue cells where the blood cannot reach directly.
- Transport of fatty materials: The lymph absorbs fat from the intestine.
- Drainage of tissue fluid and metabolites: Lymph drains away excess tissue fluid and metabolites. In this way, it keeps the volume and composition of tissue fluid constant.
- Defensive functions: The lymphocytes and monocytes present in the lymph protect the body with their phagocytic properties.
Difference between Blood and Lymph :
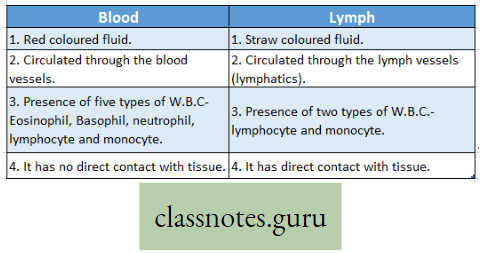
You will be surprised to know that there are approximately 6-10 liters of lymph in the body, compared to 3-5 – 5 liters of blood.
Sweat Definition: lt is the fluid secreted by the sweat glands in the skin of our body.
- Location: Sweat glands are present in the dermis of the skin of our body. Secretion of sweat depends on the weather, activity of the body, mental stress, etc. The average sweat rate in an adult is approximately 2-4 liters per day but less in children.
- Role (Function): Sweating has many functions in the body but primarily it is responsible for thermoregulation water in sweat absorbs latent heat of evaporation from the skin so the body cools down. What do you understand by ‘panting’ in a dog?
Urine Definition: It is a liquid by-product of the body secreted by the kidneys, temporarily stored in the urinary bladder, and is eliminated through the urethra outside the body.
- Location: Urine is filtered from blood in the nephron of the kidney, transported from the kidney by the ureter, and stored in the urinary bladder. Average urine production in adults is about 1-2 liters per day. However, urine volume depends on hydration, activity, environmental factors, etc.
- Role (Function): It is the primary process of elimination of toxic metabolic waste of the body.
Why is urine slightly yellowish?
CSF (Cerebro Spinal Fluid) Definition: It is a clear colorless body fluid found in the brain and spinal cord.
- Location: CSF is produced in the choroid plexus of the brain approximately 500 ml per day.
- Role (Function): CSF maintains buoyancy of being. ain, protection of the brain, transport of various substances between blood and brain, removal of metabolic waste, etc. CSF serves the function of lymph in the brain.
Synovial Fluid Definition: This is a viscous fluid found in the cavity of the synovial joint which is a movable joint of two or more bones.
- Location: The inner membrane of the synovial joint is called the synovial membrane which secretes synovial fluid into the joint cavity. There are two types of cells in the synovial membrane—type A and type B. Type A is derived from blood monocytes whereas type B produces synovial fluid. Many enzymes and acids are present in this fluid.
- Role (Function): The principal role of synovial fluid is to reduce friction (acts as a lubricant) between the articular cartilage of synovial joints during movement.
Tissue Fluid Definition: Tissue fluid (Interstitial Fluid) is a solution that is present in the intercellular space or tissue space in our body.
- Location: On average, a person has about 10 liters of tissue fluid which is filtered from blood plasma in the capillaries.
- Role (Function): Interstitial fluid flows over the cells and tissues. This provides a means of delivering materials to the cells, intercellular communication, as well as removal of metabolic waste.
Intracellular Fluid (ICF) Definition: The fluid that is present inside the plasma membrane of a cell is called intracellular fluid. It is also known as the cytosol or cytoplasmic matrix.
Cytosol = Cytoplasm—Cellular organelles
- Location: In the eukaryotic cell of the human body, the ICF is present within the cell and is part of the cytoplasm. The cytosol is a complex mixture of organic and inorganic substances. However, more than 70% of the cytosol is water but this percentage varies in different types of cells.
- Role (Function): ICF performs almost all intracellular metabolic activities and life processes like the flow of impulses through neurons, division of cells, protein synthesis (translation), glycolysis, glycogenesis, gluconeogenesis, and so on.
Composition Of Blood
Composition Of Blood Definition: Blood is an opaque, slightly alkaline, reddish-colored, salty taste, viscous specialized fluid connective tissue. The total volume of blood in an adult human is approximately 5 liters.
Color of the blood: The color of human blood is red. It is due to the presence of the respiratory pigment called hemoglobin.
Do you know plant sap is acidic but animal plasma is alkaline?
Why is blood called “specialized fluid connective tissue”?
- Blood consists of different types of cells (RBC, WBC, etc.) so it is called ’tissue’.
- Blood contains a huge matrix (plasma) and also it flows from one part of the body to another thus connecting different parts of the body it is called ‘connective tissue’.
- As it flows to different parts of the body—so it is called ‘fluid’.
- In the blood matrix (plasma), there is no connective tissue fiber (like collagen, etc.). So blood is fibreless. This is the specialty of blood. So it is called ‘specialized connective tissue’.
Composition of blood
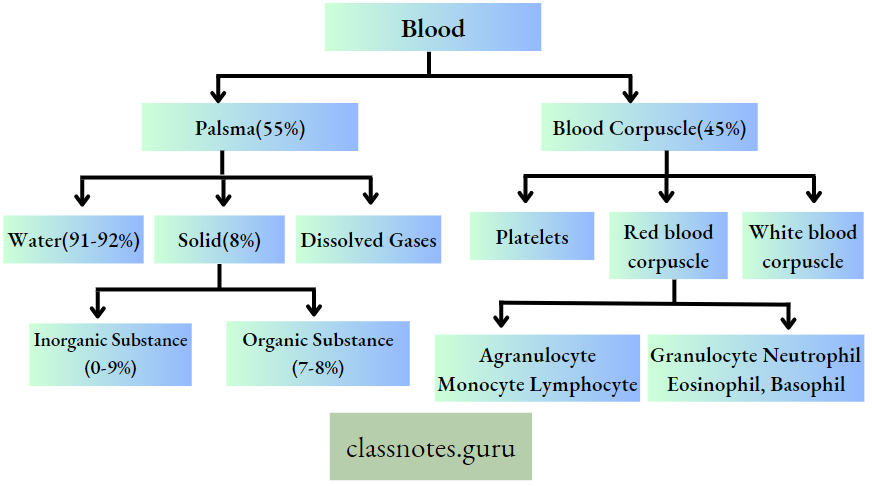
For Reference Only:
Why blood cells are called corpuscles? Blood cells (especially RBC) are called corpuscles since they are not typical cells For Example RBC is a living cell but nonnucleated and cannot divide. WBC exhibits pseudopodial or amoeboid movement. Platelets are cell fragments, also nonnucleated.
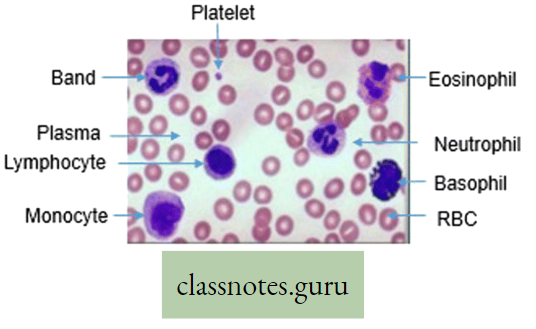
- Plasma-Definition, composition, and Functions Definition: Plasma is the pale yellow-colored fibreless intercellular alkaline fluid in which blood cells remain suspended.
- (Plasma = whole blood-cellular part of the blood)
- Composition of plasma: The total amount of plasma is 55% of total blood or about 6% of total body weight. It consists of 91-92% water and 8-9% solid substances. The solids remain dissolved in the plasma and are mainly of two types:
Inorganic constituents—The main inorganic constituents are sodium, potassium, calcium, magnesium, phosphorus, chlorine, bicarbonate, iron, copper, etc.
- Organic constituents—The important organic constituents are carbohydrates and glucose.
- Protein—Albumin, globulin, prothrombin, and fibrinogen. Fa Neutral fat, phospholipids, cholesterol, etc. Non-protein nitrogenous substances (NPN)—Urea, uric acid, ammonia, creatine, creatinine, etc. Other organic substances— Enzymes, hormones, bilirubin, antibodies, waste products, etc.
- Gases— Oxygen and carbon dioxide (in dissolved state).
Functions of plasma :
- It helps in blood clotting. The fibrinogen and prothrombin are two proteins which help in blood clotting.
- The liquid part of the plasma helps in the transport of various substances like digested food, vitamins, minerals, respiratory gases (02 and C02), hormones, etc.
- Plasma acts as a protein reservoir.
- It acts as a buffer that maintains the acid-base balance of the blood.
- It maintains the viscosity and colloidal osmotic pressure of blood. 6. Plasma helps to synthesize antibodies.
- Circulating plasma helps to regulate body temperature (thermoregulation).
- It takes part in the transport of O2 (as a physical solution) and CO2 (as a bicarbonate compound).
- It transports metabolic wastes like urea, uric acid, etc. to form urine in the kidney.
Why do we feel drowsy after lunch or dinner?
Blood Corpuscles (Formed elements of blood):
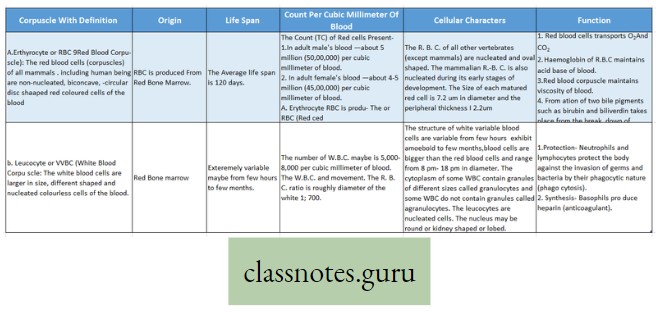
For reference only:
Flow chart of different stages of development of RBC:
Haemoncytoblast → Proerythroblast→Erythoblast→Reticulocyte→Erythrocyte
Vit B12 (cyanocobalamin) and Vit B9 (Folic acid) are responsible for the maturation of RBC. Haemoglobin is synthesized in the proerythroblast (Pronormoblast) stage during the development of RBC.
For reference only:
What is the chemical nature of granules in granulocytes?
Granules in granulocytes are clusters of hydrolytic enzymes that help in destroying the phagocytosed substance.
For reference only :
Diapedesis: The passage of WBC by pseudopodial movement through the intact capillary wall into the surrounding tissue is called diapedesis.
- That’s why WBC (larger than RBC) may come out of blood into intercellular space but RBC (smaller than WBC) cannot come out.
- Platelet count drops in Dengue and cancer chemotherapy.
- Probably you know less number of RBCs than the normal value is called Anaemia. What is called when the count of RBC increases?
- Do you know the RBC of the camel is nucleated? Since matured human RBC is non-nucleated. how can you justify whether it is a living cell or a dead cell?
Types of WBC (White Blood Corpuscle):
According to the presence and absence of granules in the cytoplasm, the white blood corpuscles are of different types.
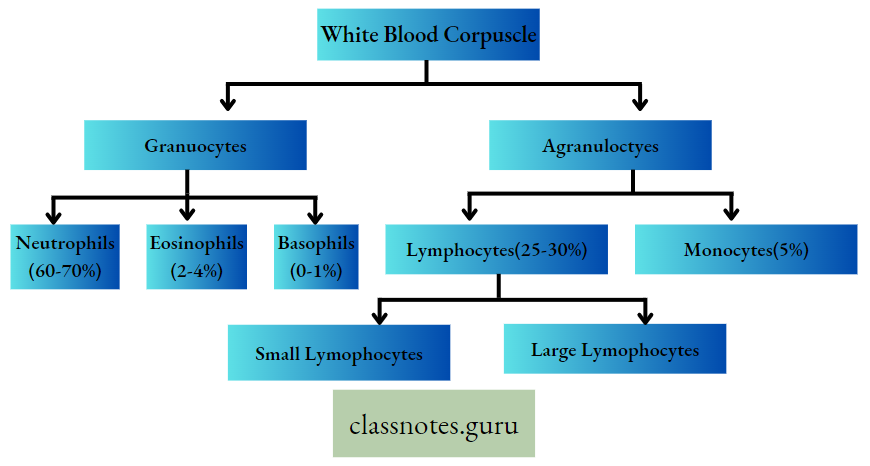
You will be surprised to know that immature lymphocytes are larger and matured lymphocytes are smaller in size.
Functions of different types of WBC:
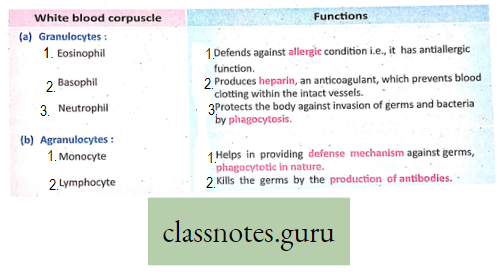
Monocytes and neutrophils are together called phagocytes since they can perform phagocytosis.
Difference between RBC and WBC:
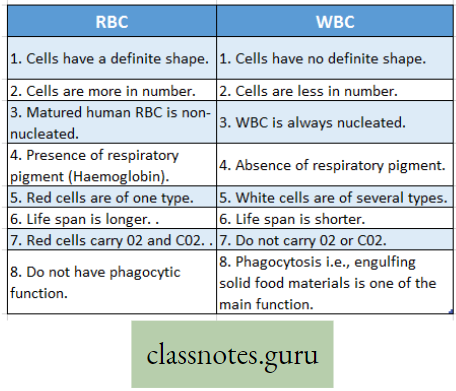
Respiratory pigments Description: Those pigments, present in the red blood cells or the plasma, that help in gaseous transport between respiratory organs and tissue cells called respiratory pigments.
Types: Two main types of respiratory pigments are found in the animals i.e., hemoglobin and hemocyanin.
Haemoglobin:
Description: Haemoglobin is an iron-containing red pigment of blood.
Composition (Chemistry): Haemoglobin is a protein (chromoprotein) and (metalloprotein) in nature. It consists of haem (4%) and globin (96%). Haem is an iron-containing part, whereas globin is a simple protein. Haemoglobin is called chromoprotein as it has a red color; it is called metalloprotein since it contains iron metal.
Location: Haemoglobin is found in the red blood cells of all vertebrates and the plasma of earthworms.
Normal amount: In normal conditions, 100 ml of human blood contains about 14-5 gm. of hemoglobin in adult males but in females slightly less (due to less number of RBC).
Functions of hemoglobin:
- Hemoglobin helps to carry oxygen from the lungs to tissue as oxyhemoglobin in the blood. It helps to carry carbon dioxide from the tissue to the lungs as carbamino hemoglobin. O2 is carried by the haem part and CO2 is carried by the globin part.
- Haemoglobin acts as a buffer which maintains the acid-base balance of the blood.
- It forms different pigments, for example, bilirubin, biliverdin, etc. after a metabolic breakdown.
For reference only:
CO (Carbon monoxide) combines with hemoglobin to form carboxyhemoglobin. One rich source of CO is cigarette smoke.
Haemocyanin :
Description: Haemocyanin is a copper-containing pigment of blood.
Location: Haemocyanin is found in the plasma of prawns, crabs, and a few other invertebrates.
Chemistry: Haemocyanin is copper copper-containing pigment which is a protein in nature. In normal conditions, it remains colorless but turns a light bluish color when it comes in contact with oxygen (Oxyhaemocyanin).
Function: Haemocyanin helps to carry oxygen in blood.
Difference between Haemoglobin and Haemocyanin :
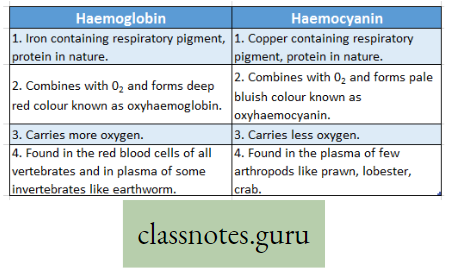
In earthworms, there is no RBC. Then where is hemoglobin present in blood?
Blood Group And Blood Donation
Blood Group :
Human Blood: Human blood can be divided into different groups according to agglu tinogen (antigen) and agglutinin (antibody)— known as blood groups.
ABO System: Human blood can be divided into four groups- A, B, AB, and 0 according to agglutinogen and agglutinin—known as ABO system.
Discovery: ABO system was discovered by Karl Landsteiner in 1900, for which he was awarded Nobel Prize later in 1930.
Agglutinogen: This is a type of antigen, present on the surface of RBC. It can be of two types—A and B. Chemically they are complex oligosaccharides.
Agglutinin: This is a type of antibody (gamma globulin—Ig), present in blood plasma. It can be of two types—a and p. Chemically they are protein in nature—mostly IgM and IgG.immunoglobulin molecules.
Naming of blood group: The blood group is named according to the type of agglutinogen present in it e.g. if agglutinogen A is present on the surface of RBC, it is called A .group and so on.
Landsteiner’s rule: “If a person contains certain agglutinogen in blood he always lacks the corresponding agglutinin”, example Blood with A agglutinogen never contains corresponding a-agglutinin; similarly blood with B agglutinogen never contains P-agglutinin.
Details of the type of blood group:
Concept of “universal donor” and “universal recipient”:’ O’ group blood does not contain any antigen (A or B) so this group may be donated to any person in an emergency. so 0 group blood is called a “universal donor”. Conversely AB group * does not contain any antibody (a or P)-so this group may receive blood from any group under emergency. so the AB group is- called a “universal recipient”.
However, the same group of blood is advised best for transfusion.
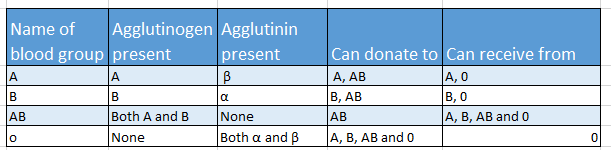
Difference between Agglutinogen (Surface antigen) and Agglutinin (Blood group antibody):
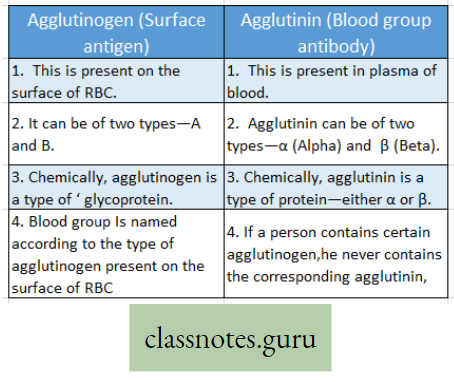
Rhesus factor (Rh factor) and its Importance: Landsteiner and Wiener (1040) discovered a substance, a type of agglutinogen, from the blood of Rhesus monkeys.
For reference only:

Rhesus monkey is a small brown macaque with red skin on the face, found in southern Asia. In India, it is found in the Himalayas, forests of Assam, and Sundarban in West Bengal. It is extensively used in iGSGaich work.
- It is also present in human red blood corpuscles of about 85% of people. Since it was first discovered in Rhesus monkeys, this agglutinogen is known as the Rhesus factor or in short Rh-factor.
- There is no corresponding agglutinin in the human plasma. Those persons who possess the Rh factor are known as Rh-positive (Rh ‘) and those who do not possess the Rh factor are known as Rh negative (Rh-).
- Complications arise when Rh+ blood mixes with Rh“ mother’s blood. In this case, the baby turns weak, anemic, and jaundiced, or in severe cases detail of the fetus occurs. This is. called Erythroblastosis foetalis.
- There are six common types of Rh antigens, each of which is called Rh factor. They are C, D, E, c, d, and e of which type D antigen is widely prevalent in your blood test report if it is written that your blood is B+ or A, then what does it mean?
For reference only: The blood group of any person is determined by genes.
Significance Of Blood Grouping :
- For blood transfusion, identification of blood groups is primarily needed.
- The blood group of a person is a very important identity of the person.
- Have you seen that the blood group is mentioned on the ID cards of different persons?
- Blood transfusion, ABO incompatibility, cross-matching, and Haemolysis :
Blood transfusion: The transfer of blood from one person to another is called transfusion. People who give their blood for transfusion are called donors and those who receive blood transfusion are called recipients.
- While testing compatibility, the agglutinogen (Antigen) of the donor’s corpuscles and the type of agglutinin (Antibody) of the recipient plasma (serum) should be taken into account.
- Because the agglutinogen of the donor will react with the agglutinin of the recipient.
Examples:
- A-agglutinogen will react with a-agglutinin (anti-A) and B-agglutinogen will react with (3-agglutinin (anti-B).
- Thus antigens of donor’s blood should be compatible with the recipient’s antibodies. Otherwise clumping (agglutination) and then hemolysis breakdown of RBC occurs.
- Agglutination: If there is a wrong incompatible blood transfusion, then corresponding agglutinogen and agglutinin may combine resulting in clumping of RBC called agglutination. In cross-matching of two blood groups, agglutination occurs when the donor’s RBC and the recipient’s plasma are incubated together—which indicates that the donor’s and recipient’s blood are incompatible.
- Cross-matching: It is the process where the donor’s red blood corpuscles are mixed with the recipient’s plasma on a slide and checked for agglutination before the actual transfusion of blood. This is a mandatory medical rule.
For reference only:
Bombay blood group: The Bombay blood group is the rarest. It was first discovered in Bombay (Mumbai), India in 1952. It is also known as the h/h blood group or Oh blood group. It is found in 1 out of every 2,50,000 persons. They can donate blood to any member of the ABO blood group but can not receive blood from any member of the ABO system (since they always contain either antigen A or B or H). (H antigen is present in the O group).
Blood Donation Definition: It is a voluntary process when someone (donor) agrees to have blood drawn from his body to give it to someone (recipient) who needs a blood transfusion. Millions of people need blood transfusions each year. Some may need blood during surgery after an accident or in any other disease.
Types of blood donation :
- Whole blood: This is the most common type of blood donation where the whole blood is donated.
- Platelets: This type of donation uses a process called apheresis where only platelets and some plasma are collected from the donor.
- Plasma: During apheresis, only plasma may be also collected.
- Double red cells: in this process, only RBCs are collected by apheresis.
Blood Bank: This is a place where blood is collected from donors, typed (A or B or AB or O), separated into components, stored, and prepared for transfusion recipients. Generally, Na-citrate is used as an anticoagulant in the blood bank for the storage of blood.
The volume of blood donation: In India, a donor would donate only 250-300 ml of blood in one donation.
Do you know that US people may donate 450 – 500 ml / per donation and that Chinese only 200 ml/per donation?
Recovery And Time Between Donations: Donors are usually kept at the donation site for 10-15 minutes after donating blood. Light refreshments or lunch is provided to the donor. The prick site of the needle is covered with a bandage.
Do you know, that during blood transfusion, a male donor’s blood can be donated to a female and vice versa (no gender specification)? Blood donation can be done after 18 years. of age with a body weight of 55 kg+.
Coagulation Of Blood
Coagulation of blood Definition: The process by which blood oozing out from the ruptured blood vessels loses Its fluidity in a few minutes and sets into a semi-solid jelly-like mass, is called coagulation.
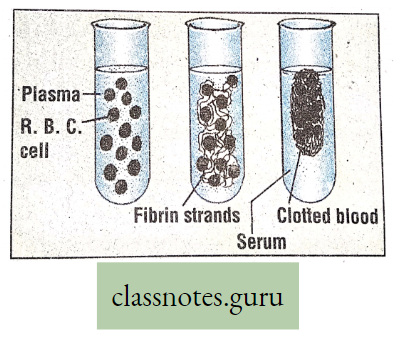
Factors Involved in Blood Coagulation: Blood coagulation is a complex process that requires about 13 factors. All these factors are present in plasma except factor-ill (thromboplastin). Except for factor IV (calcium ion), all other factors are protein in nature. Some important factors are Factor I (fibrinogen), Factor II (prothrombin), Factor III (thromboplastin), Factor IV (CaH), and so on.
Coagulation of blood depends on the type of injury. Minor injury causes quick blood clots.
Process of blood coagulation: It takes place in three steps
When blood comes out of an injured area, the platelets come in contact with ruptured blood vessels (rough surface). The platelets are broken in the presence of several clotting factors of blood and produce intrinsic thromboplastin.
- Thromboplastin is also produced from the damaged tissue cells (extrinsic thromboplastin or tissue thromboplastin) in the presence of Ca++ and other factors.
- The prothrombin of plasma is converted to thrombin in the presence of Ca++ and thromboplastin.
- Thrombin reacts with soluble fibrinogen to convert it into insoluble fibrin. In the meshwork of fibrin fibers, the blood corpuscles are entangled forming a plug which is called a clot.
Summary: Fountain reaction/Cascade reaction)
Normally in circulating blood: Uninjured Platelets + Prothrombin + Fibrinogen → no clot
After shedding of blood (after an injury):
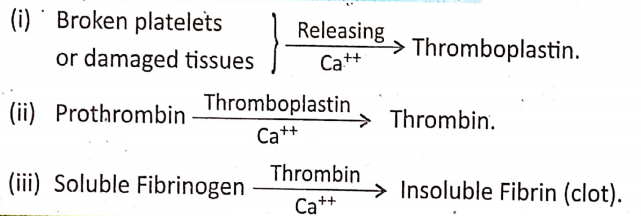
Do you know about heparin that prevents intravascular clotting of blood when blood flows through blood vessels? Try to identify some other anticoagulants that are used in the biochemical laboratory. Have you heard the name of the “Royal diseaseHaemophilia” that was first discovered in the family of Queen Victoria? Do you know what happens in Haemophilia?
Significance Of Blood Coagulation :
- It helps to stop hemorrhage.
- It blocks the entry of germs through the wounds.
- It also helps in maintaining normal blood volume.
Can you explain why flowing blood does not clot inside blood vessels?
serum: It is a light straw-colored fluid that comes out (exudes) of the fibrin network after blood clotting. It contains all the components of plasma except clotting proteins (Prothrombin and fibrinogen).
Internal Structure Of the Human Heart
Anatomical Structure: The heart is a conical muscular involuntary hollow organ of about 12cm long and 9 cm board, 250-350 gm in weight. it is situated in the throat behind the sternum and between the lungs. Its walls are made up of cardiac muscle (striated involuntary) fibers. The whole heart is covered by a double-layered membranous sac, called pericardium. It contains a small amount of serous fluid in the closed space between the two layers called pericardial fluid that acts as a shock absorber. The wall of the heart consists of the outer epicardium, middle thick myocardium, and inner thin endocardium.
Do you know the importance of pericardial fluid?
Do you know the name of the inner layer and outer layer of the pericardium?
Internal structure of Heart: The Human (mammalian) heart consists of four chambers, viz, two atria (auricle) and two ventricles; The chambers on the right side of the heart are completely separated from those on the left side by a muscular partition called septum.
The part of this septum separating the two atria is called the interatrial septum while the other part separating the two ventricles is called the interventricular septum. The two atria are separated from two ventricles by a partition called atrioventricular septum.
- The right atrium receives two great veins, the superior vena cava, and the inferior vena cava, which bring less oxygenated blood (venous blood) from all parts except the lungs. The left atrium receives oxygenated blood (arterial blood) from the four great veins called pulmonary veins, each from the two lungs.
- Blood is collected in the atria and enters into the ventricles through the apertures called atrioventricular apertures (openings). They are provided with valves that prevent the backflow of blood from the ventricle to the atrium.
- The valves at the right atrioventricular aperture are made of three cusps or folds and are called the tricuspid valves or right atrioventricular valves. At the left atrioventricular aperture, the valve is made up of two flaps or cusps and is called the bicuspid valve mitral valve, or left atrioventricular valve.
- The valves contain chordae tendinae which are attached to the papillary muscle. There are small muscular
projections, called papillary muscles present in the inner wall of the ventricles that are responsible for the attachment of valves to the wall of the ventricle. However, the wall of the ventricle is much thicker (3 times) than the auricle due to the specialized ventricular muscle called Columnqe carnage.
- From the upper parts of the ventricles arise the great arteries viz., the pulmonary trunk of the pulmonary artery from the right ventricle and the aorta from the left ventricle; The opening of these great arteries are also guarded by the valves which are called semilunar valve.
- The pulmonary semilunar valve allows the flow of blood from the right ventricle to the pulmonary artery but prevents backflow whereas the aortic semilunar valve allows the flow of blood from the left ventricle to * the aorta but prevents backflow.
- The valves of heart allow unidirectional flow of blood only’—Justify the statement.
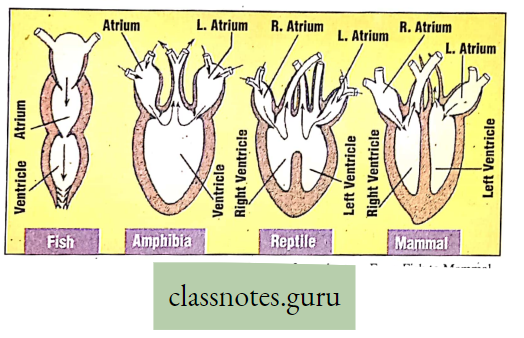
Have you heard of the modern methods of heart surgery like Open heart surgery, Bypass surgery, etc.?
You should know how many chambers are present in the heart of different vertebrates viz. in fish hearts (2 chambers), in amphibia (3 chambers), in reptilia (3 chambers), and in birds and mammals (4 chambers).
Try to construct an evolutionary tree of the heart of vertebrate series. Note down your N observation.
Heart of crocodile: Though it is a reptile, it has a 4-chambered heart but there is a special opening known as Foramen of Panizza.
Junctional Tissues Of Heart:
These are specialized cardiac tissues that are responsible for the origin of cardiac impulses and their conduction all over the heart,
Types :
- SA Node (Sino Atrial Node) SAN: It is located in the right atrium and is known as the natural pacemaker of the heart since the conduction wave starts from here.
- AV Node (Auriculo Ventricular Node) AVN: It is situated in the right atrium and is known as the reserve pacemaker of the heart.
- Internodal fibers and Buchman’s bundle: It is situated in the right atrium.
- Bundle of His: It originates from the AV node and passes down as right and left branches on both sides of the interventricular septum.
- Purkinje fiber: It originates from the Bundle of His and is situated on the wall of both ventricles.
Pathway of conduction of cardiac impulse :

A Course of Circulation of blood through the heart
Circulation of blood in man was first demonstrated by William I. Larvey (1628).
- The heart is a living pump made up of involuntary muscle. Circulation of blood through the heart is unidirectional due to the presence of valves in the several openings (orifices) of the heart.
- Due to continuous contraction (systole) and relaxation (diastole) of the heart, the blood circulates within it as well as throughout the body within the blood vessels.
- Let us discuss the circulation of blood through the human heart.
- At first, the venous blood (O2 less and more CO2– containing blood) from the upper and lower parts of the body comes through the superior vena cava and inferior vena cava respectively into the right atrium.
- From the right atrium, the venous blood passes into the right ventricle by opening the tricuspid valves.
- Thereafter contraction of the right ventricle expels the blood by opening the semilunar valves into the lungs through the pulmonary artery for oxygenation.
- In the lungs, the venous blood (less oxygenated blood) is converted into arterial blood (more oxygenated blood) after taking oxygen from the alveoli of the lungs, and CO2 enters the alveoli.
- This arterial blood returns from the lungs through the four pulmonary Veins into the left atrium.
- From the left atrium the oxygenated (arterial) blood passes into the left ventricle by opening the bicuspid or mitral valves present at the left atrioventricular opening.
- At last during ventricular contraction, the arterial blood from the left ventricle passes into the aorta by opening the aortic valves (semilunar valves) present at the base of the aorta.
Do you know that ECG is done to check the normal functions of the heart?
A circular path of Blood Circulation within the heart and body:
Double circulation :
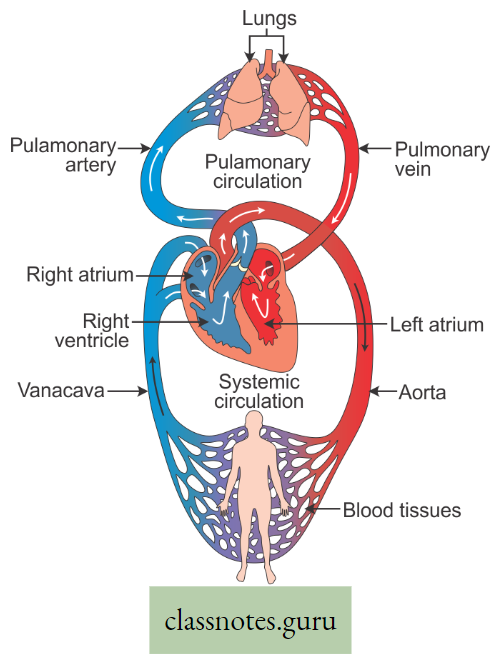
It is the process where oxygenated and deoxygenated blood flows through the heart separately but simultaneously in two different directions. Hence this type of circulation is called double circulation.
- Within the human heart, oxygenated and deoxygenated blood never get mixed. So they are separate.
- Pulmonary circulation consists of the right ventricle, pulmonary artery, lungs, pulmonary veins, and left atrium. Since blood travels a short distance between the heart to the lung and back to the heart this is called short circulation or short circuit circulation.
- On the other hand, systemic circulation consists of the left ventricle, left aorta, all over the body, superior vena cava, inferior vena cava, and right atrium. Since blood travels a long distance from the heart to all over the body and back to the heart it is called Long circulation Long circuit circulation or bigger circulation.
Do you know in the heart of fish there is only a single* circulation why? Explain why the heart “of a fish is called a venous heart.
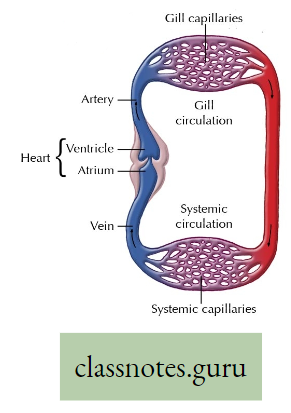
The heart of a fish is two-chambered and always contains deoxygenated blood so it is called a venous heart.
For reference only:
The function of Eosinophil:
- Granules in eosinophils contain different enzymes that help in defense of the body against diseases. Granules are modified lysosomes.
- Eosinophil plays a crucial role in killing parasites, by its enzyme Cathepsin (a proteolytic enzyme).
- Eosinophil secretes different enzymes like eosinophil peroxidase, ribonuclease (RNase), deoxyribonuclease (DNase), lipase, etc. that are toxic to parasite and host tissues.
- Eosinophil degrades histamine (released by mast cell and basophil) by histaminase and thus helps to reduce inflammation.
The function of Basophil:
- It secretes anticoagulant Heparin, (which prevents quick clotting of blood).
- It also secretes Vasodilator histamine, (which promotes blood flow to the tissues).
- Like eosinophils, basophils play a role in both parasitic infections and allergies.
Mast Cell:
It is a type of WBC. This is very much similar in both structure and function to basophil. Both are granulated cells that contain histamine and heparin. Basophils are present in blood whereas mast cells are located in connective tissue, skin, lining of the stomach, intestine, etc. They play an important role in defending these tissues from disease.
Concept Of Excretion
As a result of metabolism certain non-essential toxic chemical substances are formed 1 within the body. If these substances are allowed to accumulate, toxicity develops and the cell eventually dies. The metabolism of proteins in animal tissues produces most of the nitrogenous toxic substances.
- The rate of metabolism is directly proportional to the rate of excretion. In animals, more activity of the body causes more metabolism and so more excretion.
- The process by which the excretory materials are eliminated regularly from the body is known as excretion.
- The non-essential toxic substances that are produced during cellular metabolism are collectively called excretory products.
- The organs which are involved in the processes of excretion are called excretory organs. Therefore metabolic wastes from the body are eliminated by the process of excretion.
Excretion Definition: The process of elimination of non-essential toxic substances produced during cellular metabolism, continuously with the help of the excretory organs (in the case of animals) or by other means (in the case of plants) is called excretion. Excretion is a catabolic process, during which harmful substances are produced which are transported in the different parts of the body and afterward eliminated from the human body.
Importance or Significance of Excretion :
- Maintenance of normal health: The excretory products are harmful to the body. Excess amount of excretory substances when accumulated in the body causes toxic effects or even may cause death to the organism. Thus regular excretion of these excretory products prevents toxic effects and keeps the body fit.
- Maintenance of protoplasmic substances: Excretion has some role in maintaining the balance of different protoplasmic substances in the cell, the protoplasm in turn helps to
keep normal life processes of the organism. - Maintenance of osmosis: Excretion has some role in the regulation of osmosis.
- Role in the ecosystem: The excretory products are formed by the breakdown of nutrients.
- Economic importance: The economic importance of excretory products of the plants is vast for human as well as animal kingdom.
- Maintenance of water balance: In various organisms mainly in animals and from the human body excess amount of body water is excreted (eliminated) to maintain the water balance osmoregulation by this process.
Know the facts
Excretion is a catabolic process
It is due to the following reasons
- The formation of excretory products occurs by a catabolic process.
- Elimination of metabolic waste products from the body takes place.
- This results in a decrease in the dry weight of the organism.
Process of Excretion in Plants
In plants, there are no special excretory organs for performing excretion. The metabolic waste products are stored in roots, stems, leaves, flowers, fruits, and seeds.
- Excretory materials are less formed in plants due to the low rate of metabolism in them than in animals.
- They do not cause any harm if stored in the plant body. Hence there is no necessity for an excretory system in a plant.
- Whenever required plants get rid of their excretory products through certain processes.
Reasons that cause lesser formation of excretory materials in plants?
- Ammonia produced due. to metabolism is utilized in the formation of various nitrogenous compounds by plants.
- Carbon dioxide and water obtained due to respiration are used during photosynthesis. The oxygen obtained from photosynthesis is used in the respiration.
- In plants the metabolic process is dependent on carbohydrates, thus less nitrogenous materials are formed.
- In the plant body, the excretory materials that do not contain nitrogen are often stored within certain organs, as they are not toxic.
- In the plant body, the protein and nitrogenous compounds are broken down to ammonia which act as a useful substance.
From all these above reasons we can find that plant excretory products are less harmful than animal excretory products.
Features Of Excretion And The Processes Involved In The Removal Of Excretory Products In Plants
Some of the excretory products are stored within some specific cells of the plant body permanently. But most of them. the excretory products are stored in the cell at first temporarily and then liberated from the plant body by the following process:
- Shedding of barks: In mature tall trees, some excretory products are temporarily stored in bark (the superficial dead tissue layer of the trunk and branches of the tree). The stored excretory products are eliminated at regular intervals through the removal of bark. Examples Guava (Psidium), Arjun, Eucalyptus, etc.
- Shedding of leaves (leaf fall): In evergreen or deciduous plants, the excretory products are transported to the leaf cells where they are stored temporarily (before fall). These products are then eliminated with the abscision (fall) of the leaves. Examples- Silk Cotton, Bombax, Sirish, Albizzia, etc.
- Shedding Of Fruits: Mature fruits like apples, tamarind, and lemon, store some metabolic products sue as malic acid, tartaric acid, and citric acid respectively. These substances are eliminated through the falling of fruits.
Naming Of The Excretory Products In Plants And Animals
Excretory products are of two types, such as Nitrogenous excretory products and non-nitrogenous excretory products.
Nonnitrogenous excretory products :
- In plants: The products are resin, tannin, latex, gums, mineral crystals, organic acids, non-nitrogenous waste, essential oils, etc.
- In animals: the non-nitrogenous waste products are carbon dioxide, bilirubin, lactic acid, carbonic acid, etc.
Nitrogenous waste products :
- In plants: The nitrogenous waste products are alkaloids, such as quinine, nicotine, morphine, atropine, reserpine, strychnine, daturine, caffeine, then, etc.
- In animals: The nitrogenous waste products are urea, uric acid, creatine, creatinine, etc.
Storage of excretory products
In animals: Animal excretory products are temporarily stored in the urinary bladder as urine and then excreted.
In plants: Plant excretory products are stored within the cells of different plant organs as they have no excretory organs to liberate those non-useful products.
Know the facts
Why is stool, not an excretory substance?
The stool is not produced out of metabolism, hence it is not counted as an excretory substance.
Nitrogenous Excretory products: Alkaloid
- Nitrogenous excretory products of plants produced by protein metabolism,
- Most of them are weak bases,
- Many of them are poorly soluble in water but readily soluble in organic solvents,
- Generally, bitter.
Origin And Economical Importance / Medicinal Value Of Some Alkaloids :
Non-nitrogenous Excretory products :
Composition, Characteristics and Examples, and Uses of Excretory Materials:

- All excretory products are waste products but all waste products are not excretory products
- All excretory products are waste products because they are toxic to the body, hence eliminated from the body example, urea, uric acid, etc.
- Some waste products are eliminated from the body but they are not excretory products as they are not formed by the metabolic process, for example, stool. A stool is an undigested waste product.
- Carbon-containing excretory products tannin of plants and CO2 of animals
Process of Excretion in Animals
Various metabolic waste products are formed due to metabolic activities within the body of animals. These metabolic end products are called excretory products. Since there are more metabolic activities in the animal body, there will be more excretory products. The most important excretory products are nitrogenous Waste products e.g. urea, uric acid, ammonia, etc. These are very toxic to the organism—so must be eliminated from the body from time to time.
For reference only:
- Ureotelic, Uricotelic and Ammonotelic animals:
- The animal that mainly excretes urea as a major excretory product is called a ureotelic animal e.g. some fishes, most amphibians, and almost all mammals.
- The animal that mainly excretes uric acid as a major excretory product is called a uricotelic animal e.g. insects, reptiles (snakes, lizards), and birds.
- The animal that eliminates nitrogenous waste as soluble ammonia in water is called an ammonotelic animal e.g. most invertebrates, most fishes, tadpole larvae, etc.
Excretory Organs In Animals
Names Of Some Animals And Their Excretory Organ
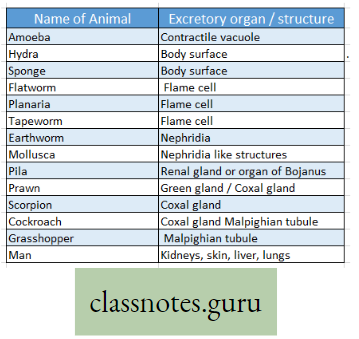
Different animals have a characteristic excretory organ that has various shapes, sizes, and structures but perform the same function of excretion as well as water balance of the body called osmoregulation some common examples of animal excretory organs are as follows:
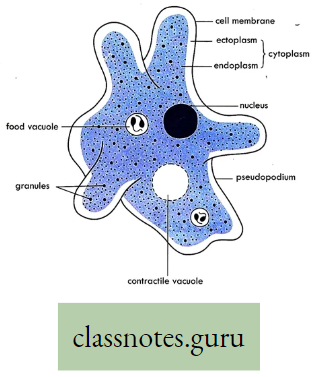
Contractile vacuole: It is a subcellular membranous organelle, mainly found in protists (for example. Amoeba, Paramoecium) and unicellular algae (for example Chlamydomonas), which primarily helps in osmoregulation. Excess water is collected in the contractile vacuole and so the vacuole swells. Then the vacuole comes in contact with the cell membrane, contracts, and releases excess water from the cell.
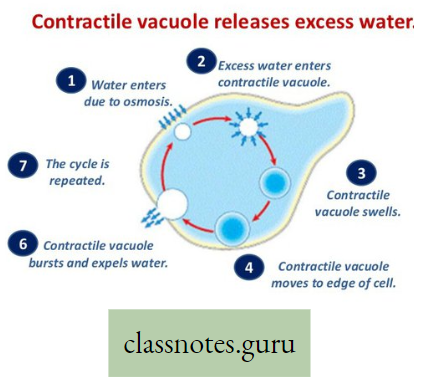
Flame cell: It is a specialized excretory cell found mainly in Platyhelminthes or flatworms (for example Taenia solium or Tape-worm), that removes an excretory product from the body.
- A bunch of flame cells are together called protonephridia.
- The flame cell is a nucleated ‘cup-shaped cell body with a bunch of flagella inside that looks like a flame of fire. Many flame cells are attached to a common excretory canal.
- Collected excretory. product from the cells is released in the excretory canal and then goes outside.
Nephridia: This is the excretory as well as osmoregulatory organ found mainly in Annelida like earthworms, leeches, etc. There are two basic types of nephridia metanephridia (larger) and protonephridia (smaller). In earthworms, metanephridia is found.
- It consists of a ciliated funnel called a nephrostome that collects excretory products from the coelom.
- The product flows through nephridia! tubule and finally goes out through the nephridiopore.
- In the tubule, however, various necessary substances are reabsorbed.
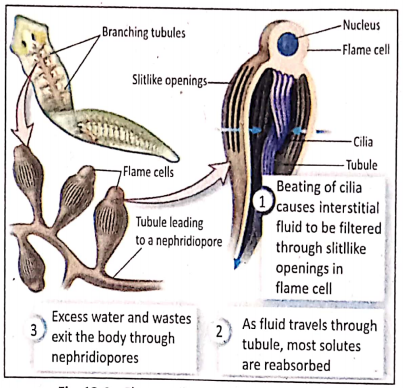
Do you find any structural and functional similarity between nephron and nephridia?
Malpighian tubule: This is a tubular excretory as well as osmoregulatory organ found in different Arthropoda (primarily in insects like cockroaches and others).
- These are a bunch of slender tubules, located at the junction of the midgut and hindgut. Most tubules are highly convoluted. The number of tubules varies.
- These tubules collect the excretory product from hemolymph and drain it into the hindgut, from where the product moves through the hindgut into the rectum and finally goes out through the anus.
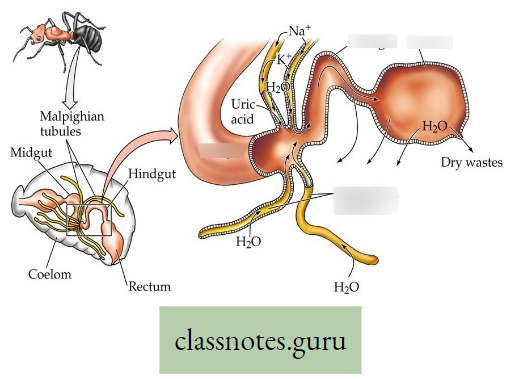
Kidney: This is the most important excretory organ in higher animals. All vertebrates have kidneys. Like human kidneys, they are made up of many nephrons (the structural and functional unit of the kidney). The kidney is mainly responsible for the elimination of toxic metabolic waste products as well as osmoregulation, mineral balance, and so on.
Do you know that based on complexity, a kidney may be pronephros, mesonephros, and metanephros in vertebrates?
For reference only: Pronephros, Mesonephors, and Metanephros kidney :
- Pronephros: It is the definitive excretory organ of primitive fishes. This is found in the embryo of higher vertebrates as a vestigial structure. It consists of several coiled tubules. It is present in Lamprey.
- Mesonephros: It is one of the three excretory organs that develop in vertebrates. It serves as the main excretory organ of aquatic vertebrates (fishes). It is present in the kidneys of fish and amphibians.
- Metanephros: The final excretory organ that develops in a vertebrate embryo is called metanephros kidney. In reptiles, birds, and mammals, metanephros • replaces mesonephros (during embryonic development) as the functional excretory organ and develops into an adult kidney.
During the embryonic development of kidneys in higher vertebrates, first pronephros develops which is replaced by mesonephros, and finally, metanephros kidney develops, which persists in adults of reptiles, birds, and mammals (including man).
Excretory System Of Human
Structure And Functions Of Excretory System Definition: The various organs that help to eliminate the toxic nitrogenous and non-nitrogenous waste products from the body (urine, sweat, etc.) form the system known as the excretory system. The important excretory organs of the excretory system are the kidney, liver, lungs, skin, etc.
Structure: The most important excretory system is the urinary system. As this system eliminates about 70 percent of excretory materials from the body—hence kidneys are known as the primary excretory organ.
Kidney: Two kidneys, which belong to the urinary system, are situated on the posterior portion of the abdominal cavity on either side of the vertebral column.
External structure: Each kidney is bean-shaped, dark brownish-red in color, and measures about 11 cm in length, 6 cm in breadth, and 3 cm in thickness. In adults, its weight is about 125—170 gm (average 150 gm). The lateral margin is convex, while the medial margin has a concavity in the middle and is called hilum. The renal artery and the renal vein enter the kidney, whereas the ureter leaves the kidney through this middle portion of the concavity.
Internal structure: If a kidney is cut through the middle in two longitudinal halves, then two regions are revealed to the naked eye an outer cortex and an inner medulla. The medulla is formed by a number (7-18) of striated conical structures called the renal pyramids. Each kidney is made up of about one million (10 lacs) of excretory units called the nephrons.
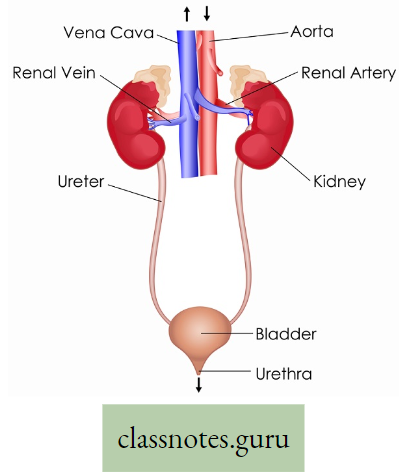
Kidney Functions:
- Kidneys eliminate toxic and metabolic waste products from the body.
- It maintains the water balance and salt balance of the body.
- It maintains the acid-base balance of the body.
- It manufactures certain substances like renin, ammonia, hippuric acid, and inorganic phosphate.
- It synthesizes erythropoietin (a local hormone) which stimulates R. B. C. formation in the bone marrow.
- It regulates blood pressure and osmotic pressure in the blood and tissues.
- Ureter: The ureters are two tubular structures. Each ureter arises from the hilum of each kidney. The ureter is about 25-30 cm long which expands at its upper end to form the broad pelvis.
Function: Urine formed in the nephrons of the kidney is carried along the ureter to the urinary bladder.
- Urinary bladder: The urinary bladder is a pear-shaped muscular bag-like structure. It is situated in the pelvic cavity in front of the rectum in males and in front of the uterus in females. It receives two ureters which run obliquely through the bladder wall in the posterior part of the urinary bladder and opens into it.
Function: It stores urine temporarily.
- Urethra: The urethra is a narrow canal that arises from the narrow neck of the bladder.
It opens to the exterior, running through the penis in the male, but separately from the vagina in the female. Its length varies from about 18 cm in men to about 4 cm in women. A thick band of smooth muscle fibers called the internal urethral sphincter, and external sphincters are present. These two sphincters control the excretion of urine through the urethra.
Function: The single urethra carries urine from the urinary bladder outside the body. Urine is formed in the kidney, passes through the ureters, and is stored temporarily in the urinary bladder. This collected urine is voided by the act of micturition (urination) when the bladder muscles contract and the urethral sphincters relax.
Do you find any difference between the ureter and urethra?
Functions of the excretory system:
- Removal of waste products: All the metabolic products that are produced in the body are excreted mainly through urine and a small amount through sweat, sebum, etc. The products are—urea, uric acid, creatinine, ammonia, etc.
- Do you know which artery and vein are carrying blood to and from the kidney?
- Maintenance of water balance: The excretory system regulates the elimination of water from the body and thus maintains water balance in the body. In summer, since there is more sweating, there is less urine formation, which is reversed in winter.
Have you heard about ‘Kidney stones’?
Out of two kidneys, a donor can donate one kidney which is transplanted into the body of the recipient. This is called kidney transplantation have you heard of it?
Nephron
Nephron Definition: The structural and functional unit of the kidney is called the nephron.
Structure: A single nephron is a coiled tubular structure with a dilated blind end. It is about 5 cm long and 0-6 mm in diameter. It consists of mainly two parts e.g., Malpighian corpuscle and Renal tubule.
Different parts of Nephron :
Structure: This is a double-walled cup-like structure situated in the cortex of the kidney. It is 0-2 mm in diameter and consists of two parts the Bowman’s capsule and the glomerulus.
- Bowman’s capsule-It is the double-walled dilated blind end of the nephron. The walls of the capsule are lined by a single layer of flat epithelial cells. The outer layer of Bowman’s capsule is called the parietal layer and the inner layer is called the visceral layer.
- Glomerulus- It is the tuft of loop-like capillaries (approximately 50 loops) invaginated within the double-walled Bowman’s capsule.
- The tuft of capillaries is formed by the division of short and wide afferent arteriole (approx 50x in diameter), a branch of the renal artery.
- The capillaries of the glomerulus reunite and form a long and narrow efferent arteriole (approx 25 in diameter).
- After leaving the glomerulus the efferent arteriole forms a second set of capillaries called the peritubular capillaries.
- These capillaries surround different parts of the renal tubule.
The function of the Malpighian corpuscle: It forms the filtering bed through which all the constituents of plasma except colloids of plasma (i.e., proteins, fats, etc.) are filtered and thus helps in urine formation. This type of filtration is known as ultra-filtration.
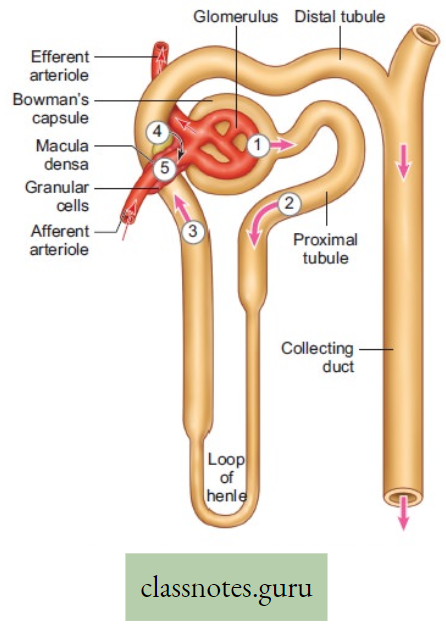
How can you compare the malpighian tubule and the malpighian corpuscle?
Renal tubule:
Structure: It is the tubular portion of the nephron. It is about 3 cm long and 0-02—0-06 mm wide originates from the lower portion of the Bowman’s capsule and consists of three parts.
- The first part of the tubule: It is the descending tubule which is highly coiled, approximately 14 mm long, and situated in the cortical region of the kidney. This part of the tubule is called the proximal convoluted tubule (PCT). The inner wall is lined by cubical epithelium.
Function: Almost all essential nutrients, 70-80% ions and electrolytes, and a huge amount of water are reabsorbed by PCT.
- The second part of the tubule -It is a U-shaped (hairpin-like) middle portion, a narrow tubular part approximately 8 mm long which is situated in the medullary region of the kidney and is known as Henle’s loop. The inner wall is lined by flat squamous epithelium.
Function: The descending limb of Henle’s loop is permeable to water whereas ascending limb is permeable to electrolytes. Thus water and electrolytes are reabsorbed here.
- The third part of the renal tubule -It is the ascending tubule approximately 6 mm long which is situated mainly in the cortical part of the kidney. It is comparatively wide and less coiled than the proximal convoluted tubule. This part of the tubule is called the distal convoluted tubule (DCT). The inner wall is lined by cubical epithelium.
Function: DCT helps in the reabsorption of Na+, H2O2, and HCO→ and maintains Na+- K+ balance in the blood.
Do you know PCT, Henle’s Loop and DCT together called Uriniferous tubule?
Why is the nephron so much convoluted?
- In a small kidney, approximately 1 million- nephrons are present. So space is scarce hence the nephron is convoluted,
- Due to convoluted nephrons, the speed of flow of urine is slow so that various essential substances can be easily absorbed,
- In the convoluted nephron, the absorptive surface area is greater which is useful for reabsorption.
The distal convoluted tubules of different nephrons open in a wide straight tubule which is known asthecollectingtubule. It passes to the medulla and opens at the apices of the pyramids into the pelvis of the kidney.
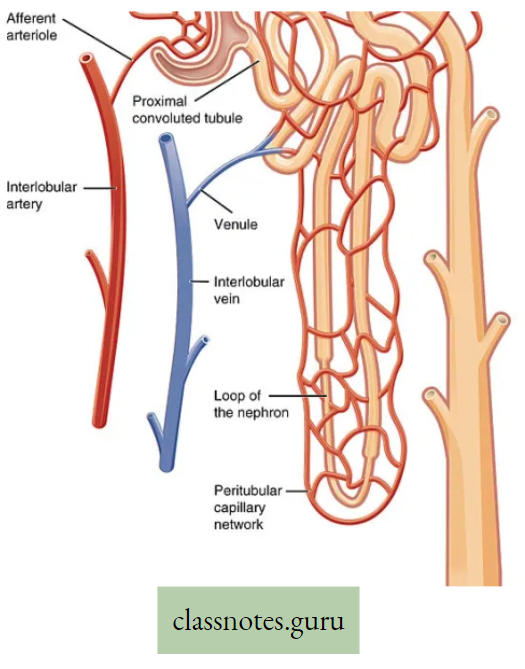
The general function of the renal tubule :
- Reabsorption: As the capsular filtrate passes through the renal tubule, almost all the essential substances of the filtrate are selectively reabsorbed
- Secretion: Some substances are not present in the capsular filtrate but are found in the urine. This shows that the renal tubular cells secrete some substances like penicillin, phenol red, creatinine, etc. which come out through urine.
- Formation of new substances: The epithelial cells of the. tubule can manufacture some substances, like ammonia, inorganic phosphate, and hippuric acid, which are also excreted through the urine.
Role of the nephron in the formation of urine:
Urine formation involves three main steps glomerular filtration, tubular reabsorption, and tubular secretion. These steps take place in different parts of the nephron.
Step 1: Glomerular filtration or Ultrafiltration or Pressure filtration: The diameter of the afferent arteriole is approximately 50p and that of the efferent is 25p.
- Hence more blood comes in the glomerulus and less blood goes out of the glomerulus.
- Thus always certain amount of blood remains stored in the glomerulus which creates a pressure that causes filtration of various materials from blood.
- This process of filtration is called ultrafiltration where crystalloids are separated from colloids.
- The amount of filtrate formed by the two kidneys per minute is called. Glomerula Filtration Rate (GFR).
- In a normal healthy person, GFR is approximately 125 ml/minute i.e. 180 litres/day. GFR is controlled by the Juxtaglomerular apparatus (JGA).
Step 2: Tubular reabsorption: Out of 180 liters of glomerular filtrate per day nearly 178-5 liters are absorbed in the tubular part of the nephron (PCT, Henle’s Loop, and DCT)—which means almost 99% of the filtrate is reabsorbed.
- This is known as tubular reabsorption. This absorption in the tubular cells is either an active or passive process.
- All necessary substances like water, glucose, amino acids, Na+, etc. are almost completely (99%) reabsorbed.
- The process of tubular reabsorption is influenced by many hormones like vasopressin (Anti Diuretic Hormone- ADH), Aldosterone, etc.
Step 3: Tubular secretion: During urine formation, tubular cells secrete substances like ammonia into the filtrate. These substances are carried over by urine. Thus tubular secretion plays an important role in urine formation as it helps in the maintenance of the ionic and acid-base balance of body fluids.
Do you find any difference between glomerular filtrate and urine from collecting tubules?
If someone suffers from diabetes, there is polyuria (frequent urine formation) why?
Have you heard of some abnormal constituents of urine that may develop in different diseases like Glycosuria, Haematuria, Bilirubinuria, etc?
Have you heard of ‘Kidney transplantation’
Accessory Excretory Organs Of Human
In addition to kidneys, man possesses additional structures which also function as excretory organs.
Skin (Sweat gland): Skin is the protective superficial layer of the body. It contains numerous sweat glands which secrete sweat. Sweat glands are coiled unbranched tubular glands. The ducts of these glands open into the surface of the skin through which sweat is eliminated. Sweat contains different excretory products like urea, lactic acid, creatinine, uric acid, ammonia, NaCI, sulfate, etc. All these excretory products, along with water are excreted. The small sweat gland is the eccrine sweat gland and the large sweat gland is the apocrine. The mammary gland is a modified apocrine sweat gland.
Lungs: Cellular metabolism produces CO2 which is a harmful (toxic) substance. CO2 which is produced by the catabolic process of respiratory substrate (glucose) in the cell reacts with water and forms carbonic acid (CO2 + H20 → H2CO2) which causes acidosis in the body. This condition is harmful to the body, hence CO2 is regarded as an excretory product. This CO2 along with water vapor and some volatile substances like alcohol, acetone, and ammonia is excreted during expiration through the lungs.
Liver: Ammonia is produced in the liver during protein metabolism. It is the most poisonous waste product. The liver converts this harmful ammonia into the relatively less harmful excretory substance called urea.
The conversion of ammonia to urea takes place by ornithine cycle or urea cycle in the liver. The liver excretes certain heavy metals, cholesterol, and bile pigments through bile.
Difference between Plant excretion and Animal excretion :