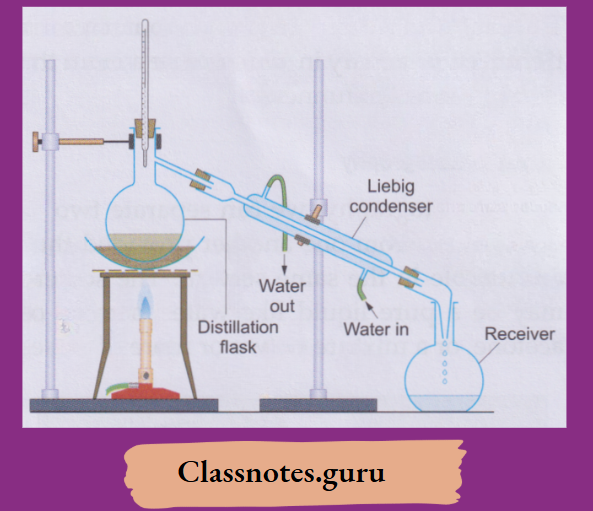
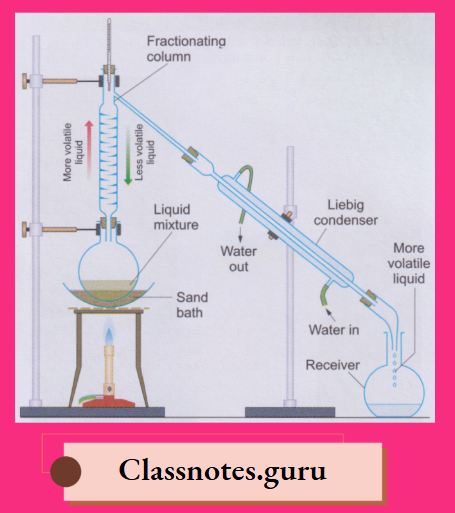
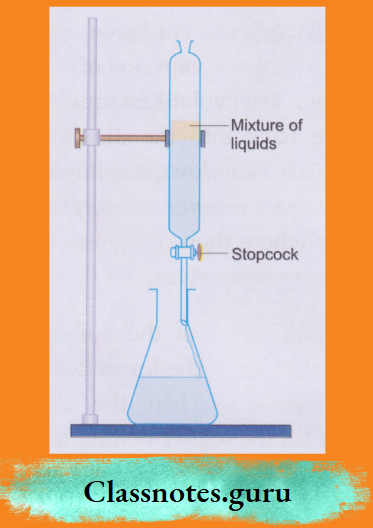
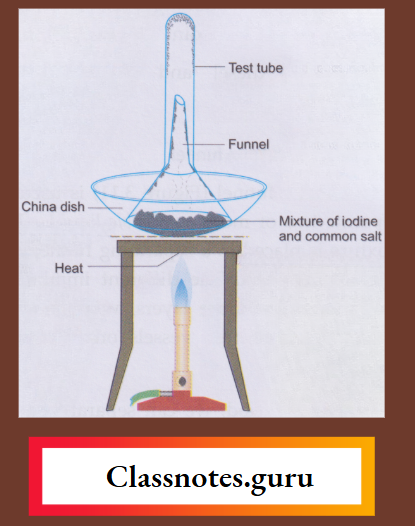
NCERT Class 8 Chemistry Chapter 8 Water Notes
Life would not have been possible on Earth without water. Water is the most abundant compound on our planet, covering three-fourths of the Earth’s surface. It constitutes about seven tenths of our bodies and more than nine tenths of fruits and vegetables.
Water can dissolve many substances —solids, liquids and gases alike. It controls the temperature of the atmosphere and also our bodies.
Read And Lean More Class 8 Chemistry
We need water for:
- Domestic purposes: Like cooking, drinking, washing and bathing;
- Agricultural purposes: Like irrigation, jute processing and dairy farming; and
- Industrial purposes: It is used as a solvent, reactant and coolant.
NCERT Solutions for Water Class 8

The sources of water are surface water bodies like ponds, rivers, lakes, seas and oceans, and groundwater. Both surface water and groundwater are replenished by the rain. In this chapter, we will discuss the properties of water as a solvent and also the types of natural water (i.e., hard and soft water).
Water—A Universal Solvent
Water is called a universal solvent as it dissolves a larger number of substances than any other.
It is this property of water that helps a river dissolve many minerals from the soil and finally discharge them into the sea. Thus, a sea or an ocean is a vast store of minerals and other soluble substances.
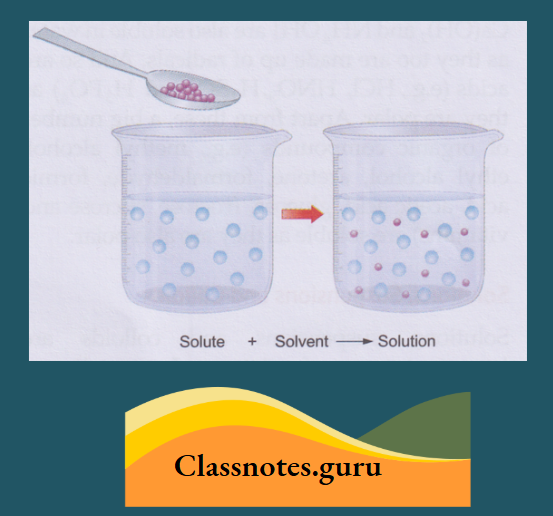
How a Solute Dissolves in Water
A solute, when mixed with water or any other solvent, separates into molecules. And these molecules hide themselves in the intermolecular space of water or the solvent and appear to have disappeared.
As the solute molecules occupy the intermolecular space of the solvent, the volume of the solvent does not change when a solute dissolves in it.
Water is Superior to Other Solvents
Water is superior to other solvents as it helps the solute fragment into molecules or radicals. A water molecule is electrically neutral.
- But there is a slight positive charge on the hydrogen atoms and an equivalent amount of negative charge on the oxygen atom, so that the opposite charges cancel each other.
- Such a molecule is called a polar molecule and thus water is a polar solvent.
- Due to polarity, water molecules experience a coulomb attraction (i.e., an electrostatic force of attraction) for polar solutes, including those containing radicals.
- When such a solute is stirred in water, the water molecules cling to the solute molecules or radicals. And take them away in a cage-like structure. Thus, the solute seems to disappear.
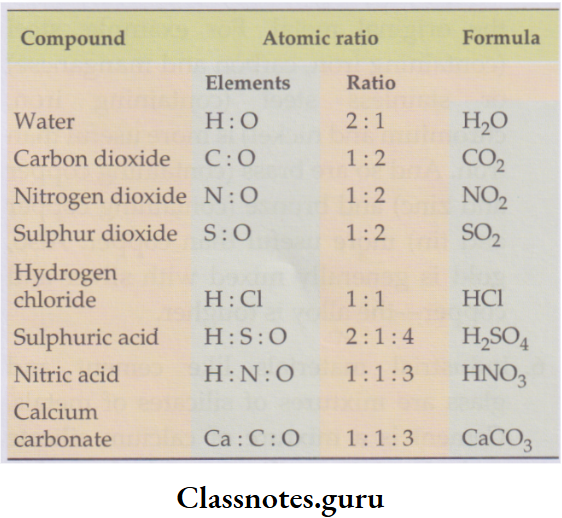
- All salts are made up of radicals. For example, sodium chloride (NaCl) is made up of Na+ and Cl– radicals, sodium sulphate (Na2SO4) of Na+ and SO2-4 radicals, magnesium sulphate (MgSO4) of Mg2+ and SO2-4 radicals, copper(H) sulphate (CuSO4) of Cu2+ and SO2-4 radicals and ammonium nitrate (NH4NO3) of NH4+ and NO–3 radicals.
- Hence, most salts are soluble in water. No wonder the sea is a vast store of salts.
- A large number of bases [i.e., NaOH, KOH, Ca(OH)2 and NH4OH] are also soluble in water as they too are made up of radicals. And so are acids
Example: HCl, HNO3, H2SO4 and H3PO4) as they are polar.
Apart from these, a big number of organic compounds
Example: Methyl alcohol, ethyl alcohol, acetone, formaldehyde, formic acid, acetic acid, glucose, fructose, sucrose and vitamins) They are soluble as they are also polar
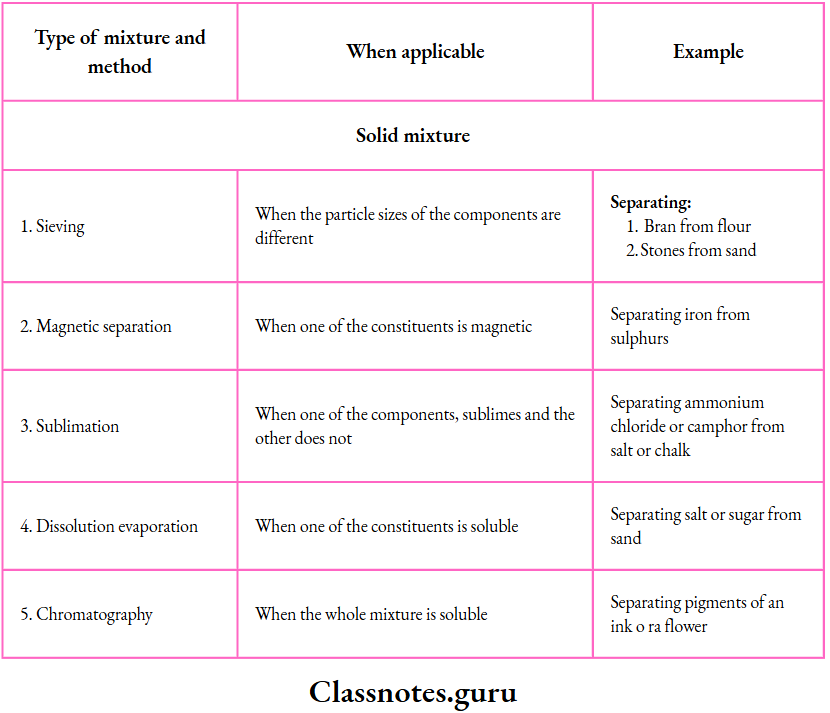
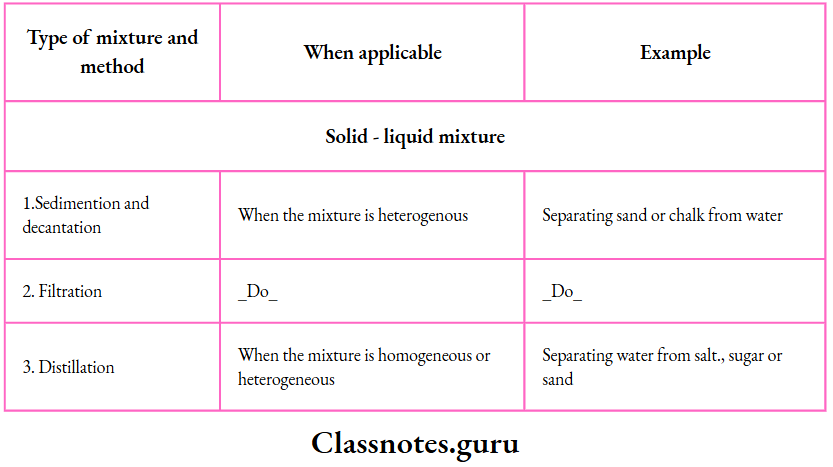
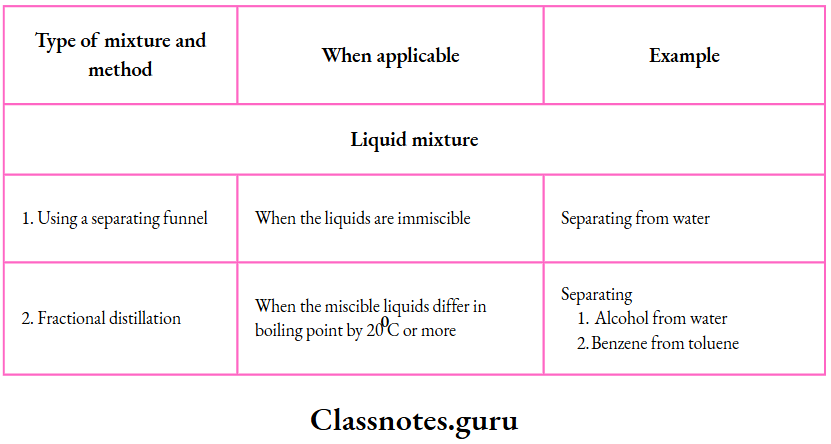
Solutions, Suspensions and Colloids
Solutions, suspensions and colloids are important types of mixtures. Let us discuss them now
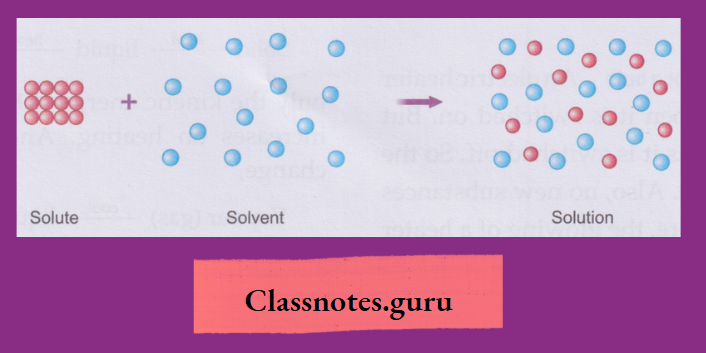
Solutions:
- A solution is a homogeneous mixture of one or more solutes with a solvent
- Common examples of solutions are the homogeneous mixtures of common salt, sugar, copper(II) sulphate, calcium chloride, potassium nitrate, potassium permanganate and ammonium chloride with water.
A solution has the important characteristics of:
- Being transparent and
- Passing through a filter paper or a membrane.
The size of the solute particles must be very small—1 nm (i.e., 10-9 m or a millionth of a millimetre) or less in diameter.

The terms solute and solvent are used in respect of a solution. They are replaced by the terms dispersed phase and dispersion (or continuous) medium, respectively, for a suspension as well as a colloid.
Suspensions
A suspension is a heterogeneous mixture of one or more dispersed phases in a dispersion medium
Muddy water is a common example of a suspension. Here, soil is the dispersed phase and water, the dispersion medium. Similarly, chalk (CaCO3) or gypsum (CaSO4.2H2O; blackboard chalk), when stirred in water, gives a suspension.
NCERT Class 8 Chemistry Chapter 8 Water
For a suspension, it is not necessary that the dispersed phase be a solid and the dispersion medium a liquid. The suspensions of
- A liquid in a liquid, called an emulsion
- Example: An oil-water emulsion
- A liquid in a gas, called fog
- Example: Water in air
- A solid in a gas, called smoke
- Example: Carbon in the air is also quite common.

The size of a dispersed particle in a suspension is much larger than that of a solute in a solution. It is 10-6 m (i.e., a millionth of a metre) or more in diameter.
A suspension is not transparent. And the dispersed particles slowly settle down because, being large, they are heavy too. You must have seen that the soil settles down from muddy water in a glass.
Colloids
A colloid is a homogeneous mixture of one or more dispersed phases in a dispersion medium. Milk is the most common example of a colloid —butterfat globules dispersed in water. Jam, jelly, whipped cream and gelatin are also common examples of a colloid.

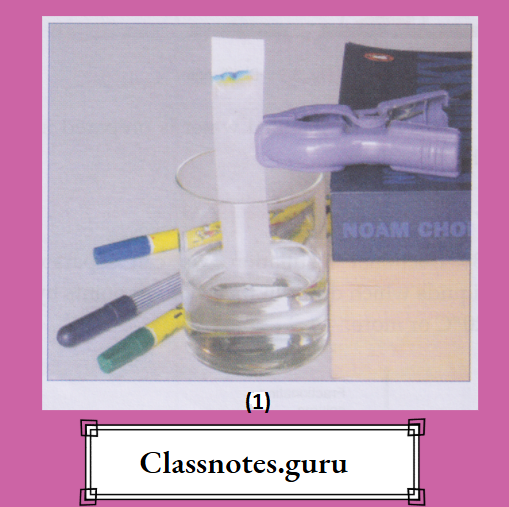
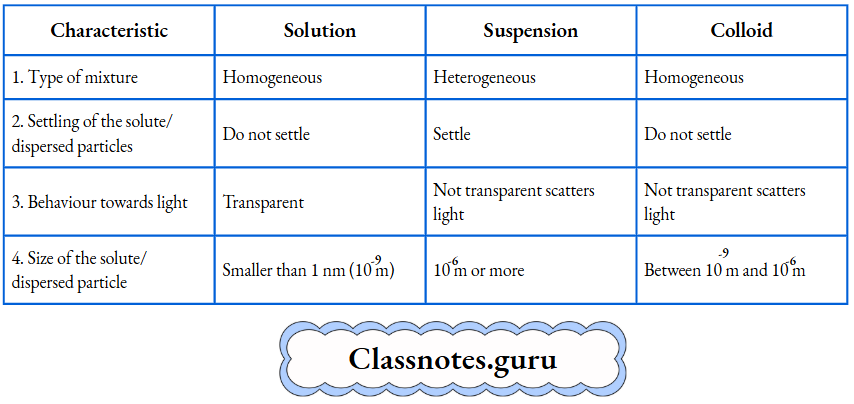
Colloids are not transparent. And the dispersed particles do not settle down. The size of a dispersed particle is in between those of a solute in a solution and a dispersed particle in a suspension, i.e., between 10-9 m and 10-6m (or greater than 1 nm and smaller than 1000 nm). The characteristics of a solution,s uspension and colloid are given in Table
Characteristics of a solution, suspension and colloid:
Solutions
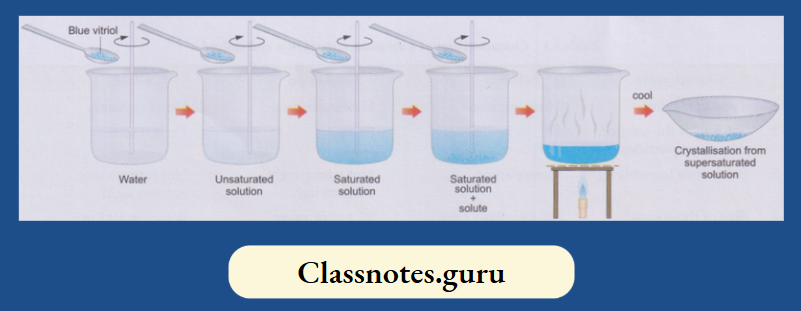
For preparing a solution, you generally add the solute in instalments (or bit by bit) to the solvent, stirring the mixture all the time. A stage comes when the mixture refuses to take in more solute. And whatever solute you add afterwards remains undissolved and settles down at the bottom.
- Now, if you heat the mixture, the undissolved solute will also dissolve. This happens because the capability of a solvent to dissolve a solute generally increases with temperature.
- In other words, the higher the temperature, the larger is the amount of a solute that can dissolve in a given amount of a solvent
- Thus, the extra amount of solute at a higher temperature should separate from the solution when the latter is cooled.
- But in most cases, the extra solute does not separate, i.e., it remains dissolved. Hasn’t the solvent retained more solute than it can dissolve at that temperature? Yes, it has.
This usually happens with those salts (as solutes) which crystallise as hydrates,
Example: Copper(II) sulphate, which crystallises as CuSO4.5H2O and iron (II) sulphate, which does so as FeSO4.7H2O. Depending on the solute content of the solution,
Water Class 8 NCERT Notes
We find that there are three kinds of solutions:
- Unsaturated
- Saturated
- Supersaturated
These are:
- Unsaturated: A solution capable of dissolving more solute at a given temperature is called an unsaturated solution.
- Saturated: A solution containing the maximum amount of solute that can be dissolved in it at a given temperature is called a saturated solution.
- Supersaturated: A solution containing more solute than the given amount of the solvent is capable of dissolving at that temperature is called a supersaturated solution
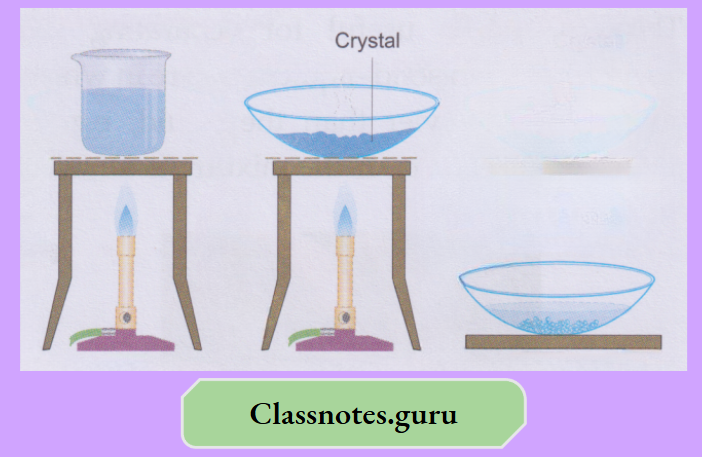
Activity:
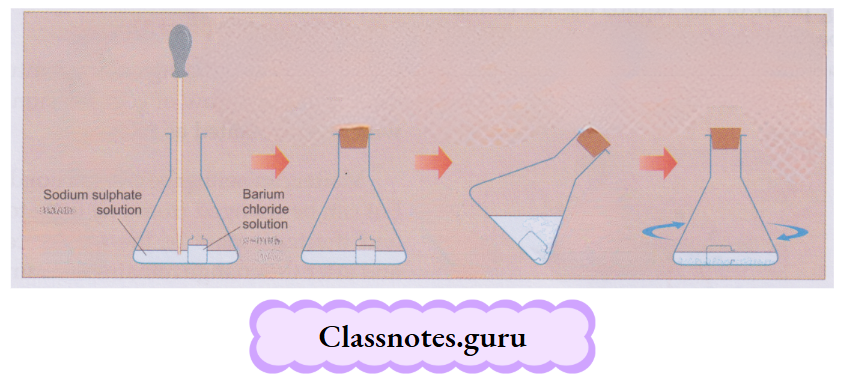
Take about 50 mL of water in a beaker and dissolve some blue vitriol crystals (CuSO4.5H2O) in it. Use a glass rod for stirring the mixture. Again, add a bit of the crystals and stir.
- That will also dissolve. You will find that if you keep adding the blue vitriol crystals to the water in small instalments and stirring, the crystals dissolve. But after a few instalments, the crystals will not dissolve any more.
- That is a saturated solution at the temperature you have prepared it. And, before that, when more and more crystals dissolved, the solution was unsaturated
- The crystals added to the saturated solution will settle down. Now heat the mixture, and you will find that the extra solute will also dissolve. Leave the solution to cool.
Nothing separates, and that solution is supersaturated. Transfer the solution to a china dish, evaporate a bit of it and leave it for a few hours (preferably overnight). Beautiful crystals of blue vitriol are obtained.
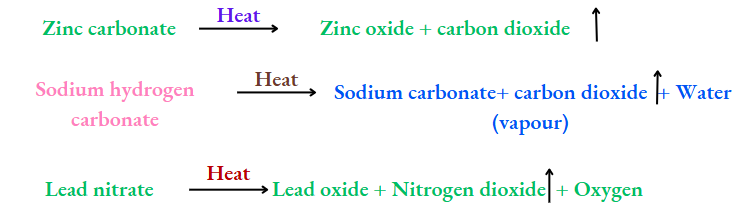
Hydrates
It has been found that whenever copper(II) sulphate is crystallised from an aqueous solution, the crystals have the formula CuSO4.5H2O.
- Similarly, iron(II) sulphate crystallises from an aqueous solution as FeSO4.7H2O.
- These water molecules appear in the same number every time and are called the water of crystallisation of a substance. A
- nd the substances (generally salts) containing such water molecules are called hydrates.
- The water molecules associated with a substance in a crystal and forming a part of the crystalline structure are referred to together as water of crystallisation.
- A substance containing water of crystallisation is called a hydrate.
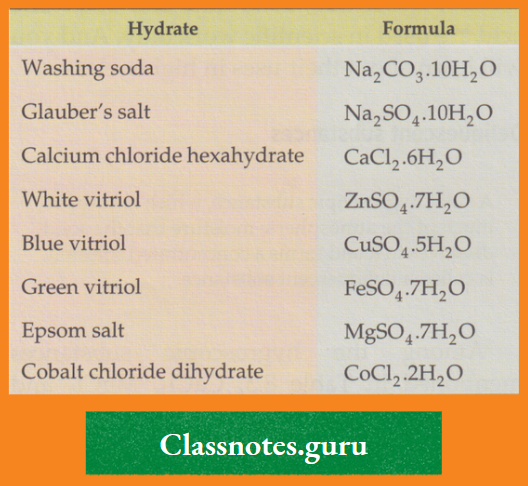
Some examples are mentioned in the Table
Some common hydrates:
The Loss of Water of Crystallisation on Heating
A hydrate, on being heated, loses its water of crystallisation. And it has been observed that it loses its crystalline structure too. You can find this for yourself by doing the following activity
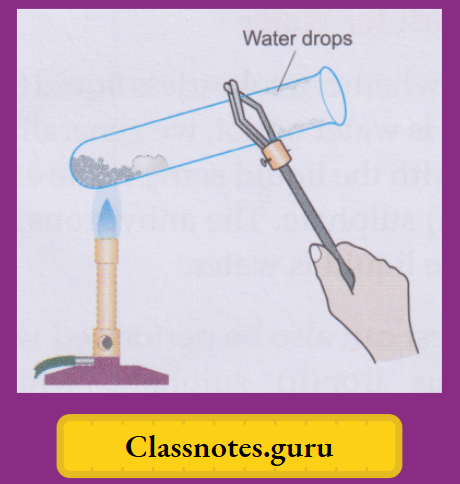
Activity:
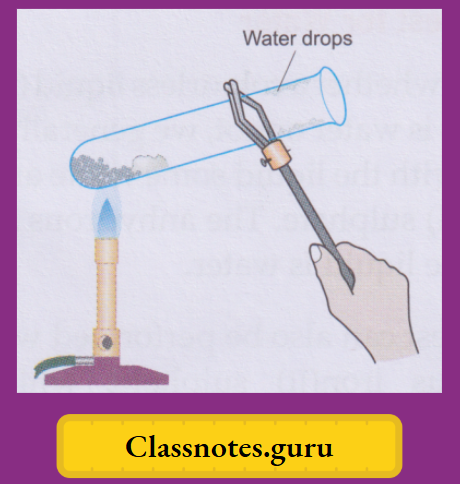
Take a few crystals of blue vitriol in a dry test tube and heat gently. You will observe that
- The salt will slowly lose its blue colour, turning white,
- The crystals will crumble down to a powdery substance, and
- Some colourless liquid drops will collect in the colder part of the test tube.
- (Tests, which we will describe soon, indicate that these are water drops.)
Cool the white powdery substance and moisten it with a drop of water. The solid turns blue again.
Water and its Properties Class 8
What happens during these changes can be summarised as follows.
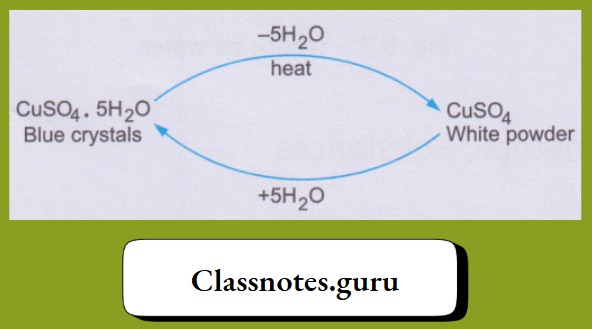
1. The blue copper(II) sulphate pentahydrate, on being heated, loses the water molecules and changes to the white anhydrous (meaning without water) copper(II) sulphate. And, on treatment with water, the anhydrous salt changes back to the hydrated salt.
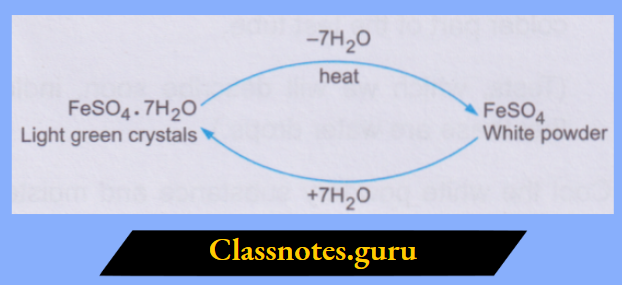
2. The crystalline structure of the hydrated salt is lost when it loses the water molecules. Thus, the water of crystallisation is a part of the crystalline structure. You can repeat the activity with the crystals of green vitriol, i.e., FeSO4.7H2O. By losing the water of crystallisation on being heated, the light green crystals change to a white, powdery solid (the anhydrous salt).
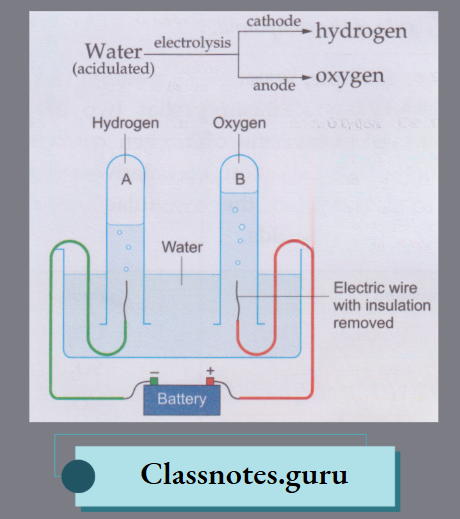
How to Test for Water
To know whether a colourless liquid (in bulk or in drops) is water or not, we generally bring in contact with the liquid some white anhydrous copper(II) sulphate. The anhydrous salt turns blue if the liquid is water. The test can also be performed with white anhydrous iron(II) sulphate, which turns green on treatment with water.
The above-mentioned anhydrous salts can be prepared by slowly heating the hydrated salts.

Hygroscopic Substances
A substance that absorbs moisture from the atmosphere is called a hygroscopic substance.
Some examples are given in the Table
Importance of Water Class 8
Some common hygroscopic substances:
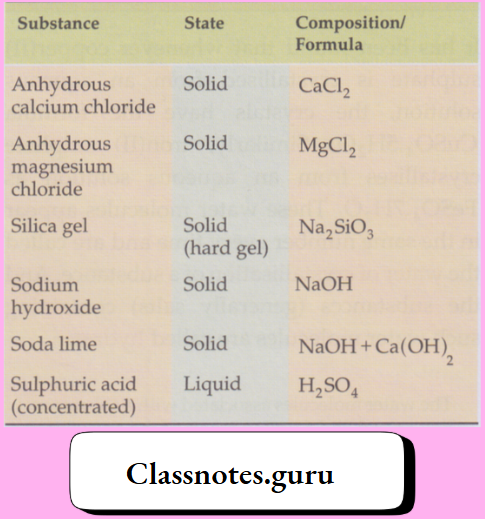
They are generally used as drying agents. You might have seen a small cloth bag, containing a solid, inside a box of medicine, thermoflask or a camera. The bag contains silica gel—a common drying agent—which keeps the air inside the box dry. Other drying agents like calcium chloride, sodium hydroxide, soda lime and sulphuric acid are used in scientific work only. And you will learn about their uses in higher classes.
Deliquescent substances
A solid hygroscopic substance, which absorbs so much of the atmospheric moisture that the solid dissolves in it and forms a concentrated solution, is called a deliquescent substance.
- Among the hygroscopic substances mentioned in Table CaCl2, MgCl2 and NaOH are deliquescent. You must have observed that rock salt or crude common salt, when left in the open in the rainy season, appears to have melted.
- It does not melt at ordinary temperature. In fact, it contains highly deliquescent substances— CaCl2 and MgCl2—as impurities. So, it absorbs so much moisture from the atmosphere in the rainy season that it gets dissolved and appears to have melted.
- However, refined table salt does not show this property as it does not contain CaCl2 or MgCl2. Similarly, solid NaOH kept open in a beaker starts looking watery within a few minutes. (Remember that after a long time, NaOH reacts with atmospheric CO2 also.)
The Action Of Metals And Metal Oxides On Water
Many metals and metal oxides react with water. To understand these reactions, we need to have an idea about the activity series. Metals along with hydrogen, have been arranged according to their activity in this series. The series consisting of some common metals is given here.
The Action of Metals on Water
Whenever a metal reacts with water, it does so with a view to displacing hydrogen from water. Obviously, only those metals can displace hydrogen from water which are more active than hydrogen, i.e., higher than hydrogen in the activity series. We can also understand that the more active the metal (i.e., the higher the metal in the activity series), the more vigorous is its reaction with water
We will discuss here the action of potassium (K), sodium (Na), calcium (Ca), magnesium (Mg) and iron (Fe) on water. We should remember that though tin (Sn) and lead (Pb) are higher than hydrogen in the activity series, they do not act on water
Water Cycle Class 8 Chemistry
Action of potassium and sodium on water:
Among the common metals, potassium and sodium are the most active ones. They are soft and get quickly affected by the moisture (and also oxygen) of the air and are, therefore, preserved in kerosene. A small piece of the metal is cut with a knife, dried by pressing between the folds of a filter paper and dropped into a trough of water. We make the following observations about the two metals
Sodium:
The metal soon changes into a silvery white globule that does not sink but darts around on the surface. A hissing sound is constantly heard. And a yellow spark flies intermittently with a ‘pop’.
The resulting solution turns red litmus blue, and so it is alkaline. We infer that sodium reacts vigorously with water to form sodium hydroxide and liberate hydrogen. At the same time, the reaction is highly exothermic, and so the metal melts to form a globule.
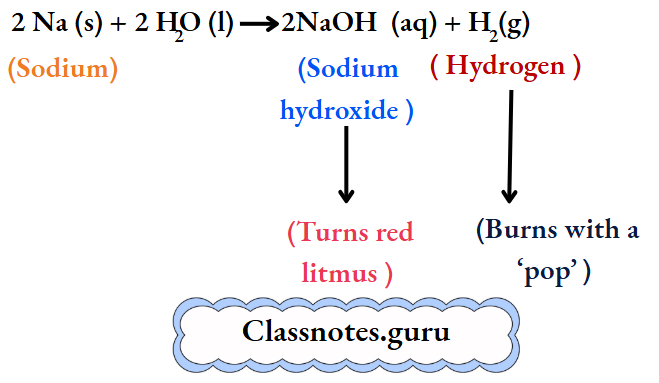
The hydrogen burns with a ‘pop’. And yellow sparks are produced by small particles of sodium. (Sodium imparts a yellow colour to a flame. Throw some common salt, i.e., sodium chloride, into the flame of a kitchen stove, and watch the colour imparted to the flame. It is yellow. Also, doesn’t a sodium vapour lamp have a yellow light?)
Potassium:
Potassium also reacts vigorously and exothermically with water to form potassium hydroxide and liberate hydrogen.
- Due to the potassium hydroxide formed, the resulting solution is alkaline and therefore turns red litmus blue.
- The only difference from the reaction of sodium is that the hydrogen liberated burns with a violet flame.
- Potassium imparts the violet colour to the flame
2K(s)(potassium)+ 2HO(l) → 2KOH(aq)(Potassium hydroxide) + H2(g)
The action of calcium on the water ribbon
Calcium is heavier than water and a piece of the metal sinks in it. The evolution ofhydrogen starts briskly but slows down soon as the lime produced forms a coating on the metal. Calcium hydroxide (slaked lime) is much less soluble than sodium hydroxide or potassium hydroxide and makes the solution turbid. The solution is alkaline, turning red litmus blue.
Ca(s)(calcium) + 2HOH(l) → Ca(OH)2 (aq) (calcium hydroxide (alkaline) + H2(g)
The action of magnesium on water
Magnesium, being less active than calcium, displaces hydrogen from water very slowly at room temperature. However, the reaction is fast with steam.
⇒ \(\mathrm{Mg}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{MgO}+\mathrm{H}_2\)
When magnesium powder is mixed with water, the evolution of hydrogen starts slowly and stops soon because the MgO forms a coating over the metal particles. But you can verify for yourself how fast the reaction with steam is
Activity:
Boil some water in a conical flask to replace the air inside with water vapour. Continue boiling and introduce a burning piece of magnesium ribbon into the mouth of the conical flask.
- The ribbon continues to burn in steam/water vapour, though the nature of the flame changes.
- In air, magnesium burns with a dazzling white flame, but in steam, it gives a smaller, orange flame due to the burning of the liberated hydrogen
The particles of magnesium oxide falling into the water make it alkaline —the solution or the mixture turns red litmus blue.
Water Purification Class 8 Chemistry
The action of iron on water
Though iron is above hydrogen in the activity series, it is much less active than magnesium. Iron displaces hydrogen from water only when steam is passed over the red-hot metal. A black oxide triiron tetroxide(Fe3O4), also called ferrosoferric oxide, is formed.
3 Fe (iron)+ 4H2O(steam) → Fe3O4 (Triiron tetroxide) (black) + 4H2 (hydrogen)
(Triiron tetroxide is considered a mixed oxide of iron(II) and iron(III), i.e., FeO. Fe2O3.)
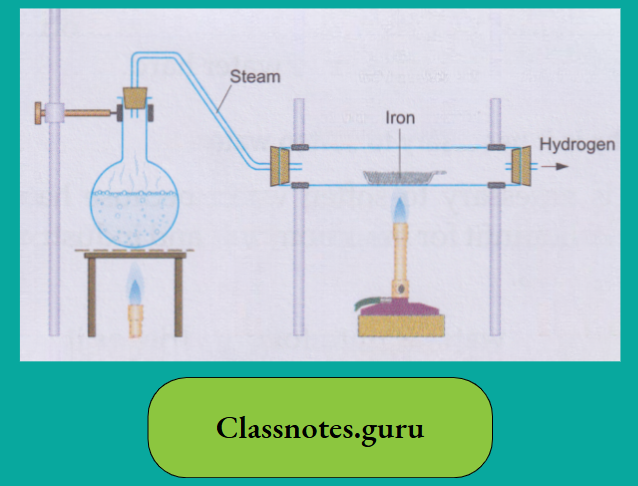
The Action of Water on Metal Oxides
The oxide of the highly active metal, like K, Na and Ca, reacts vigorously and exothermically with water to form the hydroxide of the metal.
K2O + H2O→ 2KOH
Na2O + H2O→ 2NaOH
CaO + H2O → Ca(OH)2
You know that these hydroxides are alkalis.
However, as we move down the activity series, the reactivity of the metal oxide with water sharply decreases. For example, MgO reacts with water to form Mg (OH)2 to a small extent and Al2O3, ZnO and Fe2O3, etc., to a still smaller extent.
Hard Water And Soft Water
You may have noticed that the water of some places forms lather easily with soap whereas that of other places does not lather easily.
- Water that lathers easily with soap is called soft water
- Water that does not lather easily with soap is called hard water.
What Makes Water Hard?
The presence of soluble salts (like hydrogen-carbonates, sulphates or chlorides) of calcium and magnesium in a sample of water makes it hard
Soap contains sodium salts of fatty acids. (Fatty acids are organic acids containing a large number of carbon atoms.) These salts produce lather with water. However, the calcium and magnesium salts of these fatty acids are insoluble. So, when a soap is treated with hard water, the calcium and magnesium salts of the fatty acids precipitate in the form of a scum. As a result, the soap is consumed, but no lather is produced
2Na (Ft) ( sodium salt of the fatty acid) + Ca (HCO3)2(calcium hydrogencarbonate) → 2 NaHCO3 (sodium hydrogencarbonate) + Ca(Ft)2 ↓ (calcium salt of the fatty acid)
Precipitation over clothes leaves dirty stains, and that over your body irritates the skin.
The hardness of water increases with the amount of dissolved calcium and magnesium salts. But remember that dissolved sodium or potassium salts
Example: NaCl, K2SO4, etc.) do not make water hard. This is because the sodium and potassium salts of fatty acids do not precipitate. And water containing sodium and potassium salts does lather with soap.
Temporarily and Permanently Hard Water
The hardness of some water samples can be removed by boiling, but not of all. On this basis, hard water is classified into two types.
- If the hardness ofa watersample can be removed by boiling, it is called temporarily hard water.
- If the hardness of a water sample cannot be removed by boiling, it is called permanently hard water
Temporary hardness is caused by the dissolved hydrogencarbonates of calcium and magnesium carbonates. Permanent hardness is caused by the dissolved sulphates and chlorides of calcium and magnesium.
Softening of Water
If the hardness of water is removed, soft water is produced, and the process is called softening of water.
Physical and Chemical Properties of Water Class 8
The following methods are used to soften water:
1. Boiling:
Temporarily hard water can be softened by boiling it. When such water is heated, the hydrogencarbonates of calcium and magnesium are decomposed to the carbonates. Being insoluble, the carbonates precipitate out
Ca(HCO3)2 (calcium hydrogencarbonate)→ CaCO3 + CO2↑ + H2O
2. Treating with washing soda. Permanent hardness of water is removed by treating with washing soda (Na2CO3.10H2O). A solution of washing soda is added to the water, and the carbonates of calcium and magnesium are precipitated
CaSO4(calcium sulphate) + Na2CO3 (sodium carbonate) → CaCO3 ↓ (calcium carbonate) + Na2SO4 (sodium sulphate) (in solution)
CaCl2(calcium chloride)+ Na2CO3 (sodium carbonate) → CaCO3 (calcium carbonate) + 2 NaCl (sodium chloride) (in solution)
The sodium sulphate and sodium chloride formed will not make the water hard
Water Resources and Conservation Class 8
Why is it necessary to soften water?
It is necessary to soften water because hard water is unfit for most domestic and industrial purposes.
- Hard water is unfit for laundries as it
- Consumes too much soap, and
- Leaves dirty stains of calcium and magnesium salts of fatty acids on the cloth.
- Hard water is not very suitable for bathing. The precipitates of calcium and magnesium salts of fatty acids, formed on reaction with soap, irritate the skin.
- It is not possible to properly cook hard foodstuffs, like pulses, in hard water.
- Though not injurious to health, hard water does not have an agreeable taste.
- When used for industrial purposes (mainly in boilers), hard water produces white deposits of insoluble substances, called scales.
- The scales consist mainly of CaCO3, MgCO3 and CaSO4. They deposit on the walls of the boiler and do not allow proper conduction of heat.
- They also block the pipes, which may cause serious accidents.