Anti viral Agents Introduction
Anti virals are a class of medications that are used to treat viral infections. Most viral infections resolve spontaneously in immune-competent individuals.
- Antiviral therapy aims to minimize symptoms and infectivity as well as to shorten the duration of illness. These drugs act by arresting the viral replication cycle at various stages.
- Currently, antiviral therapy is available only for a limited number of infections.
- Most of the currently available antiviral drugs are used in the therapy of infections caused by HIV, herpes viruses, hepatitis Band C viruses, and influenza A and B viruses.
- Because viruses are obligate, intracellular parasites, it is difficult to find targets for the drug that interfere with viral replication without also harming the host cells.
- Unlike other antimicrobials, none of the antiviral drugs deactivate or destroy the microbe (in this case, the virus); rather they act by only inhibiting replication.
- Thus, they prevent the viral load from increasing to a point where it could cause pathogenesis, leaving it susceptible to neutralization by the body’s innate immune mechanisms.
Anti-Retroviral
1. Nucleoside Reverse Transcriptase Inhibitors (NRTIs): Abacavir, Didanosine, Lamivudine, Stavudine, Tenofovir, Zalcitabine, Zidovudine, Emtricitabine
2. Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs): Delavirdine, Efavirenz, Nevirapine
3. Protease Inhibitors: Nelfinavir, Amprenavir, Saquinavir, Ritonavir, Indinavir, Lopinavir, Atazanavir, Fosamprenavir, Tiranavir
4. Fusion Inhibitors: Enfuvirtide
Anti-Herpes Virus
Iodoxuridine, Acyclovir, Penciclovir, Famciclovir, Ganciclovir, Fomivirsen, Cidofovir, Valaciclovir, Foscarnet
Anti-Influenza Virus
Amantadine, Rimantadine, Oseltamivir, Zanamivir
Read and Learn More Medicinal Chemistry III Notes
Anti-Hepatitis B
Adefovir, dipivoxil, Entecavir
Drugs For Other Viral Infections
Interferon a, Peginterferon a-2b, Ribavirin
1. Anti – Retroviral
Nucleoside Reverse Transcriptase Inhibitors (NRTIs):
NRTIs in the presence of host cell thymidine kinase convert into active triphosphate metabolite (nucleotides) → compete with corresponding nucleotide for incorporation into viral DNA → inhibit reverse transcriptase enzyme and termination of viral DNA synthesis.
Abacavir:
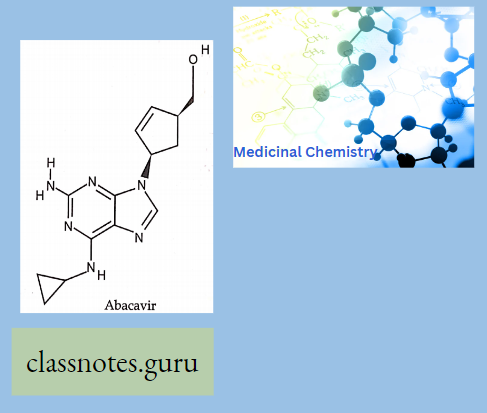
- Abacavir (ABC) is a powerful nucleoside analog reverse transcriptase inhibitor (NRTl) used to treat HIV and AIDS, Chemically
- it is a synthetic carbocyclic nucleoside and is the enantiomer with IS, 4R absolute configuration on the cyclopentene ring. In vivo, ab. David sulfate dissociates from its free base.
Didanosine:
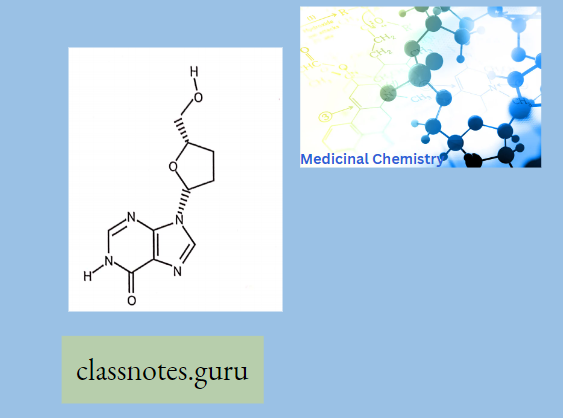
A dideoxynucleoside compound in which the 3′-hydroxy group on the sugar moiety has been replaced by hydrogen.
- This modification prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains.
- Didanosine is a potent inhibitor of HIV replication, acting as a chain terminator of viral
- DNA by binding to reverse transcriptase is then metabolized to deoxyadenosine triphosphate, its putative active metabolite.
Lamivudine:
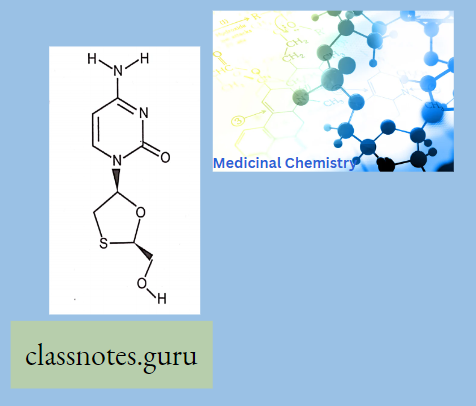
- Lamivudine is a Hepatitis B Vims Nucleoside Analog Reverse Transcriptase Inhibitor. The mechanism of action of lamivudine is as a Nucleoside Reverse Transcriptase Inhibitor.
- The chemical classification of lamivudine is the Nucleoside Analog Zalcitabine
Zidovudine:
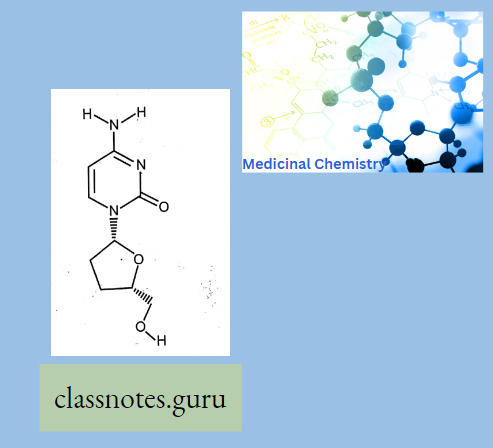
A dideoxynucleoside compound in which the 3′-hydroxy group on the sugar moiety has been replaced by hydrogen.
- This modification prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains.
- The compound is a potent inhibitor of HIV replication at low concentrations, acting as a chain-terminator of viral DNA by binding to reverse transcriptase.
- Its principal toxic side effect is axonal degeneration resulting in peripheral neuropathy.
Zidovudine (AZT):
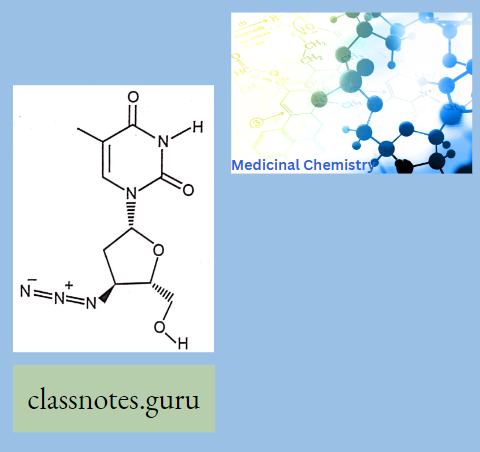
It is a thymidine analog and is also known as azidothymidine. It is converted into active triphosphate metabolite and incorporated in the viral DNA that inhibits reverse transcriptase enzyme and terminates the DNA chain synthesis.
- A dideoxynucleoside compound in which the 3′-hydroxy group on the sugar moiety has been replaced by an azido group.
- This modification prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains.
- The compound is a potent inhibitor of HIV replication, acting as a chain-terminator of viral DNA during reverse transcription. It improves immunologic function, partially reverses the HIV-induced neurological dysfunction, and improves certain other clinical abnormalities associated with AIDS.
- Its principal toxic effect is dose-dependent suppression of bone marrow, resulting in anemia and leukopenia.
- Zidovudine treatment significantly reduces the incidence of in-utero transmission of HIV from infected mother to fetus.
- Zidovudine and Stavudine are not used together because they appear to be antagonistic actions.
Adverse Effects:
- All NRTIs show lactic acidosis, hepatic steatosis, and lipodystrophy (all higher with stavudine).
- Zidovudine: Bone marrow suppression-anemia, neutropenia; gastrointestinal intolerance, headache.
- Didanosin: pancreatitis, peripheral neuropathy, gastrointestinal intolerance.
- Stavudine: pancreatitis, peripheral neuropathy
- Tenofovir (diphosphonate diester of nucleoside): Headache, gastrointestinal intolerance, renal impairment.
Adverse Effects:
- All NNRTI:
- Rash (most common) Rash can progress to Stevens-Johnson syndrome.
- Nevirapine: Hepatotoxicity, rash including Stevens-Johnson syndrome, induces the metabolism of protease inhibitors and oral contraceptives.
Efavirenz:
- Neuropsychiatric reactions and teratogenic Protease inhibitors Protease inhibitors bind to the active site of protease enzyme → prevent the cleavage of gag-pol polyprotein → inhibit the maturation of virus resulting production of immature, non-infectious viral particles.
- The administration of Ritonavir with another PI is known as boosted therapy. All PI is metabolized in the liver and excreted in the fecal.
Adverse Effects:
- All protease inhibitors: lip dystrophy (fat accumulation Fat redistribution- Buffalo Hump), hyperlipidemia, insulin resistance and diabetes, elevated liver function tests, inhibits metabolism of other protease inhibitors.
- Amprenavir, Fosamprenavir: Gastrointestinal intolerance, rash
D. Fusion Inhibitors: Enfuvirtide
Fusion inhibitors gll20 and gP41 Enfuvirtide is a synthetic polypeptide and binds with viral surface glycoprotein 120 and 41 to inhibit the fusion of HIV with host cells (CD4+ T helper cell) before the virus enters the cell and begins its replication process. Adverse reaction Injection site reaction, hypersensitivity reactions.
Anti-Herpes Virus:
Iodoxuridine, Acyclovir, Penciclovir, Famciclovir, Ganciclovir, Fomivirsen, Cidofovir, Valaciclovir, Foscarnet
Mechanism Of Action:
- Nucleoside analogs drugs in the presence of viral thymidine kinase are converted into mono phosphate nucleoside which converts into active triphosphate nucleotide active nucleotide inhibits the DNA polymerase and inhibits DNA synthesis.
- Foscarnet directly blocks DNA polymerase reverses transcriptase enzyme and inhibits DNA synthesis.
Anti Influenza Agent
Amantadine, Rimantadine, Oseltamivir, Zanamivir
Anti-Influenza Agent:
- Amantadine and Rimantadine prevent the uncoating of the influenza A virus and entry into the host cells.
- Oseltamivir and Zanamivir inhibit the neuraminidase (sialidase) in Influenza A and B → preventing the release of virions from host cells (spreading of virus).
Amantadine:
It is the organic compounds-adamantylamine or 1-amino adamantane, meaning it consists of an adamantane backbone that has an amino group substituted at one of the four methyne positions. Rimantadine is a closely related derivative of adamantane with similar biological properties.
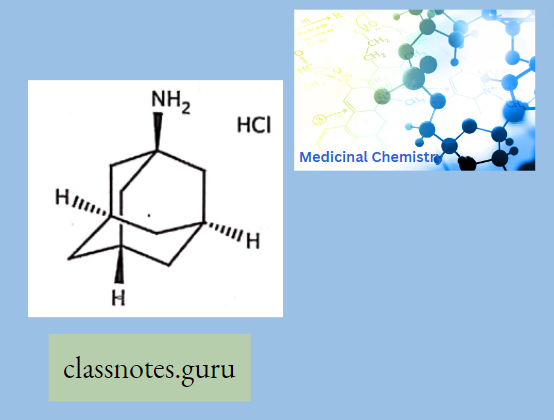
- Amantadine Mechanism Of Action: The mechanism by which Amantadine exerts its antiviral activity is not clearly understood. It appears to mainly prevent the release of infectious viral nucleic acid into the host cell by interfering with the function of the transmembrane domain of the viral M2 protein. In certain cases, Amantadine is also known to prevent virus assembly during virus replication. It does not appear to interfere with the immunogenicity of inactivated influenza A virus vaccine.
- Rimantidine: Rimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenza virus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the severity of influenza. Both rimantadine and the similar drug amantadine are derivates of adamantane.
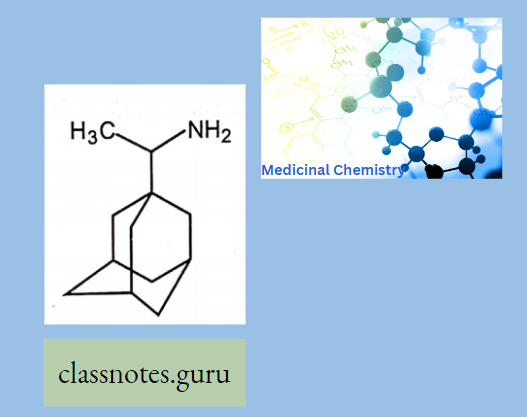
Rimantidine Mechanism Of Action: The mechanism of action of Rimantadine is not fully understood.
- Rimantadine appears to exert its inhibitory effect early in the viral replicative cycle, possibly inhibiting the uncoating of the virus.
- Genetic studies suggest that a virus protein specified by the virion M2 gene plays an important role in the susceptibility of influenza A virus to inhibition by Rimantadine.
Oseltamivir:
- Oseltamivir is an antiviral medication that blocks the actions of influenza virus types A and B in your body.
- Oseltamivir is used to treat influenza in people 2 weeks of age and older who have had flu symptoms for 2 days or less.
- Oseltamivir may also be given to prevent influenza in people who are at least 1 year old, and who may be exposed but do not yet have symptoms.
- This medicine will not treat the common cold.
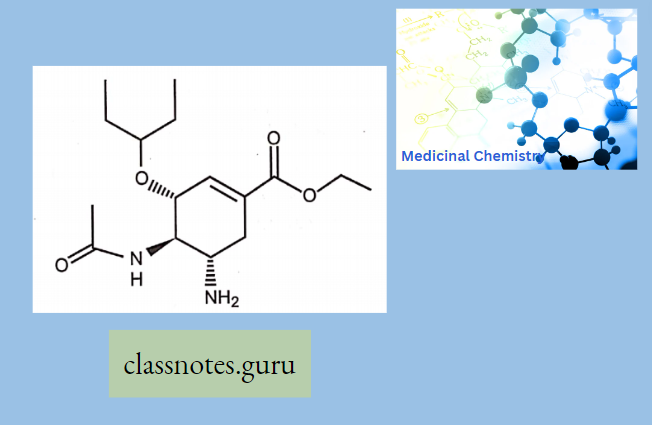
Oseltamivir Mechanism of Action: Oseltamivir inhibits the neuraminidase enzyme, which is expressed on the viral surface. The enzyme promotes the release of virus from infected cells and facilitates viral movement within the respiratory tract. In the presence of neuraminidase inhibitors, virions stay attached to the membrane of infected cells and are also entrapped in respiratory secretions.
Anti-Hepatitis B: Adcfovir, dipivoxil, Enlecavir
Drugs For Other Viral Infections: Interferon a, Peginterferon a-2b, Ribavirin
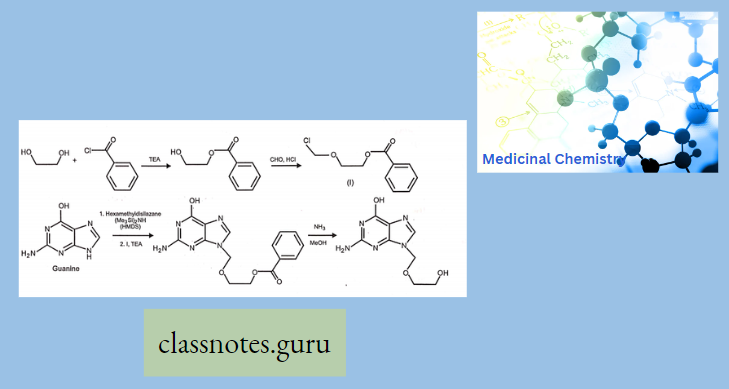
Synthesis Of Acyclovir
Anti viral Agents Multiple Choice Question And Answers
Question 1. Isoniazid is
- Pyridine derivative
- Trioxane derivative
- Guanidine derivative
- None
Answer: 1. Pyridine derivative
Question 2. Isoniazid act on
- Mycolic acid
- Arabino galactose
- Cox V
- All
Answer: 1. Mycolic acid
Question 3. Ethambutol act on
- Mycolic acid
- Arabino galactose
- Cox
- All
Answer: 2. Arabino galactose
Question 4. Neurotoxicity is the adverse effect of…
- Isoniazid
- Ethambutol
- Both
- None
Answer: 1. Isoniazide
Question 5.Visual disruption Adverse effect of
- Isoniazid
- Pyrazinamide
- Ethambutol
- None
Answer: 3. Ethambutol
Anti viral Agents Short Question And Answers
Question 1. Write the mode of action of isoniazid.
Answer:
Mode Of Action Of Isoniazid: INH inhibits Mycolase Synthase, an enzyme necessary for the biosynthesis of mycolic add (essential constitute of mycobacterial cell wall).
Question 2 Write the mode of action of pyrazinamide.
Answer:
Mode Of Action of Pyrazinamide
- PZA enters the cell wall of M. tuberculosis via passive diffusion and it is converted to pyrazinoic acid (Active metabolite) by pyrazinamidase enzyme.
- Then later it inhibits mycobacterial fatty acid synthase-I enzyme and disrupts mycolic acid synthesis needed for mycobacterium cell wall synthesis.
Question 3 Write the adverse effect of Cycloserine.
Answer:
The Adverse Effect Of Cycloserine
- Peripheral Neuritis
- Tremors
- Psychotic
- Behavioral changes
Question 4 Write the adverse effect of isoniazid.
Answer:
The Adverse Effect Of Isoniazid
- Peripheral Neuritis-Co administration of Pyridoxine (Vit B6) with INH prevents the symptoms of peripheral neuritis.
- GIT disturbance (Constipation, Loss of appetite)
- Hepatotoxicity
Question 5 Write the mode of action of Rifampicin.
Answer:
The Mode Of Action Of Rifampicin
- It strongly binds to the (3 subunit of bacterial ‘DNA dependent RNA polymerase’ enzyme.
- Thereby inhibits the RNA synthesis of bacteria. Mammalian RNA polymerase does not bind to rifampicin.
Question 6. Write the adverse of Rifampicin.
Answer:
The Adverse Of Rifampicin
- Hepatitis risk may increase when used in combination with INH.
- The flu-like syndrome is characterized by fever, chills, myalgias, and thrombocytopenia. Rifampicin imparts a harmless red-orange color to urine.
Anti Fungal Agents Introduction
Fungal infections are caused by eukaryotic organisms and for that reason, they generally present more difficult therapeutic problems than bacterial infections.
There are relatively few agents that can be used to treat fungal infections. The fungal cell wall may be considered to be a prime target for selectively toxic antifungal agents because No clinically available inhibitor of chitin synthesis analogous to the b-lactams exists at present, even though much effort is being directed toward developing such agents. Other targets are currently being exploited.
Classification Of Antifungal Agents
Polyenes:
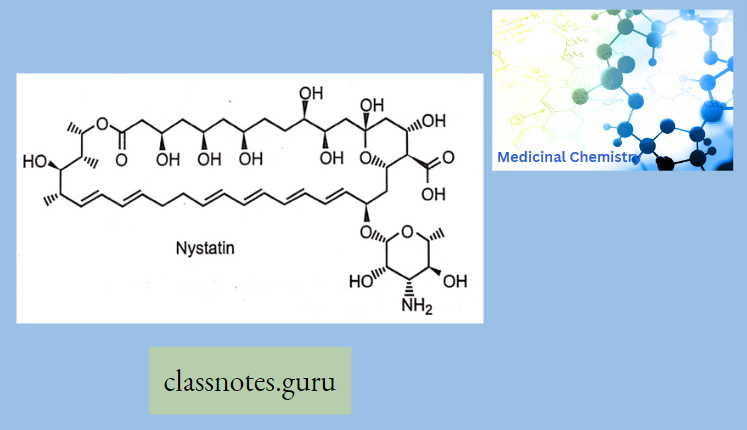
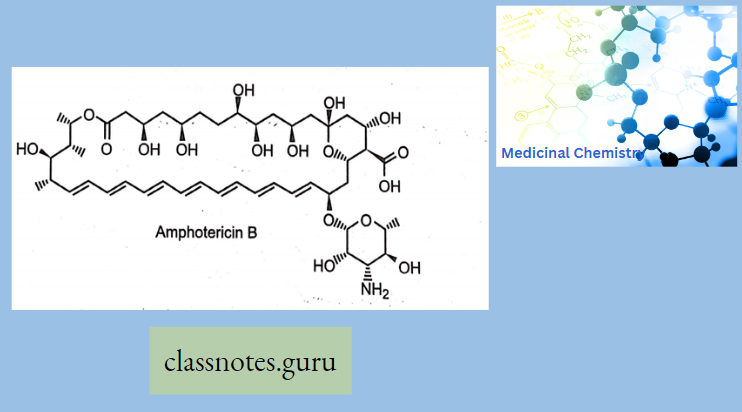
Amphotericin B (Amb): It is obtained from Streptomyces nodosus. Amphotericin B is an amphoteric polyene macrolide (polyene = containing many double bonds; macrolide = containing a large lactone ring of 12 or more atoms) and gives fungicidal action.
- It is nearly insoluble in water (orally for topical infections), and is therefore prepared as a colloidal suspension of amphotericin B and sodium desoxycholate for intravenous injection (for systemic infections)
Mechanism Of Action: Amphotericin B binds with ergosterol (a component of the fungal cell membrane) → AMB- ergosterol complex alters the membrane permeability →creates pores in the membrane → pores allow the leakage of intracellular ions, amino acids, and micromolecules→ cell death.
Clinical Uses: AMB is topically applied for oral, vaginal, and cutaneous candidiasis and partially used for systemic infections. AMB is the most effective drug for resistant cases of kala-azar.
Adverse Effects: Infusion-related fever, chills, muscle rigor, and hypotension (histamine release) occur during i.v. infusion (a test dose is advisable) and can be alleviated partly by pretreatment with NSAIDs, antihistamines, meperidine, and adrenal steroids.
Greisofulvin:
- Heterocyclic benzofuran Greisofulvin
- It is isolated from Penicillium griseofulvum and cures infections due to dermatophytes (ringworm) when administered orally.
- It is ineffective against Candida albicans.
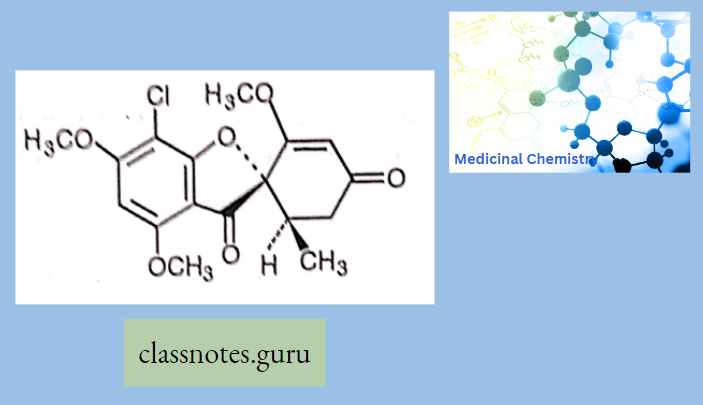
Greisofulvin Mechanism Of Action
- Griseofulvin interacts with microtubules of the mitotic spindle and with cytoplasmic microtubules →disorientation of mitotic microtubules and interferes in the mitosis → inhibits the growth of fungal hyphae.
- Griseofulvin has very low water-soluble damage → low absorption → absorption improved by taking it with fatty meals and microfinancing the drug particles.
- Now use ultrafine microparticles →increase Griseofulvin absorption and reduce to Vi dose compared to microfine particle formulations.
Greisofulvin Adverse Effect: Headache, GIT disturbances, peripheral neuritis, rashes, leukopenia.
Greisofulvin Interactions: Griseofulvin induces warfarin metabolism and reduces the efficacy of oral contraceptives.
Clotrimazole: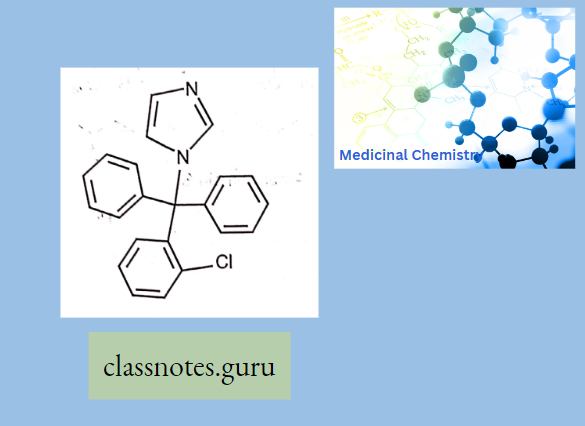
Clotrimazole Mechanism Of Action:
- Clotrimazole works by inhibiting tire growth of individual Candida or fungal cells by altering the permeability of the fungal cell wall.’ It binds to phospholipids in the cell
- membrane and inhibits the biosynthesis of ergosterol and other sterols required for cell membrane production.
Econazole
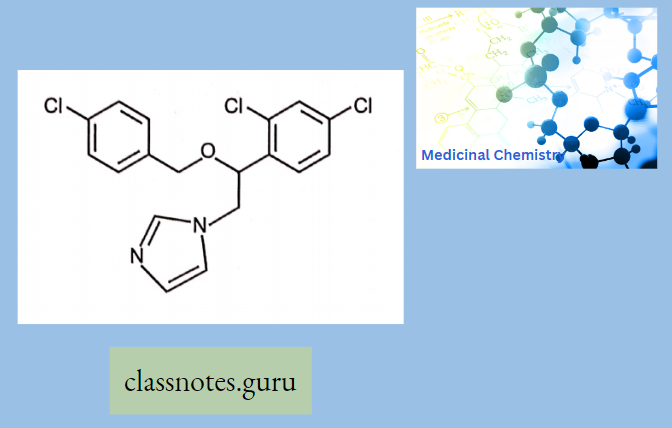
Econazole Mechanism of Action: Econazole interacts with 14-ct demethylase, a cytochrome P-450 enzyme necessary to convert lanosterol to ergosterol. As ergosterol is an essential component of the fungal cell membrane, inhibition of its synthesis results in increased cellular permeability causing leakage of cellular contents.
Miconazole:
![]()
Miconazole inhibits the synthesis of ergosterol, a major component of fungal cell membranes. This interferes with the barrier function of the membrane and with membrane-bound enzymes.
Ketoconazole:
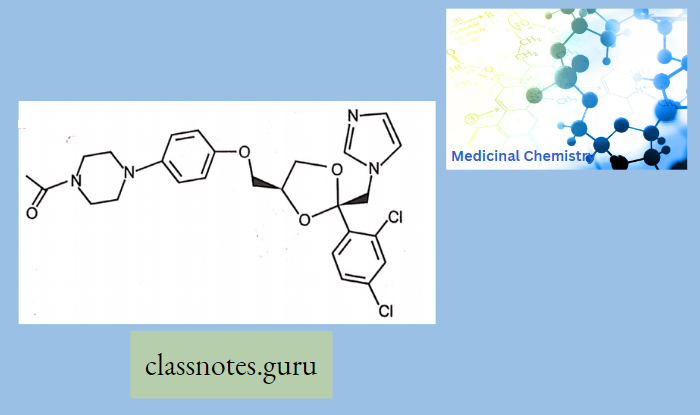
As an antifungal, ketoconazole is structurally similar to imidazole and interferes with the fungal synthesis of ergosterol, a constituent of fungal cell membranes, as well as certain enzymes. This enzyme participates in the sterol biosynthesis pathway that leads from lanosterol to ergosterol.
Itraconazole:
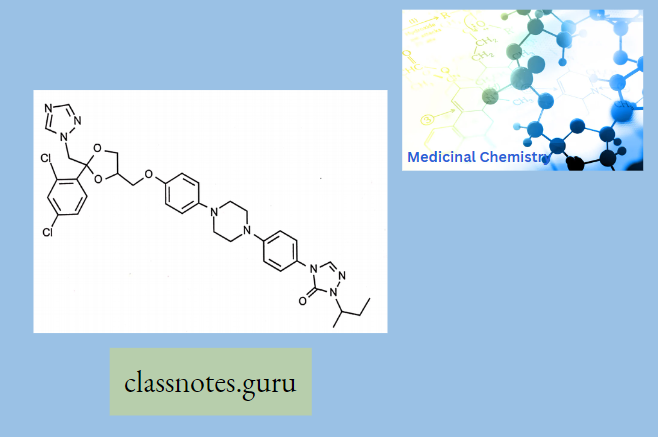
The mechanism of action of itraconazole is the same as the other azole antifungals: it inhibits the fungal-mediated synthesis of ergosterol, via the inhibition of lanosterol 14a demethylase.
Metabolites: Hydroxy-itraconazole, keto-itraco.
Fluconazole:
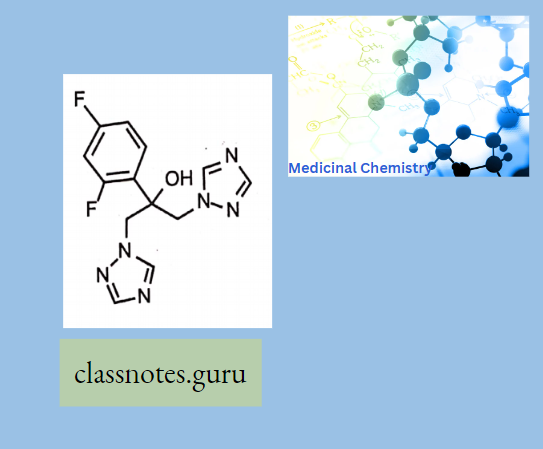
Mechanism of action:
Like other imidazole- and triazole-class antifungals, fluconazole inhibits the fungal cytochrome P450 enzyme 14a-demethylase.
Fluconazole is primarily fungistatic; however, it may be fungicidal against certain organisms in a dose-dependent manner, specifically Cryptococcus
Naftifine:
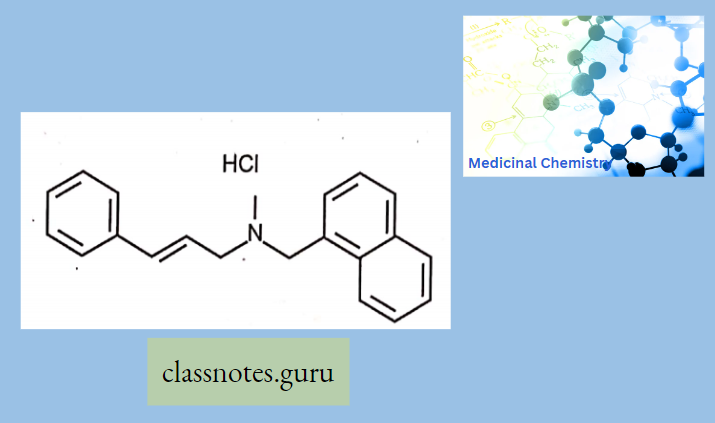
Naftifine has a triple action: antifungal, antibacterial, and anti-inflammatory. Its precise mechanism of action is unknown but may involve selectively blocking sterol biosynthesis via inhibition of the squalene 2,3-epoxidase enzyme.
Tolfnatate:
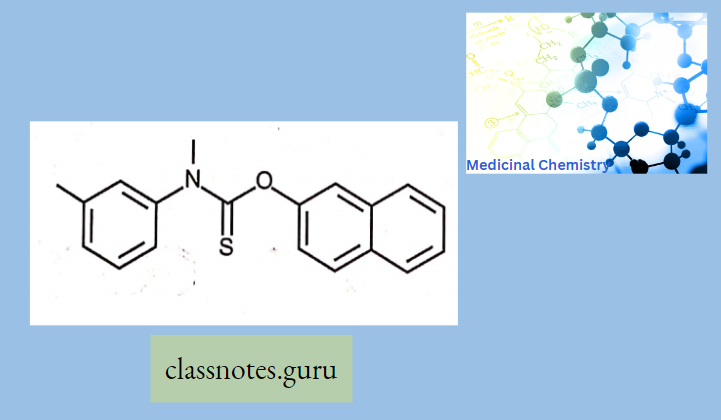
Mechanism of action is not entirely known, it is believed to inhibit squalene epoxidase, an important enzyme in the biosynthetic pathway of ergosterol (a key component of the fungal membrane) in a similar way to allylamines.
Synthesis Of Miconazole
In hexamethylphosphoramide (an aprotic solvent) which was then extracted with nitric acid to give miconazole nitrate.
![]()
Synthesis Of Tolfnatate
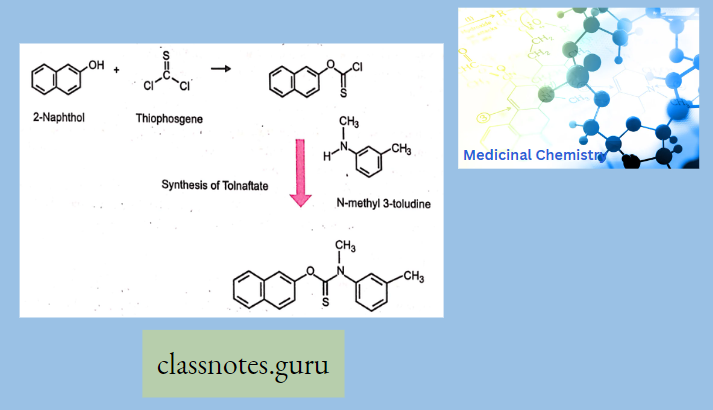
Anti Fungal Agents Multiple Choice Question And Answers
Question 1.Griesofulvin is
- Benzofurnn
- Trioxane derivative
- Guanidine Derivative
- None
Answer: 1. Benzofurnn
Question 2.Gricsofulvin net on
- Microtubule
- Arabino galactose
- Cox
- All
Answer: 1. Microtubule
Question 3.Fluconazoleact on
- Mycolic acid
- Cytochrome P450 enzyme 14a-demethylase
- Cox
- None
Answer: 2. Cytochrome P450 enzyme 14a-demethylase
Question 4. Polyenesderivntive of…
- Amphotericin
- Ethambutol
- Both
- None
Answer: 1. Amphotericin
Anti-Fungal Agents Short Question And Answers
Question l. Write the mode of action of Griesofulvin.
Answer:
Mode of Action of Griseofulvin: Griseofulvin interacts with microtubules of the mitotic spindle and with cytoplasmic microtubules → disorientation of mitotic microtubules and interferes in the mitosis→inhibits the growth of fungal hyphae.
Question 2. Write the mode of action of fluconazole.
Answer:
Mode of Action of Fluconazole: Like other imidazole- and triazole-class antifungals, fluconazole inhibits the fungal cytochrome P450 enzyme 14a-demethylase.
Question 3. Write the adverse effect of amphotericin β.
Answer:
Adverse Effect of Amphotericin β: Infusion-related fever, chills, muscle rigor, and hypotension (histamine release) occur during i.v. infusion (a test dose is advisable) and can be alleviated partly by pretreatment with NSAIDs, antihistamines, meperidine, and adrenal steroids.
