Chapter 3 Elements, Compounds And Mixtures Notes
As you know, substances are classified as elements, compounds and mixtures. This classification is of great interest to chemists as it helps describe the kind of matter anything contains
Elements
An element cannot be split into simpler substances by chemical means. For example, hydrogen, nitrogen, oxygen, carbon, sulphur, phosphorus, iron, copper, silver and gold are elements as they cannot be split into simpler substances by chemical means.
Read And Lean More Class 8 Chemistry
- Remember that a chemical means is much more powerful than a physical means like filtration, sublimation or distillation.
- Thus, a substance which cannot be split by a chemical means will not be broken down by filtration, sublimation or distillation either.
- A substance that cannot be split into simpler substances, by a physical means like filtration, sublimation or distillation a pure substance
NCERT Solutions For Elements Compounds And Mixtures
So, an element is a pure substance.

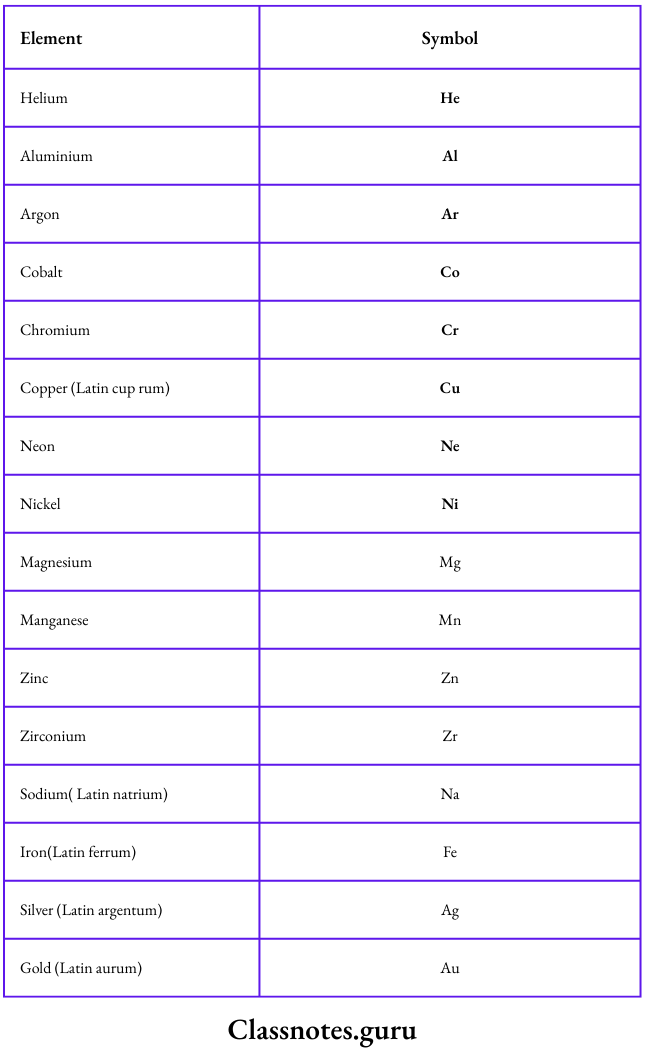
Symbols represent elements
- A one-letter or a two-letter symbol represents an element.
- The one-letter symbols are used for elements like hydrogen (H), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), sulphur (S), phosphorus (P) and iodine (I).
- These symbols are the first letters of the English names of the elements.
- Two-letter symbols were introduced to distinguish between those elements whose names start with the same letter of the alphabet
Examples:
- Hydrogen/helium, carbon/ cobalt, chromium/copper, nitrogen/nickel, iodine/indium, magnesium/manganese, etc..
- Such a symbol is made up of the first and one more letter of the English or Latin name of the element.
Elements And Compounds NCERT Notes
Some examples are given below:
Compounds
- A compound is a substance that can be split into simpler substances by chemical means.
- A compound can be split into simpler substances only by a chemical means, and not by a physical means like filtration, sublimation or distillation.
- A compound is formed by the combination of two or more elements.
For example, the compound:
- Water is formed by the combination of hydrogen and oxygen,
- Carbon dioxide, by that of carbon and oxygen,
- Sulphur dioxide, by that of sulphur and oxygen,
- Calcium carbonate, by the use of calcium, carbon and oxygen, and
- Sugar, by the way of carbon, hydrogen and oxygen
Splitting of a Compound into Simpler Substances
As we have just said, a compound can be split into simpler substances by chemical means. Some examples are given below.
NCERT Class 8 Chemistry Chapter 3 Elements, Compounds, And Mixtures
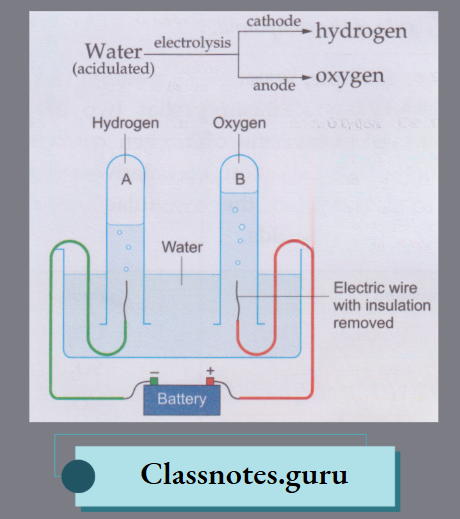
Electrolysis of Water:
Electrolysis is chemical decomposition brought about by passing an electric current through a liquid or a solution.
- Pure water is a bad conductor of electricity, i.e., it does not allow an electric current to pass through it.
- But water mixed with a small amount of an acid (hydrochloric or sulphuric acid)—called acidulated water—is a good conductor of electricity.
- When an electric current is passed through acidulated water using a setup of this kind, water decomposes to give hydrogen at the negative electrode (cathode) and oxygen at the positive electrode (anode).
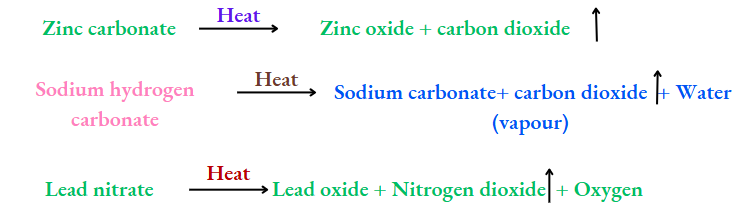
Thermal decomposition:
The splitting of a substance into simpler substances by the action of heat on it is called thermal decomposition.
For example:
Chalk, i.e., calcium carbonate (which contains calcium, carbon and oxygen), on being heated, gives calcium oxide (containing calcium and oxygen) and carbon dioxide (containing carbon and oxygen).
Both products are simpler than calcium carbonate, and so the latter is a compound.

There are other examples too:
Compounds are Represented by Formulae
A compound is represented by a formula, which shows how many atoms of which element constitute a molecule of the compound.
Elements, Compounds And Mixtures NCERT Notes
For example:
Water is represented by the formula H2O, indicating that two atoms of hydrogen and one of oxygen constitute a molecule of it. You can derive similar information from the formulae of the following compounds.

Important Characteristics of a Compound
You should remember some important characteristics of a compound.
1. A compound can be split into its constituent elements only by chemical means and not by physical means like filtration, sublimation and distillation.
For example:
Water cannot be split into hydrogen and oxygen by any of the physical means mentioned above.
But it can be split by a chemical means like electrolysis. Hydrogen can also be obtained from water by the chemical action of an active metal like sodium on water.
⇒ \(\begin{aligned}\text { Sodium }+ \text { water } \rightarrow \text { sodium hydroxide + hydrogen} \uparrow\end{aligned}\)
Similarly, hydrogen can be obtained from an acid (like hydrochloric or sulphuric acid) by the action of a metal like magnesium, zinc or iron on the acid.
Magnesium + Hydrochloric acid( dilute) → MAgnesium chloride + Hydrogen ↑
Zinc + Sulphuric acid(dilute) → Zinc sulphate + Hydrogen ↑

2. The properties of a compound are entirely different from those of the constituent elements.
For example:
Hydrogen (a combustible gas) reacts with oxygen (a supporter of combustion) to form water (which is neither combustible nor a supporter of combustion).
You can also compare the properties of carbon dioxide with those of its constituent elements, carbon and oxygen.
- Again, the bright yellow compound mercurous iodide is a product of two constituent elements—mercury and iodine. Mercury is a silver-white liquid metal and iodine, a violet-black solid.
- The constituent elements of copper(II) sulphate pentahydrate (CuSO4.5H2O—a blue solid) are the red metal copper, yellow solid sulphur and colourless gases hydrogen and oxygen.
3. Elements combine in a fixed proportion of atoms to form a compound.
For example:
2 atoms of hydrogen combine with 1 atom of oxygen to form
Atomic ratio of elements in some compounds:
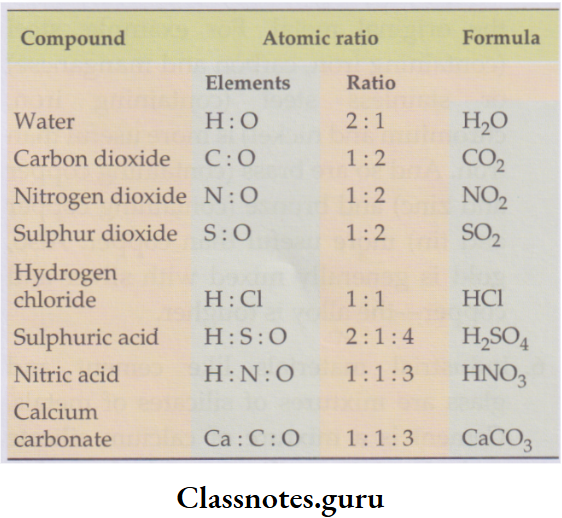
1 molecule of water. And the atomic ratio of hydrogen to oxygen in any sample of water will be 2:1.
- Thus, 10 billion hydrogen atoms will require 5 billion oxygen atoms to form 5 billion molecules of water.
- The atomic ratios of elements in some compounds are given in Table 3.1, from which you can easily guess the formulae of the compounds.
4. A compound contains the constituent elements in a fixed proportion of mass.
For example:
By mass, water contains 1 part of hydrogen and 8 parts of oxygen.
- So, the mass ratio of H to O in water is 1 : 8. Can you guess how many grams of water will be formed by 1 g of hydrogen and 8 g of oxygen?
- The law of conservation of mass gives you the answer.
- It is 9 g (1 g + 8 g) as matter is neither created nor destroyed.
- Also, the mass ratio of C and O in CO2 is 3:8. This suggests that every 11 g (3g + 8g) of carbon dioxide will contain 3 g of carbon and 8 g of oxygen.
You will learn in higher classes how to calculate the mass ratios of elements in a compound
Elements Compounds Mixtures Class 8
Mixture
Elements and compounds are pure substances, but rarely are they found in the pure state in nature.
- They are generally mixed with other elements, compounds or both. For example, air contains
- The elements nitrogen, oxygen and the noble gases (mainly argon), and
- The compounds are carbon dioxide and water.
- And natural water contains
- Dissolved air, and
Some salts (chlorides, sulphates, hydrogencarbonates, etc.) of metals like magnesium and calcium are dissolved in it.

Similarly, sea water contains so many dissolved substances that it is unfit for industrial, agricultural or domestic purposes.
- Thus, these substances are impure and are called mixtures. The different pure substances, which a mixture is made up of, are called the components of the mixture.
- These components can be separated by simple physical means
- A mixture is a substance which can be split into two or more pure substances by a physical means such as filtration, sublimation and distillation.
Examples of Mixtures
Most substances we come across in nature are mixtures. Fluman beings have also made many useful mixtures. Some examples are given below.
1. Air is the most commonly found mixture in nature. It contains nitrogen, oxygen, noble gases, carbon dioxide and moisture. We can separate the components by the physical means mentioned above.
2. Natural water is a mixture containing water, dissolved air and some salts which can be separated by physical means.
3. Foodstuffs
Example: Rice, wheat, pulses, vegetables, fruits, milk, butter, meat, and fish are all mixtures. They contain substances like starch, proteins, vitamins, salts, sugars and water in different proportions.
4. Medicines are mixtures containing some active ingredients in minute quantities (generally in milligrams) mixed with some other substances.
- The purpose of adding some other substances is to lessen the harshness of the chemical activity of the active ingredients.
- At the same time, the bulk increases, and it becomes easier to dispense the medicine.
5. Alloys are very common examples of mixtures. It has been found that a metal, when alloyed with other metal(s) or nonmetal(s), is generally more useful than the original metal.
For example:
Steel (containing iron, carbon and manganese) or stainless steel (containing iron, chromium and nickel) is more useful than iron. And so are brass (containing copper and zinc) and bronze (containing copper and tin) more useful than copper. Also, gold is generally mixed with silver and copper—the alloy is tougher.
6. Industrial materials like cement and glass are mixtures of silicates of metals. Cement is a mixture of calcium silicate and aluminium silicate, and glass, that of sodium silicate, potassium silicate and lead silicate.
7. All minerals are mixtures
Characteristics of a Mixture
A mixture has the following characteristics.
1. The components of a mixture may be present in any proportion
For example:
Regardless of whether you dissolve one or two spoons of sugar in a glass of water, the resulting solution will be a sugar-water mixture.
- Again, air—a gaseous mixture—contains more carbon dioxide in cities than in the countryside.
- Also, the moisture content of air is greater during the rains than in the dry season.
2. The components of a mixture coexist without chemically reacting with one another.
Types Of Mixtures Class 8 Chemistry
For example:
- Nitrogen, oxygen, carbon dioxide, noble gases and moisture coexist in air as they are and do not react among themselves to form any new substances.
- Similarly, water does not react with the soil in mud or with sugar in a sweet drink.
3. The components of a mixture retain their properties. The constituent elements of a compound do not retain their properties, but the components of a mixture do.
For example:
Hydrogen and oxygen do not show their properties in water, but water and sugar do in a sugar solution. Isn’t a sugar solution as sweet as well as watery?
4. The components of a mixture can be separated by simple physical means. By simple physical means, we mean here methods like filtration, sublimation, distillation, etc. We will discuss these methods soon
Types of Mixtures
Mixtures are usually classified based on the state of their components.
- A mixture containing two or more solids is called a solid mixture, two or more liquids, a liquid mixture, and two or more gases, a gaseous mixture.
- Besides, we can have solid-liquid, solid-gas and liquid-gas mixtures too.
- Further, a mixture can be homogeneous or heterogeneous, as described below.
- A mixture which has the same composition and properties throughout is said to be homogeneous.
- A mixture which has different compositions and properties in different parts of it is said to be heterogeneous.
One can see separately or distinguish between the different components of a heterogeneous mixture, but not ofa homogeneous mixture.

Thus, a fizzy drink that contains carbon dioxide, water and a sweetener in the same proportion throughout is a homogeneous mixture. But an oil-water mixture is a heterogeneous mixture.
Lists various kinds of mixtures, along with examples:
Types of mixtures with examples:

Separating The Components Of Mixtures
To obtain a pure substance, we often need to separate the components of a mixture.
It is only by doing so that we obtain
- Common salt from seawater,
- Drinking water from ordinary water,
- Petrol, diesel and kerosene from crude oil.
Principles of Separation
For separating the components of a mixture, we take advantage of the property of the components in which they differ the most. Such a distinguishing property can be the state, size, magnetic behaviour, solubility, melting point, boiling point or adsorbability.
Thus, it will be easy to separate
- A solid from a liquid or a gas, and a liquid from a gas,
- Smaller particles from larger ones,
- An amagnetic substance from a nonmagnetic substance,
- A soluble substance from an insoluble one
- A high-melting solid from a low-melting one
- A high-boiling liquid from a low-boiling liquid, and
- A strongly adsorbing substance from a weakly adsorbing substance.
- We will see how the methods of separation are based on these principles
Difference Between Elements and Compounds
Methods of Separation
Scientists use varied methods of separation, from simple to sophisticated. But here we will discuss some simple ones only.
Separating the Components of Mixtures
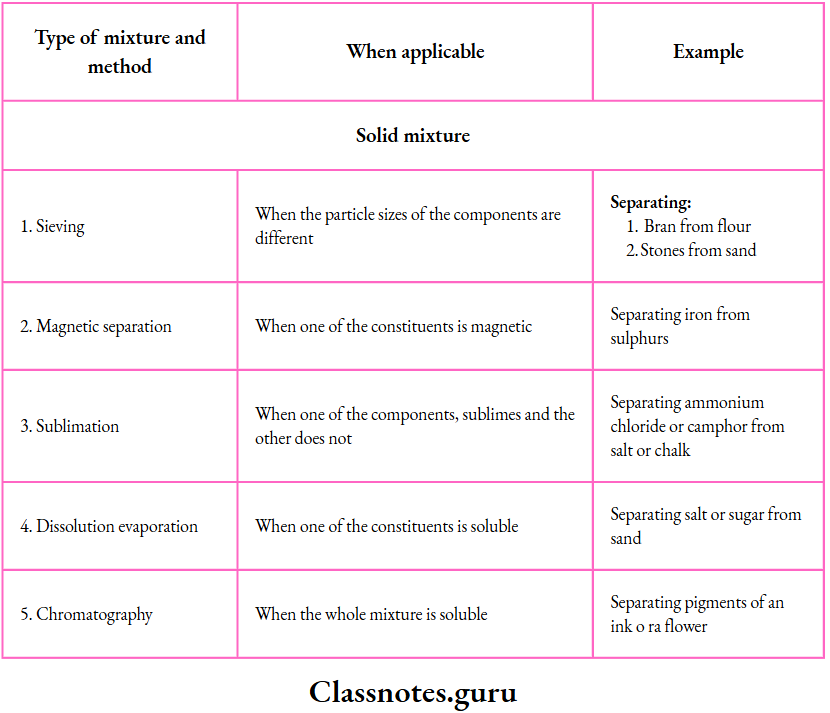
1. Sieving:
This method is based on the difference in the particle size of the components.
- When a solid mixture is stirred or shaken on a mesh, called a sieve, the particles smaller than the holes of the mesh pass through.
- And the larger ones remain on the mesh
- Fine sieves are used in the kitchen to separate bran from flour, and bigger ones at construction sites to separate stones from sand.
- Thus, sieves of different sizes are used for different solid mixtures
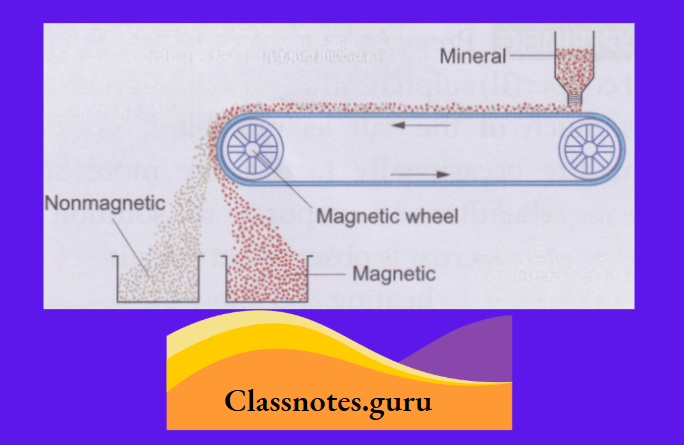
2. Magnetic separation:
This method is used to separate a magnetic substance from a nonmagnetic substance.
- You have learnt in the previous classes that iron, being magnetic, can be separated from sulphur or chalk, which is nonmagnetic, by moving a magnet through the mixture.
- The method, in a modified form, is highly useful in separating magnetic minerals from nonmagnetic ones. The mixture is placed on a conveyor belt moving around a magnetic wheel.
- While falling from the belt, the mixture gets separated into two heaps.
The outer heap consists of the nonmagnetic component and the inner heap, of the magnetic component (as it is attracted by the pole).
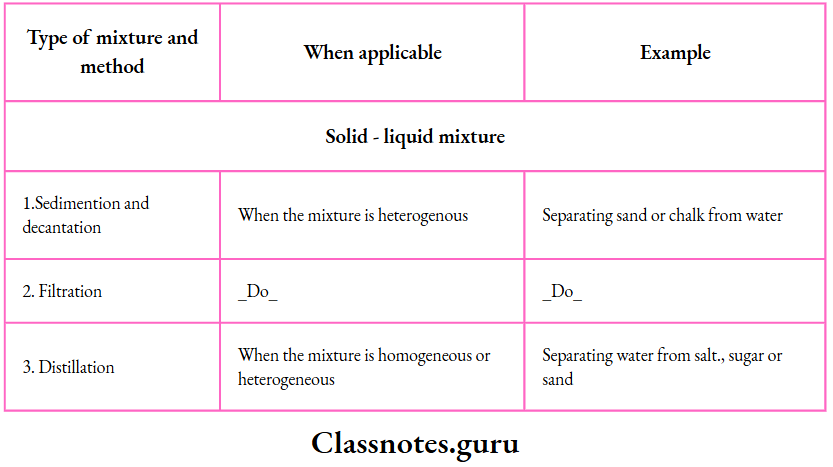
3. Sedimentation and decantation:
This method is useful for separating the components of a solid-liquid mixture in which the solid is heavier than the liquid.
Example:
- A sand-water mixture. If the mixture is allowed 3. Sedimentation and decantation.
- This method is useful for separating the components of a solid-liquid mixture in which the solid is heavier than the liquid.
Example: A sand-water mixture. If the mixture is allowed.


The process can be used to separate immiscible liquids from one another.
However, it is not suitable for the separation of the components of a solid-liquid mixture in which the solid is lighter than the liquid, as in a husk-water mixture
4. Filtration:
If you want to separate a liquid from an insoluble solid, filtration is a better method than decantation.
You have learnt how a filter paper cone is prepared and fitted to a funnel.
- The apparatus is set up as shown.
- The solid-liquid mixture is allowed to stand for some time.
- The supernatant liquid is poured along a glass rod into the funnel.
- The liquid passes through, and the solid collects on the filter paper.
- The clear liquid thus obtained is called the filtrate.
You can easily separate sand or chalk from water by filtration.

5. Dissolution followed by evaporation or crystallisation:
This method is useful for a solid mixture of which one component is soluble in a chosen solvent but the other is not.
Example: A mixture containing
- Salt or sugar (soluble in water) and sand, chalk or sawdust (insoluble in water), or
- Sulphur (soluble in carbon disulfide) and iron filings (insoluble in carbon disulfide).
Stir a salt-sand mixture in water and warm so that the salt dissolves. Filter the mixture. The salt passes into the filtrate, and the sand remains as residue.
- The residue is washed with warm water to make the sand free of salt.
- The filtrate, along with the washings, is slowly heated in a dish, and the water evaporates, and the salt behind.
- Prepare a concentrated solution of copper (II) sulphate in a beaker by dissolving as much of the salt as possible.
Heat the mixture occasionally to dissolve more and more salt. Filter and evaporate the solution in a basin till a crust is observed at the surface of the liquid.
Mixtures And Their Types Class 8
- Stop heating and allow the solution to cool. Beautiful blue crystals of copper(II) sulphate pentahydrate will appear slowly and will grow in size with time.
- A purer sample of the salt can be obtained by crystallisation from the filtrate.
- The filtrate is heated to obtain a concentrated solution (i.e., a solution containing a smaller proportion of the solvent than usual).
- When the concentrated solution is left to cool slowly, the white crystals of the salt separate.
- Remember that a purer solid is obtained from a solution by crystallisation rather than by evaporation to dryness.
- Crystallisation sets in better and faster if the concentrated solution is seeded with a small crystal of the pure solid. For seeding, you may simply have to add the solid to the concentrated solution while cooling.
- You have learnt in the previous class how crystals of candy can be obtained from a concentrated solution of sugar.
- Here you can try to obtain the crystals of copper(II) sulphate pentahydrate (CuS04.5H20), also called blue vitriol
Experiment:
Prepare a concentrated solution of copper (II) sulphate in a beaker by dissolving as much of the salt as possible.
- Heat the mixture occasionally to dissolve more and more salt.
- Filter and evaporate the solution in a basin till a crust is observed at the surface of the liquid.
- Stop heating and allow the solution to cool.
Beautiful blue crystals of copper(II) sulphate pentahydrate will appear slowly and will grow in size with time.
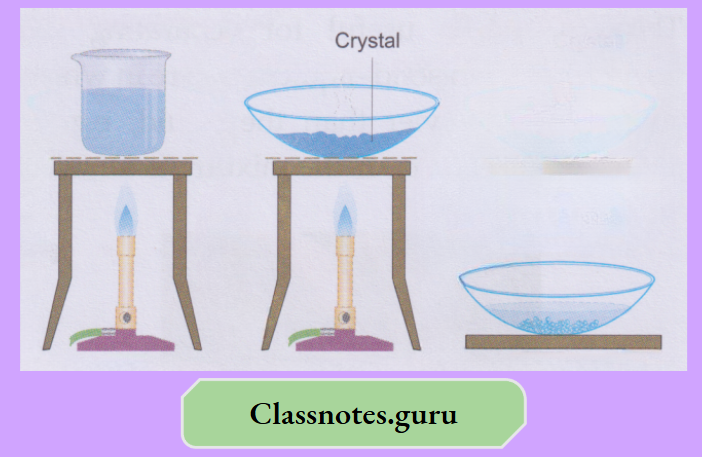
Similarly, sulphur can be separated from iron filings by stirring the mixture with carbon disulfide. The sulphur comes out with the solvent. Iron is filtered, and the filtrate gives sulphur on evaporation or crystallisation.
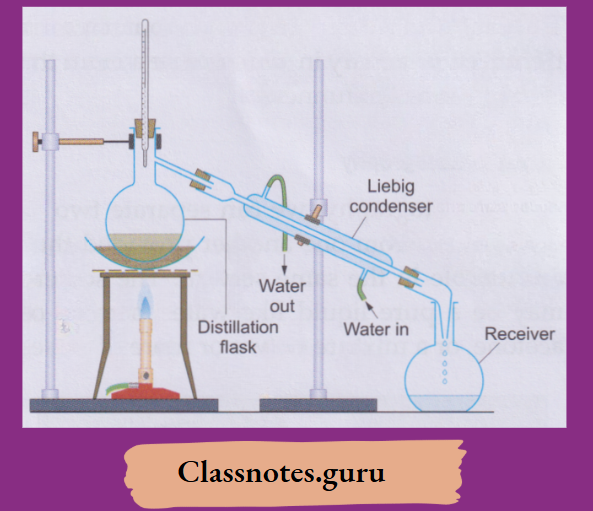
6. Distillation:
By distillation, we can separate a solid-liquid mixture—homogeneous
Example: A solution of salt in water) or heterogeneous
Example: A sandwater or a chalk-water mixture.
The apparatus is set up as shown. The mixture is boiled in a distillation flask.
The vapours coming out condense while passing through a Liebig condenser in which they are cooled by the water circulating in the outer jacket.
- The pure liquid (Example: Water) is collected in a receiver.
- The solid—soluble (Example: Salt) or
- Insoluble (Example: Sand or chalk) remains in the distillation flask.
This is how distilled water is prepared in the laboratory
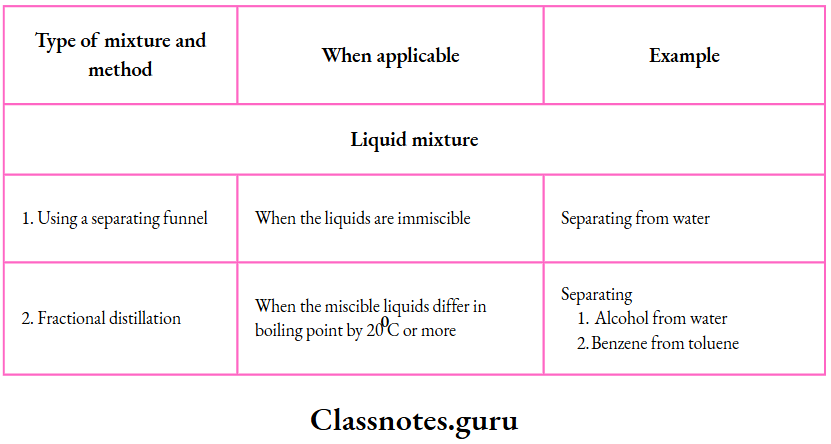
7. Fractional distillation:
By fractional distillation, we can separate liquids which differ in their boiling points by 20°C or more
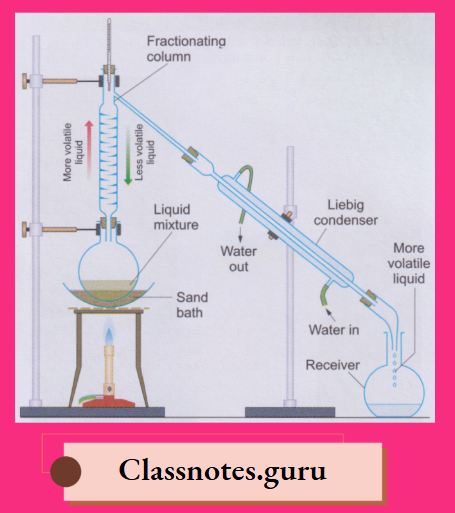
The liquid mixture is boiled in the distillation flask fitted with a fractionating column and a Liebig condenser
- The mixed vapours enter the fractionating column, where the vapours of the higher¬ boiling (i.e., less volatile) liquid condense and trickle back into the distillation flask.
- The vapours of the lower-boiling (i.e., more volatile) liquid, however, pass into the Liebig condenser, where they condense; the liquid is collected in the receiver
- The temperature of the boiling mixture remains constant till the lower-boiling liquid distils completely.
- Then the temperature again rises till the higher-boiling liquid starts distilling.
- The receiver is quickly changed to collect the higher-boiling liquid.
- The liquids obtained by boiling a mixture at different temperatures are called fractions, and the method of fractional distillation is also called fractionation
By this method, we can separate
- Benzene (boiling point 80°C) is from toluene (boiling point 110°C),
- Ethyl alcohol (boiling point 78°C) from water (boiling point 100°C), and
- Petrol, diesel and kerosene from crude oil.
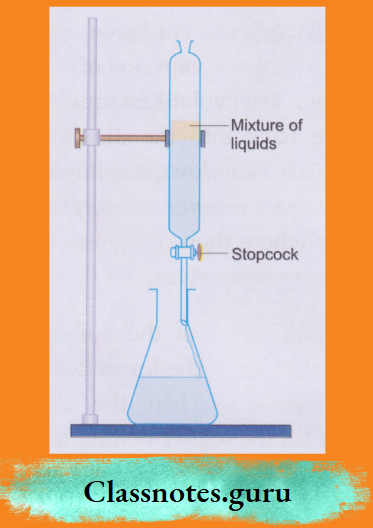
8. Using a separating funnel:
A separating funnel is used to separate two or more immiscible liquids.
- The mixture is placed in a separating funnel and allowed to stand.
- The different immiscible liquids form separate layers, which can be collected in different vessels one after the other.
- By this method, we can separate an oil, benzene, toluene or ether from water.
9. Sublimation:
Using this method, we can separate a substance that sublimes
Example: Ammonium chloride, camphor or iodine)
From one that does not (Example: Salt, sand or chalk).
A funnel is inverted over the mixture placed in a china dish. A dry test tube is also inverted over the outlet of the funnel. The outlet of the funnel is loosely plugged with cotton. The mixture is heated.
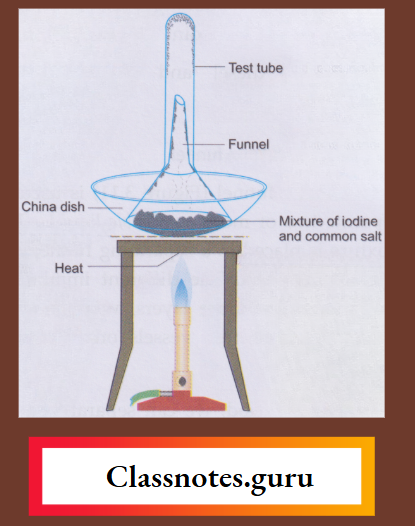
The sublimable component vapourises and the vapours solidify in the test tube and on the cooler part of the funnel.
10. Chromatography:
By chromatography, we can separate two or more solids from one another, provided they are soluble in the same solvent.
The solvent may be a pure liquid like water, alcohol or acetone, or a mixture of two or more of these.

The method works on the principle of adsorption.
- One should understand the difference between adsorption and absorption.
- In absorption, a substance gets equally distributed over the entire bulk of another substance, like dissolved air in natural water or carbon dioxide in a fizzy drink.
- Adsorption, however, is a surface phenomenon in which a substance is held at the surface of another by a weak force.
An example is a dye held on the surface of a fibre.
The substance that is adsorbed
(Example: A dye) is called the adsorbate, and the surface on which it is adsorbed
(Example: A fibre), The adsorbent.
In chromatography, we generally use cellulose, silica or alumina as an adsorbent.
- Cellulose is conveniently used in the form of blotting paper, filter paper (generally Whatman 41) or specially made chromatographic paper. We will now discuss the technique of paper chromatography.
- You must have observed that blotting paper soaks up a liquid that spreads fast over the paper.
- The liquid moves even against gravity, i.e., upwards on a vertically placed blotting paper.
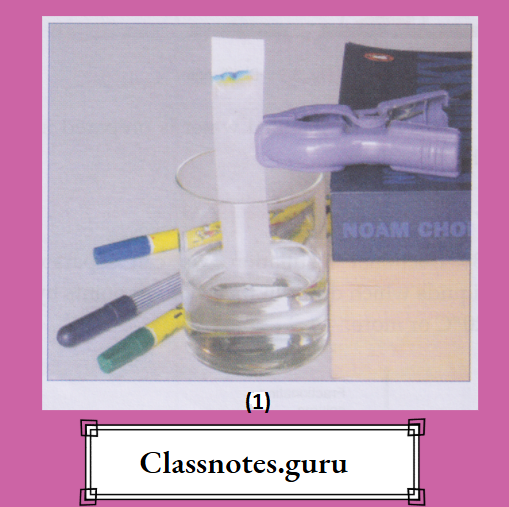
- A long strip of chromatographic paper or a good-quality filter paper is cut out.
- A drop of a solution of the mixture (say the ink of a green sketch pen) is placed about a centimetre from one end of the strip and dried.
- A very small amount of the solvent is taken in a jar.
- The paper strip is suspended in the jar such that the end near which the mixture is placed just touches the solvent.
- The whole set-up is left undisturbed. After some time, one can observe that the green-ink spot has moved up the strip and separated into two colours— blue and yellow.
- (In fact, the blue and yellow make up the green.)
This happens because the different dyes (pigments), i.e., the different colouring substances, are held (i.e., adsorbed) by the adsorbent with different forces—some by stronger and some by weaker forces.
- The one that is held less strongly is driven faster by the solvent than that held more strongly by the adsorbent.
- As a result, the different pigments move with different speeds over the adsorbent surface under the influence of the solvent. And so they get separated.
The array of colours on a chromatographic paper is called a chromatogram.
- One can take a mixture of inks of different colours to have a more colourful chromatogram.
- The smaller strips of different colours are now cut out from the main strip.
- And the colouring matter can be obtained from each strip by dissolving it out in the solvent and evaporating the solvent.
In chromatography, the adsorbent part is called the stationary phase, and the things that move, i.e., the solvent and the solution, are collectively known as the mobile phase.
- Different types of chromatographic techniques have been developed and named based on the types of phases.
- Column chromatography is a commonly used technique in which the stationary phase is a column of adsorbent, i.e., the adsorbent is packed in a vertically placed wide tube.
- Other well-known types are gas-liquid chromatography (GLC) and high-performance liquid chromatography (HPLC)
Separation Methods: A Summary
A summary of the methods ofseparationofthe components of mixtures is given in Table 3.3.
Methods of separating the components of different types of mixtures:
Separation of mixtures—a few examples
Through the following examples, you will learn how to choose a method for separating the components of a given mixture.
1. A sand-water mixture:
Sand can be separated from water by filtration or distillation. In distillation, the water distils out, leaving the sand as residue.
2. A salt solution:
By distillation, the water can be obtained as the distillate and the salt as the residue. (By evaporation to dryness, the salt can be obtained, but the water will be lost.)
3. A salt-sand mixture:
The salt can be dissolved in water, and the sand filtered out. The filtrate, on evaporation to dryness, yields the salt.
4. A sugar-chalk mixture:
Sugar is soluble in water, but chalk is not. So, the sugar can be dissolved in water, leaving the chalk behind. The mixture, on filtration, will give the chalk as the residue and the filtrate, on evaporation or crystallisation, will yield the sugar.
5. An iron filings-sawdust mixture:
As iron is magnetic and sawdust is not, magnetic separation will be a convenient method to separate them.
6. An iron filings—sulphur mixture:
Two methods can be used.
- Magnetic separation (Iron is magnetic, but sulphur is not.)
- Dissolution of the sulphur in carbon disulfide, followed by the recovery of the sulphur from the solution by evaporation or crystallisation.
7. A carbon-sulphur mixture:
Knowing that sulphur is soluble in carbon disulfide but carbon is not, you can suggest the method.
8. A water-oil mixture.:
As water and oil are immiscible, they will form separate layers and can, therefore, be separated by using a separating funnel.
- Remember that, like oil, chloroform, carbon tetrachloride, and ether are also immiscible with water.
- So, the method will be useful for a mixture containing water and any of these liquids.
9. A benzene-toluene mixture:
As the difference in the boiling points of benzene (80°C) and toluene (110°C) is more than 20°C, the two miscible liquids can be conveniently separated by fractional distillation.
10. An ink mixture: By paper chromatography.
11. A salt-sand-sulphur mixture:
Among the three components, only sulphur is soluble in carbon disulphide and only salt in water, but sand in either of the two solvents.
- So, the sulphur can be dissolved in carbon disulfide. From the residue containing salt and sand, the salt can be dissolved out in water, leaving the sand behind.
- The sulphur and salt can be recovered from their solutions by evaporating the solvents.
- Alternatively, first the salt canbe dissolved out in water and then sulphur in carbon disulfide.
12. A carbon-sulphur-nitre mixture (gunpowder):
Gunpowder is an explosive containing carbon, sulphur and potassium nitrate (nitre). Only sulphur is soluble in carbon disulphide, and only nitre in water.
- So, the sulphur and nitre can be dissolved out successively in carbon disulfide and water, and recovered from the solutions by evaporating the solvents or by crystallisation.
- After the final dissolution, carbon will be left as the residue.
- Alternatively, the nitre can be dissolved out first and then the sulphur.
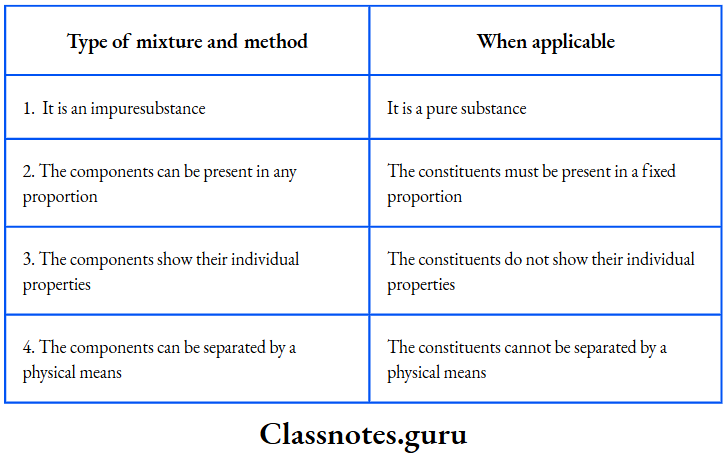
Difference between a Mixture and a Compound.
We can now conclude that a mixture differs from a compound, as shown in Table
How a mixture differs from a compound: