Chapter 2 Physical And Chemical Changes Notes
Changes occur every moment around us. They are an integral part of nature. In this chapter, we will see how changes are classified and study physical and chemical changes systematically.
How Changes Are Classified
Let us now discuss some ways in which changes are classified
Reversible and Irreversible Changes
A change is said to be reversible when the opposite change can be brought about by reversing the conditions.
Read And Lean More Class 8 Chemistry
Changes in state of matter are the most common examples of reversible change
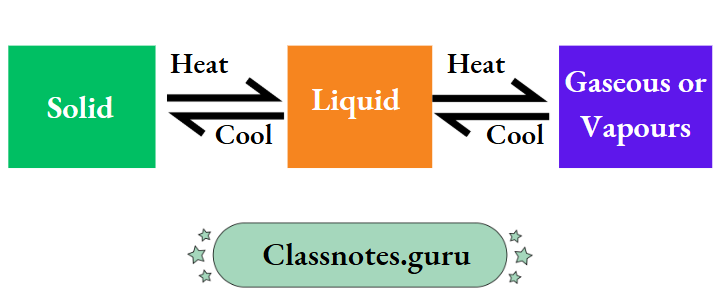
A solid melts on being heated and the melt solidifies (i.e., freezes) on being cooled. So, the melting of a solid and also the freezing of a liquid are reversible changes.
And so are the vaporisation of a liquid (on being heated) and the condensation of vapours (on being cooled). Also, the heating and cooling of the coil of an electric heater when the heater is switched on and off, respectively, are reversible changes
NCERT Solutions For Physical And Chemical Changes

A change is said to be irreversible when the opposite change cannot be brought about by reversing the conditions
For example:
Sugar, when heated, swells to form a brown substance which finally gets charred, i.e., forms a black mass. But the charred mass, on being cooled, does not give back sugar.
Similarly, milk can be changed into cottage cheese by boiling it with some lemon juice added to it. But cottage cheese, or paneer, cannot be changed into milk. Thus, the charring of sugar and the curdling of milk are irreversible changes. And so are the growth of plants and animals, digestion, photosynthesis, and the burning of a fuel
Activities:
1. With the help of an adult, place some sugar crystals in a china dish. Heat the solid slowly and observe the changes. It swells and becomes brown and finally black. The black mass is carbon.

2. Add a few drops of lemon juice to the milk and boil the mixture. In a short while, a thick white substance, called curd, is formed. This is called the curdling of milk. Curd is entirely different from milk. You will not be able to reverse this change.

Periodic and Nonperiodic Changes
Periodic changes are those which take place at fixed intervals of time.
- You may have seen a clock with a pendulum. The pendulum moves from side to side, or to and fro, continuously, at fixed intervals of time.
- The motion of the pendulum is an example of periodic change.
- As you know, the moon changes phase gradually from full moon to new moon and then again to full moon.
- This change takes place over 28 days. The same change is repeated over the next 28 days. So, the phases of the moon are an example of periodic change.
NCERT Class 8 Chemistry Chapter 2 Physical And Chemical Changes
- Nonperiodic changes are those which do not take place at fixed intervals of time.
- The melting of ice, vaporisation of water, curdling of milk and charring of sugar do not repeat themselves at fixed intervals of time.
- So, they are non-periodic changes.
Desirable and Undesirable Changes
The changes that are useful to us are called desirable changes whereas those that are harmful are called undesirable changes.
- Thus, the digestion of food, the ripening of fruit, the growth of living beings and the changes involved in cooking are desirable changes.
- But the rusting of iron and the spoiling of food are undesirable changes.
- Earthquakes, cyclones and volcanic eruptions are such undesirable changes that cause damage on a large scale.
Physical And Chemical Changes
Chemists are very interested in the classification of changes into physical and chemical changes. Therefore, we will study physical and chemical changes separately.
Physical Changes
A change in which no new substances are formed and which can be reversed by reversing the conditions is called a physical change. We will now discuss some examples of physical change.
Physical And Chemical Changes NCERT Notes
The glowing of a heater or a bulb:
An electric heater or a bulb glows when it is switched on.
- But the glow vanishes as it is switched off. So the change is reversible. Also, no new substances are formed.
- Therefore, the glowing of a heater or a bulb is a physical change.

Thermal expansion of a substance:
On being heated, a substance expands but on being cooled, it contracts. Also, no new substances are formed, and so the change is physical.
- You must have observed that an inflated balloon often bursts on its own at a birthday party.
- Why so? Because the air inside the balloon expands when the latter remains near a hot bulb for a while.
Changes in state of matter:
You have learnt that any change in the state of matter is reversible. And also that the kind of matter remains the same in any change in state of matter, be it solid-liquid or liquid-vapour (gas) interconversion.
For example:
The melting of ice is reversed by the freezing of water, and the vaporisation of water is reversed by the condensation of water vapour.
At the same time, the substances in the three states are the same, i.e., water (H2O). This is true for other substances too.
Example: Sulphur, sodium, iron, copper and zinc.
The kinetic theory of matter tells us that in the change
Physical vs Chemical Changes Class 8
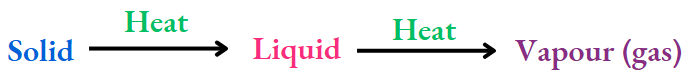
Only the kinetic energy (KE) of the molecules increases when heating. And in the opposite case,
The KE of the molecules decreases on cooling.

Sublimation:
This is also an example of state change. Insublimation, a solid like ammonium chloride, naphthalene, camphor or iodine directly forms vapours, without melting. And when cooled, the vapours are directly converted into the solid. Thus, the change is reversible. No new substances are formed either. So, sublimation is a physical change.
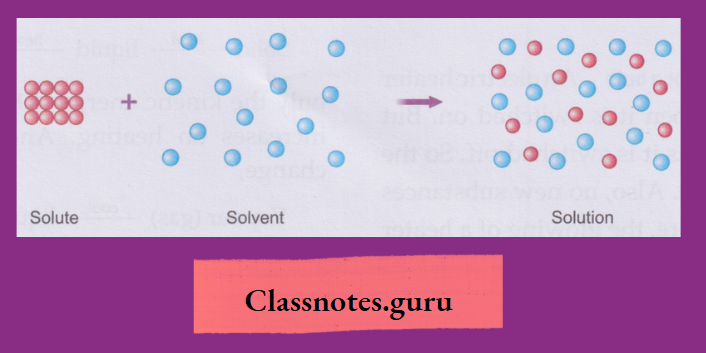
The dissolving of a substance in a liquid:
You know that a solid like sugar, salt, or glucose dissolves in water.
- The substance that dissolves is called thesoluteand the liquid in which the substance dissolves is called the solvent.
- Thus, a solute dissolves in a solvent to form a solution.
- As the solute can be obtained back from the solution by evaporating the solvent, dissolution is a physical change.
- While dissolving in a liquid, a solid breaks up into molecules inside the liquid.
- And these molecules hide themselves in the intermolecular space of the liquid.
- So the volume of a liquid does not change if II I something is dissolved in it
Chemical Changes
- A change in which new substances are formed and which cannot be reversed by reversing the conditions is called a chemical change.
- Some examples of chemical change are mentioned below.
Types Of Changes In Matter Class 8
Burning:
When lighted, a combustible substance (Example: Hydrogen, coal, wood, paper, kerosene, diesel, petrol, LPG, CNG, spirit) burns in air.
- You have learnt that burning is a fast reaction between the combustible substance and the oxygen of the air.
- When kindled, hydrogen burns in air to form a new substance, water. On being cooled, water does not give back hydrogen, so the change is irreversible.
- Thus, the burning of hydrogen is a chemical change.
- Coal contains mainly carbon. When burnt, the carbon of the coal combines with the oxygen of air to form a new substance, carbon dioxide. Carbon dioxide is a gas and is liberated into the air.
This change is irreversible —on being cooled, carbon dioxide does not give back carbon and oxygen. Hence, the burning of coal is a chemical change.
Wood or paper, when burnt, combines with the oxygen of the air and forms new substances —carbon dioxide and water vapour.
- On being cooled, carbon dioxide and water vapour do not give back to the wood or paper. Thus, the burning of wood or paper is a chemical change.
- Kerosene, diesel, petrol, LPG (liquefied petroleum gas) and CNG (compressed natural gas) are all hydrocarbons, i.e., they are made up of carbon and hydrogen only.
- When lit, they burn in air to form the new substances, carbon dioxide and water vapour.
The changes are irreversible, too. So, the burning of these fuels is a chemical change

Ethanol (i.e., ethyl alcohol, commonly called alcohol) is a compound containing carbon, hydrogen and oxygen. It is a liquid that catches fire when near a flame and burns to give carbon dioxide and water vapour. This is also a chemical change.
Physical And Chemical Changes Examples
Rusting
Iron rusts in moist air, forming a red-brown solid, called rust. The rust formed cannot be changed back to iron by reversing the condition, i.e., the change is irreversible. Thus, rusting is a chemical change.

The cooking of food
The taste of vegetables and pulses changes when they are cooked because new substances are formed in the process. Also, we cannot obtain green vegetables from a vegetable curry or grains from cooked pulses. So, the cooking of food is a chemical change.
The curdling of milk:
- Once milk is curdled
- A new substance (curd) is formed, and
- Milk cannot be obtained back from the curd, i.e., the change is irreversible. Thus, the curdling of milk is a chemical change.
The charring of sugar:
Sugar belongs to a class of compounds called carbohydrates. A carbohydrate contains carbon and the elements of water. So, when sugar is heated, water gets loosened and vaporised, and the black residue of carbon is left behind. You have learnt that the change is also irreversible and is, therefore, a chemical change.
Digestion
Food, on being digested inside our body, is changed into several different things—some of which are taken up and some expelled by the body. The change cannot be reversed either. Thus, digestion is a chemical change.
Fermentation
Fermentation is a process employed for preparing alcohol from fruits containing sugar. In the presence of enzymes, a sugar solution changes into alcohol, liberating carbon dioxide.
- As a result, a froth is formed, and the liquid appears as if it were boiling.
- The process is known as fermentation (derived from the Latin word fervere, meaning ‘to boil’).
- It is an irreversible process in which new substances are formed. Therefore, it is a chemical change
Photosynthesis:
Green plants prepare their food (glucose) from carbon dioxide and water in the presence of chlorophyll in sunlight
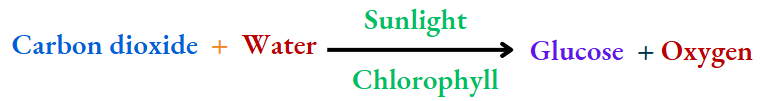
New substances (glucose and oxygen) are formed in the process. Also, the change is irreversible. So, photosynthesis is a chemical change.
Chemical Reactions NCERT Notes
Respiration:
In respiration, the glucose (a carbohydrate) formed by a living being combines with oxygen of the air, forming the new substances carbon dioxide and water. Energy is also released in the process, and the change is irreversible. So, respiration is a chemical change
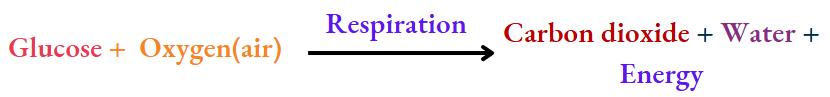
Conservation of mass in a chemical change
The mass of the individual substance(s) undergoing a chemical change is altered. But the total mass of the reactants is the same as that of the products.
For example:
When carbon is burnt in air, the mass of carbon is reduced, and finally, the carbon vanishes.
- All the carbon gets converted to carbon dioxide.
- But the mass of the carbon used plus the mass of the oxygen taken up from the air is the same as that of the carbon dioxide formed
The mass of an iron nail increases when the nail rusts. But the mass of the original nail plus that of the oxygen and moisture taken up from the air is the same as that of the rusted nail.
Thus, a chemical change obeys the law of conservation of mass.
Comparison between physical and chemical changes:
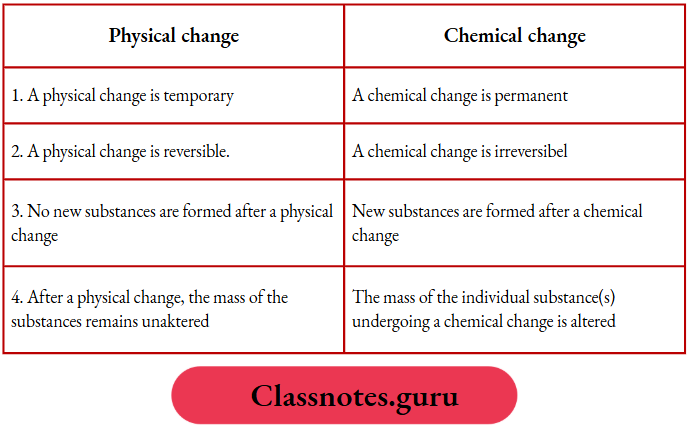
Chemical Change NCERT Notes
Can Physical and Chemical Changes Occur Together?
Yes, they can. Let us examine the changes that occur when a candle burns.
1. The wax under the wick melts. The molten wax flows down and solidifies. These are changes in state and, therefore, physical changes

A part of the molten wax that vaporises burns to form carbon dioxide and water vapour. The burning of wax is a chemical change
Physical and Chemical Changes Involve Energy Change
There is a change in energy when a physical or chemical change occurs. Energy, usually in the form of heat, is either taken in (i.e., absorbed) or given out (i.e., evolved) by a substance undergoing a change
- A change during which heat is given out (i.e., evolved) is called an exothermic change.
- A change during which heat is taken in (i.e., absorbed) is called an endothermic change
There are many changes which are neither exothermic nor endothermic. The formation of a heterogeneous mixture is a common example of this type of change. When sand is mixed with salt or water, no heat is given out or taken in.
Energy change in a physical change
State changes:
- Energy changes take place during a state change. A solid absorbs heat to melt, and a liquid absorbs heat to change into a vapour. Thus, these are endothermic changes.
- On being cooled, gases and vapours condense and liquids freeze. Heat is given out in these processes. So, these are exothermic changes.
Activity:
Take a little water in a steel or glass bowl. Pour boiling water into a mug. Hold the bowl over the mug. Steam coming out of the mug will condense as it comes in contact with the bowl. After some time, you will find that the water in the bowl has become warmer than before.
On being boiled, water changes into water vapour. The water vapour condenses, transferring heat to the cold water in the bowl. So, the water in the bowl becomes warm.

Why an energy change in a change of state:
This happens because the kinetic energy possessed by the molecules of a substance is different in different states.
- It is the lowest in the solid state, higher in the liquid state and the highest in the gaseous state.
- So, energy (heat) will be absorbed by a solid to melt and a liquid to boil or vaporise. And heat will be evolved by vapours to condense and a liquid to freeze.
- Dissolution. Many substances evolve heat, whereas some others absorb heat while dissolving in a solvent. You can test this by doing the following activity.
NCERT Class 8 Physical And Chemical Changes
Activity:
Hold a glass in your hand. Pour some water into the glass and stir it after adding a couple of spoons of glucose. The glass will become slightly colder. Thus, the dissolution of glucose in water is an endothermic change. You can get back the glucose by evaporating the water.
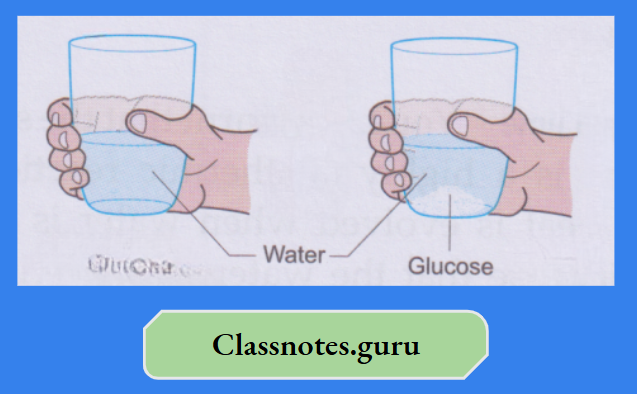
When you put some glucose on your tongue, your tongue feels cool. While dissolving in the moisture of the tongue, glucose absorbs heat from it. So, your tongue feels the cooling effect. On the other hand, if you add bathroom acid (concentrated hydrochloric acid) to water, heat is evolved.
Why an energy change in dissolution:
During dissolution, the solute particles break up from the main bulk and hide themselves in the intermolecular space of the solvent.
- This affects the motion and so the KE of the molecules. The KE ofthe particles is dependent on temperature.
- And so, a change in the KE will result in the absorption or evolution of heat.
Energy change in a chemical change
All chemical changes involve a change in energy.
Burning:
You know that both heat and light are emitted when anything burns

Photosynthesis:
In photosynthesis, chlorophyll —the green pigment of leaves—absorbs sunlight (energy), and helps the plant produce glucose and oxygen from water and carbon dioxide.
Slaking of lime. You have learned that the slaking of lime is a highly exothermic reaction. So much heat is evolved when water is added to quicklime that the water boils during the reaction.
Quicklime + water→slaked lime + heat
Why an energy change in a chemical change:
A chemical change involves a rearrangement of atoms. The reactant molecules break up, and the product molecules are formed.
- These processes are not only opposite but also involve different amounts of energy.
- So, in the overall process, there is either a surplus or a deficit of energy.
- If there is a surplus, energy will be given out, and if there is a deficit, energy will be taken in. So, there is a change in energy in a chemical change.
Energy Requirement for the Completion of a Change
The energy requirements are different for the completion of the two kinds of change: endothermic and exothermic
Endothermic change:
You know that energy is absorbed in an endothermic change. In other words, a substance essentially requires energy from outside to undergo an endothermic change. Hence, an endothermic change continues only till energy is pumped in.
For example:
A solid melts or a liquid boils only when it is heated. And the reaction between nitrogen and oxygen forming nitric oxide (NO)—a highly endothermic reaction—takes place only in the presence of an electric spark. So, the reaction takes place in the sky only when there is lightning
Exothermic change:
Heat is evolved in an exothermic change. So, at first instance, it appears that no energy should be required from outside by the substance undergoing such a change. And actually, there are many exothermic changes—especially the exothermic physical changes—which do not need energy from outside at any stage of the change.
A common example is the condensation of vapour or the freezing of a liquid. Similarly, when dilute hydrochloric acid is added to a solution of sodium hydroxide, an instant reaction takes place (forming sodium chloride and water), liberating heat.
However, many exothermic reactions do not take place without being initiated by heating, igniting, illumination and sparking. But once initiated, exothermic reactions go to completion on their own.
NCERT Class 8 Physical And Chemical Changes
Some examples are given below:
- Hydrogen does not react with oxygen when the two are only mixed, but it reacts explosively when the mixture is kindled or sparked. This is a highly exothermic reaction.
- A piece of coal, wood or paper (in fact, any combustible substance) does not burn unless lit. But once the substance starts burning, it burns out completely.
- The reaction between iron and sulphur, forming iron(II) sulphide, is an exothermic process.
- When iron filings and sulphur are mixed in the mass ratio 7 : 4, nothing happens. But once the mixture is heated in a hard-glass test tube for a short while, it begins to glow, showing that a reaction has started.
- Now you can stop heating, but you will find that the solid continues to glow, and the glow spreads throughout the mass.
- After the glow dies out and the test tube cools down, you will find that a greyish black solid is formed, different from the original mixture. This is a compound, iron(II) sulphide.
Thus, we can conclude that an exothermic reaction, once initiated, continues. The heat evolved is more than enough to sustain it.
